Back to Journals » Biologics: Targets and Therapy » Volume 17
Effectiveness of RCTs Pooling Evidence on Mesenchymal Stem Cell (MSC) Therapeutic Applications During COVID-19 Epidemic: A Systematic Review
Authors Kandula UR , Wake AD
Received 11 January 2023
Accepted for publication 9 May 2023
Published 18 May 2023 Volume 2023:17 Pages 85—112
DOI https://doi.org/10.2147/BTT.S404421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Usha Rani Kandula, Addisu Dabi Wake
Department of Clinical Nursing, College of Health Sciences, Arsi University, Asella, Ethiopia
Correspondence: Usha Rani Kandula, Adult Health Nursing, College of Health Sciences, Arsi University, Oromia Region, P.O. Box-396, Asella, Ethiopia, Tel +251-939052408, Email [email protected]; [email protected]
Background: Global pandemic identified as coronavirus disease 2019 (COVID-19) has resulted in a variety of clinical symptoms, from asymptomatic carriers to those with severe acute respiratory distress syndrome (SARS) and moderate upper respiratory tract symptoms (URTS). This systematic review aimed to determine effectiveness of stem cell (SC) applications among COVID-19 patients.
Methods: Multiple databases of PubMed, EMBASE, Science Direct, Google Scholar, Scopus, Web of Science, and Cochrane Library were used. Studies were screened, chosen, and included in this systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flowchart diagram and PRISMA checklist. Included studies’ quality was assessed employing Critical Appraisal Skills Programme (CASP) quality evaluation criteria for 14 randomized controlled trials (RCTs).
Results: Fourteen RCTs were performed between the years of 2020 to 2022, respectively, with a sample size n = 574 (treatment group (n = 318); control group (n = 256)) in multiple countries of Indonesia, Iran, Brazil, Turkey, China, Florida, UK, and France. The greatest sample size reported from China among 100 COVID-19 patients, while the lowest sample of 9 COVID-19 patients from Jakarta, Indonesia, and the patient’s age ranges from 18 to 69 years. Studies applied to the type of SC were “Umbilical cord MSCs, MSCs secretome, MSCs, Placenta-derived MSCs, Human immature dental pulp SC, DW-MSC infusion, Wharton Jelly-derived MSCs”. The injected therapeutic dose was 1 × 106 cells/kg, 1 × 107 cells/kg, 1 × 105 cells/kg, and 1 million cells/kg as per the evidence from the different studies. Studies focused on demographic variables, clinical symptoms, laboratory tests, Comorbidities, respiratory measures, concomitant therapies, Sequential Organ Failure Assessment score, mechanical ventilation, body mass index, adverse events, inflammatory markers, and PaO2/FiO2 ratio were all recorded as study characteristics.
Conclusion: Clinical evidence on MSC’s therapeutic applications during COVID-19 pandemic has proven to be a promising therapy for COVID-19 patient recovery with no consequences and applied as a routine treatment for challenging ailments.
Keywords: COVID-19, nCoV-19, novel coronavirus, SARS-CoV-2, severe acute respiratory distress syndrome-coronavirus-2, stem cell management, Mesenchymal stem cell applications
Introduction
The treatment (TX) of severe Coronavirus disease 2019 (COVID-19) is extremely difficult.1 COVID-19, induced by SARS-CoV-2 infection,2 causes significant lung damage ranging from moderate respiratory symptoms to SARS and death.3–5 COVID-19 caused widespread fear and apprehension since it is extremely communicable.6–8 The World Health Organization (WHO) has termed COVID-19 pneumonia a global pandemic and a public health emergency.9 Because of a lack of clear awareness in the initial days of the epidemic, the number of infected individuals rapidly rose, quickly spreading to more and more continents.10,11
Globally, the number of infected victims is growing constantly.12 COVID-19 has an incubation period that can differ from 0 to 14 days but is commonly around 3 and 7 days.13 Fever, headache, dry cough, and breathlessness are the most frequent symptoms.14–16 A sore throat, diarrhea, nasal congestion, and rhinorrhea are other significant complaints among patients.17,18 Expiratory Hypertension (HTN) and dyspnea are common in severe individuals one week following the commencement of the illness. According to a biopsy and autopsy investigation, individuals in the most severe instances might have Acute Respiratory Distress Syndrome (ARDS), severe acute lung damage (LD), septic shock, metabolic acidosis, and coagulopathy.5
The leading causes of mortality include severe pneumonia, ARDS, pulmonary edema, or multiple organ failure.4 ARDS is a severe lung injury characterized by an uncontrolled inflammatory process that creates significant alveolar destruction and capillary basement membrane leaking, leading to progressive respiratory distress. There is currently no effective TX for ARDS; however, a variety of TXs, including cell-based therapies, have been proposed.19,20 Successful repair and regeneration of endothelium and alveolar cells21 and regulation of excessive inflammatory immune responses may be critical aspects of ARDS recovery in afflicted individuals. COVID-19 has the potential to cause expiratory dyspnea and ARDS. 13.8% of COVID-19 patients had severe cases, 6.1% had critical cases, and roughly 2.3% had fatal outcomes.22,23
There are different vaccinations which were approved by the Food and Drug Administration (FDA) for avoiding COVID-19 infections, and a breakthrough in the therapeutic strategy is crucial for the TX of COVID-19, particularly in severely or critically ill patients who may develop ARDS and/or expiratory dyspnea.24,25 Currently, a few medicines such as remdesivir and dexamethasone have demonstrated intriguing early outcomes in randomized, controlled, open-label clinical studies.26,27
In addition to this, some specific medicines have been proven to be successful in the TX of COVID-19. Furthermore, SARS-CoV-2-induced secondary infections have been attributed to multiple organ dysfunction syndrome (MODS) in extremely or critically sick patients and this remains a significant concern globally.12 Immune dysregulation in both the innate and adaptive immune systems (AIS) has been implicated in disease intensity, lung injury, and long-term functional disability.28–31
There are presently some preemptive vaccinations or effective antiviral medicines available to address COVID-19, and COVID-19 patients are managed primarily with symptomatic and supportive therapy.32 As a result, there is an immediate need for safe and viable TX approaches to reduce inflammatory organ damage. Currently, immunotherapeutic methods in clinical studies include convalescent plasma treatment (CPT), monoclonal antibodies against interleukin-6 (IL-6), including cell therapies.26,33,34
The FDA has established forward regulatory procedures to enable the development of COVID-19 vaccines that fulfills the FDA’s stringent scientific specifications. The different kinds of vaccinations were as follows:
Pfizer-BioNTech COVID-19 Vaccines: On August 23, 2021, the FDA revealed the first clearance of a COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and the approved vaccine is sold as Comirnaty (monovalent COVID-19 vaccine), for the protection of COVID-19 among individuals who are 12 years of age and older.35
Moderna COVID-19 Vaccines: The FDA declared the second clearance of a COVID-19 vaccine on January 31, 2022. The vaccine, previously known as the Moderna COVID-19 Vaccine, will now be sold as Spikevax (monovalent COVID-19 vaccine) for the protection of COVID-19 in people aged 18 and older.36
Janssen COVID-19 Vaccine: The Janssen COVID-19 Vaccine is obtainable under the EUA to prevent COVID-19 in individuals 18 years of age and older for whom other FDA-authorized or accepted COVID-19 vaccines are not available or clinically appropriate, as well as individuals 18 years of age and older who select to receive the Janssen COVID-19 Vaccine because they would not otherwise acquire a COVID-19 vaccine.37
Novavax COVID-19 Vaccine, Adjuvanted: Novavax COVID-19 Adjuvanted Vaccine is offered under emergency use authorization (EUA) to avoid COVID-19 in people aged 12 and older. The SARS-CoV-2 spike protein and Matrix-M adjuvant are both included in the Novavax COVID-19 Vaccine, Adjuvanted. Adjuvants are added to some vaccines to improve the immune reaction of the immunized person.38
In addition to this, remdesivir has shown clinical advantages such as reduced hospitalisation time, lower progression to artificial breathing, and lower utilisation of other hospital facilities; it is uncertain whether it lowers mortality, but one randomised controlled study indicated potential longevity benefits. According to the accessible information, remdesivir has been approved (or authorised for early use) in 48 different countries.39 Other medications are hydroxychloroquine/chloroquine and azithromycin, favipiravir, interleukin (IL)-6 pathway inhibitor, lopinavir/ritonavir, histamine 2 receptor antagonist (H2RA), interferon (IFN) beta, convalescent plasma (CP), plasma adsorption and exchange.40
Stem Cells (SC)
Cellular TXs have considerable potential for the TX of COVID-19.41 SC TXs are emerging as viable therapeutic techniques that can reduce inflammation and repair LD caused by COVID-19, either alone or in conjunction with existent therapy regimens.22,42 The SC secretome has been determined to exhibit strong antifibrotic, anti-inflammatory assets, immunomodulatory (IMD), and angiogenic biological functions.43,44
The secretome, which can be studied in conditioned medium, consists of growth factors, cytokines, and extracellular vesicles such as microvesicles and exosomes.45 These components have a multitude of biological functions which can be targeted by a variety of procedures. The secretome is emerging as a potential alternative treatment due to the absence of conventional therapeutics.
These are multi-potent cells that are being applied extensively in regenerative medicine. MSC TX has a large body of preclinical evidence and early, preliminary clinical data indicating its ability to repair and restore the function of injured tissues and organs.46 MSCs are being studied for their potential application in the TX of ARDS induced by SARS-CoV-2 infection.47 MSCs are a therapeutic agent that is generally accessible, safe for the patient, and exempt from ethics issues due to their successful acquisition from a wide range of human body tissues, notably bone marrow (BM), adipose cells (AC), synovial surfaces of joints (SSJ), umbilical cord (UC), and placenta.48
Mesenchymal Stem Cells (MSCs)
MSCs show extraordinary IMD features, as well as minimal immunogenicity, paracrine characteristics, and the potential to develop into diverse cell lines. These abilities make them attractive therapeutic possibilities for the TX of neurological, cardiovascular, and pulmonary ailments, which may be occupational problems.49 Preclinical research utilizing experimental animal designs has shown that MSCs have therapeutic implications in illnesses such as silicosis and pulmonary disease. Currently, MSC TXs have the potential to improve the management of the new illness COVID-19.49 MSCs are non-hematopoietic cells (n-HPC) with a strong proliferative capacity and multi-lineage differentiation abilities.50,51
MSCs have been employed for almost 3 decades and have demonstrated tremendous advances.52 MSCs may help with COVID-19 consequences such as cytokine storm (CS), ARDS, and acute lung injury (ALI).53 MSCs were observed to generate exosomes that had strong IMD ability for tissue restoration.54 Intravenous (IV) injection is a common way of administering SC therapy.55,56 MSCs and their generated extracellular vesicles (ECVs) have the potential to treat COVID-19 because of their capacity to modify the immune response, enhance pathogen clearance, and reduce the degree of organ damage.57
Many clinical investigations have found that MSCs and their exosomes (MSCs-Exo) effectively alleviated lung inflammation caused by various forms of LD. So far, they have been employed successfully in multiple trials to treat a variety of illnesses, including Systemic Lupus Erythematosus (SLE), Amyotrophic Lateral Sclerosis (ALS), Graft-Versus-Host Disease (GVHD), and ARDS. In this context, several studies on the usage of these cells for COVID-19 clients have been officially registered, but only a portion of them have been finalized and released their findings.58
By the International Society for Cell & Gene Therapy (ISCGT) guidelines for 2019, mesenchymal stem cells should be renamed as mesenchymal stromal cells (MSCs).59,60 Due to the cells’ self-renewing ability, multi-potent possibility, low immunogenicity, anti-inflammatory efficacy, and ability to adhere to injured tissue, MSC-based cellular TX has been the focus of an increasing number of studies.61,62 More significantly, MSCs have distinct IMD capabilities that affect both innate and adaptive immune responses (AIRs), making them a highly appealing cell TX tool.62,63
MSCs primarily control AIRs by targeting T- and B-lymphocytes, antigen-presenting cells (APCs), dendritic cells (DCs), natural killer (NK) cells, and regulatory T-cells (Tregs).62 MSCs also have a role in innate immune responses, primarily by targeting DCs, NK cells, innate TH17 cells, neutrophils, monocytes, macrophages, and mast cells.63 Furthermore, MSC-based TXs have demonstrated promising effects in several clinical investigations across a wide range of diseases.64,65
Researchers have observed that after injecting MSCs, the human body activates the host’s innate immune cascade mechanism, such as complement and blood coagulation, which is described as the immediate blood-mediated inflammatory response (IBMIR).66 IBMIR is crucial given the extremely procoagulant state of many critically and severely ill patients requiring MSC treatment.67
MSCs can be derived from a variety of sources, such as BM, AT, UC, placenta, menstrual blood (MB), muscles, the dental pulp (DP), Wharton’s jelly (WJ), fetal liver, amniotic membranes, amniotic fluid, urine, and others.65,68,69 Additionally, MSC-based therapy has shown favorable outcomes in investigations on inflammatory lung illness, limiting alveolar collapse, cell death, and collagen aggregation in lung tissues.70 The angiotensin-converting enzyme-2 (ACE-2) has been recognized as a receptor for SARS-CoV-2 passage into target cells.71,72 Similarly, studies have shown that MSCs do not express ACE-2 and that MSCs are resilient to SARS-CoV-2 invasion as well as when confronted with SARS-CoV-2 infected cells.73,74
Wilson et al75 also reported no pre-specified adverse effects after infusion of allogeneic MSCs into 9 patients with ARDS, including cardiac arrhythmia (CA), hypoxemia, and ventricular tachycardia (VT). Moreover, the study team revealed that MSC transplantation dramatically reduces mortality in patients with pandemic Influenza A (H7N9) induced ARDS.76 MSCs may be beneficial in treating COVID-19, particularly in severe and critical patients.12
MSCs are n-HPC that have immunological modulatory, regeneration, and differentiation capabilities.65 In animal models and clinical studies, MSC therapy decreased lung pathology and inhibited the cell-mediated immune inflammatory response caused by the influenza virus.76–78 MSC has also been studied for its safety and possible effectiveness in ARDS patients.75,79–81 MSCs’ IMD and regenerative characteristics provide a possible cellular TX strategy for lung injury in COVID-19 patients, but they must be validated in RCTs. Since the emergence of the COVID-19 pandemic, several SC TX clinical studies have been conducted, and the findings have shown that MSCs not only reduce LD and recovery time but also enhance patient survival with good tolerance in the early phase.1,82,83
The earlier double-blind, randomized, placebo-controlled trial noted the short-term safety and efficacy of UC-MCS TX, from base point to day 28 after TX, and noticed that UC-MCS management greatly decreased the proportions of solid constituent lesion volume in the lungs and quantitatively increased the 6-minute walk test (6-MWD), especially in comparison to the placebo control.1 MSCs’ potential advantages make them suitable for potential new therapy in ARDS patients.50
MSCs are safe, ACE-2 negative, and capable of effectively suppressing the overactive immune system in COVID-19 individuals. Furthermore, IV injections of MSCs can rapidly transport a large number of them to the lungs, which are the primary damaged organs in ARDS.58 Existing clinical trials suggested the potential effectiveness of MSCs-Exo in the treatment of COVID-19.57 MSCs might be used as an alternative or supplemental therapy. To present, therapeutic trials utilizing MSCs in COVID-19 patients have raised hopes for the safe and beneficial use of this SC type. MSCs might be a viable therapeutic or supplemental agent for COVID-19 therapy.49
They are widely used because of factors such as their low immunogenicity. MSCs are not identified by the human Immune System (IS) due to poor expression of Major Histocompatibility Complex (MHC) particles and an undetectable level of MHC-II.84 Clinical investigations on COVID-19 patients with MSCs indicated their utility in the acute period of the disease.85
Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs)
UC-MSCs may have IMD capabilities.86,87 Regarding the COVID-19 outbreak, MSCs from various sources, particularly UC-MSCs, have been employed in clinical trials.74,88,89 These UC-MSC features, which may be due to their more primitive origin as compared to adult tissue-derived MSCs,90 may explain why cord-derived MSCs were the most often employed in a recent evaluation of registered studies investigating MSCs in COVID-19 patients.91 Another justification for employing UC-MSCs in the setting of SARS-CoV-2-induced severe ARDS is that they do not exhibit the ACE-2 receptor.74
Previous study findings indicated that IV of human UC-MSCs was safe and well tolerated in patients with mild to severe COVID-19 in a phase-1 trial.88 Another study published the findings of a randomized, double-blind, placebo-controlled trial conducted at 2 medical institutions in Wuhan, China, to assess the safety and effectiveness of IV therapy with UC-MSCs in severe COVID-19 patients with LD.1 A study of 11 patients with COVID-19-associated ARDS found that IV (60x107 cells) of human UC-MSCs or placental MSCs (PLMSCs) immediately reduced respiratory distress (RD) while also lowering the excessive inflammatory response.92
Several randomized, double-blind, placebo-controlled studies found that UC-MSC (a total of 10–12 × 107 cells) enhanced lung lesion repair in COVID-19 patients without posing any safety risks.86,93 Anecdotal case reports, small-scale non-randomized or open-label studies, and only 2 recent, single-center, double-blind, Placebo-Controlled Trials (PCTs) have been published thus far in studies investigating UC-MSCs in COVID-19 patients. Overall, the results of those studies corroborated the great tolerance of IV MSC infusions and showed improved clinical outcomes (COs), even though they were small-scale non-randomized or open-label studies, with just 2 contemporary, single-center, double-blind, PCTs. Overall, the findings of those investigations supported the high tolerability of IV MSC infusions and suggested improved COs.86
So far, the evidence suggests that allogeneic UC-MSC TX is safe for a wide range of diseases.94 These cells can be generated from UC that have been discarded after birth and rapidly grown to clinically significant amounts. They display low amounts of human leukocyte antigen (HLA) class-I and class-II, which may diminish alloreactivity.95
Menstrual Blood-MSCs (MB-Derived MSCs)
Considering SC-based TX is dose-dependent, and human clinical research normally necessitates millions of SC, a high proliferation rate is critical in therapeutic trials. MB-derived MSCs double in approximately 20 hours, while BM-derived MSCs double in around 40–45 hours. Thus, MSCs obtained from MB had a higher output in a shorter amount of time at early passages.96,97 More significantly, MB-derived MSCs provide a painless solution that is devoid of the ethical problems that may come with BM-MSC contributions.98 Thus, MSC-based therapy produced from MB may be a potential TX for COVID-19, especially in fighting the inflammatory CS shown in severe and critical patients.12
This is an exploratory trial to determine whether MB-derived MSCs may benefit COVID-19 patients who are severely and chronically unwell. To that effect, researchers evaluated the safety, therapeutic effectiveness, and tolerability of transplanted MSCs during a one-month follow-up following SARS-CoV-2 infection. The research specifically looked at any improvements in pulmonary function. The findings of the study not only shed light on MSCs’ potential to cure COVID-19 patients but also imply that MSCs are a promising method for treating acute or chronic pneumonia in future clinical applications.11 MB-derived MSCs have lately gained a lot of interest due to their exceptional proliferation capacity and absence of ethical issues.12
MSC transplantation from MB considerably reduces the death rate of severe and critical SARS-CoV-2-induced patients with COVID-19.14 MSC-based therapy might be used as an alternate TX for COVID-19 in the future. MB-derived MSCs have been viewed in numerous pre-clinical and clinical studies, including the TX of pulmonary illnesses, in recent decades due to their unique features, such as high proliferation rate, low immunogenicity, and non-invasive periodical acquisition.99,100
Bone Marrow MSCs (BM-Derived MSCs)
In children, allogeneic hematopoietic stem cell transplantation (allo-HSCT) from a Human Leukocyte Antigen (HLA) identical family donor is the initial therapy.101 BM-MSCs have been found to promote lung repair following ventilator-induced LD, to aid in inflammation resolution, and to restore lung function and structure in ARDS patients.102
Adipose Tissue-Derived MSCs (AT-Derived MSCs)
Sanchez-Guijo et al revealed the effects of IV injection of AT-derived MSCs in 13 severe COVID-19 pneumonia cases on mechanical ventilation (MV), and the patients were followed up on 16 days following the infusions.93 The advantages of perinatal MSCs over adult MSCs include their ease of availability, lack of donor site morbidity, cell naivety, the quantity of SC in the main tissue, and a high potential for proliferation.103
Given the global severity of COVID-19, one research study evaluated the safety of aerosol inhalation of exosomes derived from human AT-derived MSCs-Exo in the TX of patients with severe COVID-19-related pneumonia, intending to determine the optimal dosage and delivery route of MSCs-based therapy for acute respiratory diseases (ARD). Research utilizing a mouse model of occupational bronchial asthma found that TX of AT-derived MSCs dramatically lowers lung neutrophilic infiltrates and immunoglobulin-E (IgE) levels.104 AT-derived SC, a common kind of MSCs, has been offered as a therapy option for COVID-19 to minimize morbidity and mortality. AT-derived MSC therapies have the potential to alleviate the strain on crucial hospital resources41 (Table 1).
 |
Table 1 Search Databases and Strategies About the Stem Cell Applications Among the COVID-19 Population |
Research Questions
- What is the pooled effectiveness of MSC applications among the COVID-19 population?
- What are the study characteristics of MSC applications among the COVID-19 population?
Methods
This systematic review (SR) included studies from all across the world. This study was registered in the PROSPERO (CRD42022380088).
Search Strategies
Scopus, Web of Science, PUBMED, EMBASE, Science Direct, Cochrane Library, and Google Scholar were utilized to search for relevant literature. During this time, papers published until December 31, 2022, were sought with key terms on COVID-19, SARS-CoV-2, Novel Coronavirus, nCoV, and Severe Acute Respiratory Syndrome, coronavirus-2, Coronavirus Disease-2019 Virus, 2019-nCoV, 2019 New Coronavirus, Coronavirus, Stem Cells, Stem Cell Management, Therapeutic Stem Cell Management, Stem Cell Applications, Mesenchymal Stem Cell Applications, Mesenchymal Stem Cell Management, Therapeutic Mesenchymal Stem Cell Applications”, and Boolean operators were employed.
Eligibility Criteria
Inclusion Criteria
Studies were included in the SR if they fulfil: Studies that reported outcome variables on SC management on COVID-19 patients, studies reported on RCTs, articles published in the English language, and articles published up to December 31, 2022, across all countries.
Exclusion Criteria
Articles that did not assess the outcome variables, articles that were not fully accessible, articles published in a non-English language, articles with non-RCTs, observational studies, case studies, editorials, perspectives, commentary, and poor quality studies were excluded from this SR.
Outcome Interest
In this SR, the primary outcome was the effectiveness of MSCs applications among COVID-19 patients. Effectiveness refers to studies that explained positive results on MSC applications among COVID-19 patient studies which were included in this SR.
The secondary outcome was study characteristics that reported on MSC applications among the COVID-19 population.
Data Extraction and Quality Assessment
The retrieved articles from all databases were exported to “Thomson Reuters EndNote version-8”. The titles and abstracts of all possible articles to be included in this SR were checked. The standardized data extraction format prepared in a Microsoft Excel worksheet was used to extract the data from the selected articles according to the present inclusion criteria. Author name, publication year, study period, study country, participants, sample size, study design, type of intervention, outcome measurement, study findings, and conclusion of the study were used for the extraction of data from each article.
This SR has included 14 RCTs. The CASP, a critical appraisal tool for SR that could be RCTs on MSC applications among COVID-19 patients was used to assess the included articles, and the CASP for RCTs was used to include the articles in this SR, whereas all articles fulfilling CASP criteria are considered high-quality RCTs and included in this SR and given as a Supplementary 1 File. The CASP methodological quality assessment checklist has been included for each article.
Data Processing and Analysis
This SR adopted a narrative synthesis of the RCTs pooling evidence on MSC therapeutic applications among COVID-19 patients during the epidemic. The data are prepared in the tabular column which includes
Author, Year, Country, Study Design, Study Duration, Setting of The Study, Population, Sample Size, Sampling Technique, Method of Randomization, Type of SC Applied, Type of Intervention, Duration of Intervention, Study Outcome Measurement, Study Characteristics, Results and Conclusion of the Studies.
Data Synthesis and Reporting
This SR was conducted on the effectiveness of pooling evidence on MSC’s therapeutic applications during the COVID-19 epidemic. During this, PRISMA 2020 flowchart diagram and PRISMA 2020 checklist were used for the study screening, selection, and inclusion into this SR. PRISMA 2020 checklist is given in (the Supplementary 2 Files).
Results
Search Results
All related studies done across the world were identified by using diverse databases. From the search made through the mentioned databases, 12,567 studies were found. From this, only 14 studies were meeting the predefined eligibility criteria and were included in this SR (Figure 1).105
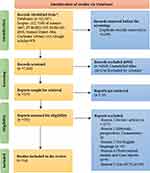 |
Figure 1 PRISMA flowchart diagram of the study selection for a systematic review on Mesenchymal stem cell therapeutic applications during the COVID-19 epidemic. |
Study Characteristics
This SR focused on the studies conducted on MSC applications among COVID-19 patients. In this SR, a total of 14 studies were included, comprising studies done on MSC applications on COVID-19 patients.
Results
The study overall records (12,567) are extracted from various databases (Scopus, Web of Science, PUBMED, EMBASE, Science Direct, Cochrane Library, and Google Scholar), and 14 RCTs were included in this SR,1,58,83,86,88,93,106–113 whereas remaining records were excluded with various reasons and are explained in Figure 1 in detailed. The CASP checklist criteria were considered for inclusion of the studies and considered as high-quality studies (CASP check attached as Supplementary File 1).
Effectiveness of RCTs on MSC Applications Among COVID-19 Patients
In this systematic review, the included studies conducted on MSC therapeutic applications among COVID-19 patients with RCT clinical trials. The outcome of the each included study was clearly evidenced with positive outcome of the patient prognosis. The RCTs on MSC therapies among COVID-19 patients was progressing and showing promising results with no complication, and these study effective results may evidence early patient recovery with harmful ailments (Table 2).
 |
Table 2 RCTs Pooled Evidence on Stem Cell Applications During the COVID-19 Epidemic |
Demographic Characteristics
The studies were conducted by
Murdani Abdullah, Hamid Reza Aghayan, Najmeh Kafash Farkhad, Rodrigo Pinheiro Araldi, Muhammad Karyana, Antoine Monsel, Carmen Lucia Kuniyoshi Rebelatto, Ayca Sultan Sahin, G Adas, Smail Hadisoebroto Dilogo, Giacomo Lanzoni, Lei Shi, Lei Shi, Fanping Meng.1,58,83,86,88,93,106–113
RCT published studies included in the year 2022 were 8; 58,86,106–111 in 2021 were 5; 1,83,93,112,113 in 2020 one study.88 The RCTs published from different countries were Indonesia-3; 106,109,112 Iran-2; 58,107 Brazil −2; 108,110 Turkey-2; 83,111 China-2; 1,113 Florida-1; 93 UK-1.88
The total pooled sample size was 574 (treatment group (318), control group (256)).1,58,83,86,88,93,106–113 The highest sample size was reported from China, ie, 100 (100 severe COVID-19 patients with lung damage with UC-MSCs (n = 65) or placebo (n = 35)).1 The lowest sample size was recorded from Jakarta, Indonesia, ie, 9 patients with low clinical risk COVID-19 infection, and 9 subjects were enrolled and randomly assigned to 1 of the 3 groups: TL, TH, and C.109
The reported highest age of the patient category was 69 years,108 and the lowest age category was 18 years,106 and the identified age (mean ± SD) is intubated/no comorbidity (48 ± 10.8781), intubated/with comorbidity (62.2857 ± 8.2606), no intubated (56.8571 ± 13.2718); UC-MSC group (n = 65) 60.72 (9.14), placebo group (n = 35) 59.94 (7.79).111,113 The recorded BMI (body mass index), Kg/m2, of the patient is UC-MSC group (n = 65) 24.71 (3.19), placebo group (n = 35) 25.01 (3.12); UC-MSC (n = 12) 34.5 ± 4.5, control (n = 12) 29.6 ± 3.5; UC-MSC (n = 21) 28.6 (3.5), placebo (n = 24) 28 (5.5).1,86,93
Different study measurements were recorded on comorbidities; 1,58,88,93,106,107,110,112,113 SOFA score; 86 PaO2/FiO2 ratio; 86,93 laboratory values; 1,88,106,107,109–111,113 mechanical ventilation; 86,88 clinical symptoms; 88,110 inflammatory markers; 83,106,111,112 body mass index; 1,86,93,113 concomitant treatment; 1,93,110,113 adverse events.1,86,113
The type of MSCs applied in the studies was MSCs-secretome (1); 106 MSCs (1); 111 PL-MSCs; 107 human immature dental pulp stromal cells (hIDP-SCs) (1);108 DW-MSC infusion (1); 109 WJ-MSCs (1); 83 majority of the studied transfused UC-MSCs (8).1,58,86,88,93,110,112,113 The transfused interventional dosage was 1 × 106 cells/kg (6); 58,83,86,93,107,112 1 × 107 cells/kg (4); 1,88,108,109 1 × 105 cells/kg (1);110 1 million cells/kg (1).111
The reported laboratory measurements are haemoglobin, haematocrit, white blood cells (WBC), platelets, erythrocyte, basophils, eosinophils, neutrophils, lymphocytes, monocytes, neutrophils lymphocytes ratio, ureum, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, direct bilirubin, indirect bilirubin, procalcitonin, C-reactive protein (CRP), albumin, glomerular filtration rate (GFR), D-dimer, sodium (Na+), potassium (K+), chloride, ferritin, troponin-I.1,106,110
The recorded clinical symptoms are fever, fatigue, cough, shortness of breath (SOB), nausea or vomiting, diarrhea, loss of taste or smell, disorientation, and confusion.88,110 The documented inflammatory markers are interleukin (IL)-6, IL-10, LIF, VEGF, and ferritin.1,106,113 The findings on the Sepsis-related Organ-Failure Assessment score (SOFA) are UC-MSC (n = 21) 5.5 (2.7) and placebo (n = 24) 5.9 (2.7).86 The used concomitant medications are antiviral drugs, immunomodulatory drugs, steroids treatment, antibiotics, corticosteroids, heparin (anticoagulant), only prophylactic dose heparin, therapeutic dose heparin, remdesivir, convalescent plasma, corticosteroids, tocilizumab, hydroxychloroquine, alteplase.1,86,88,93,110,113
The observed comorbidities are obesity, diabetes mellitus (DM), heart disease, hypertension (HTN), atrial fibrillation, coronary artery disease (CAD), dyslipidemia, encephalitis, stroke, asthma, chronic obstructive pulmonary disease (COPD), chronic bronchitis, tuberculosis (TB), stress ulcer, dyspepsia syndrome with alarm sign, kidney disease, sepsis, autoimmune diseases, adenomyosis, mild anemia, fatty liver disease, schizophrenia, cancer1,86,93,106,110,113 (Table 2 and Table 3).
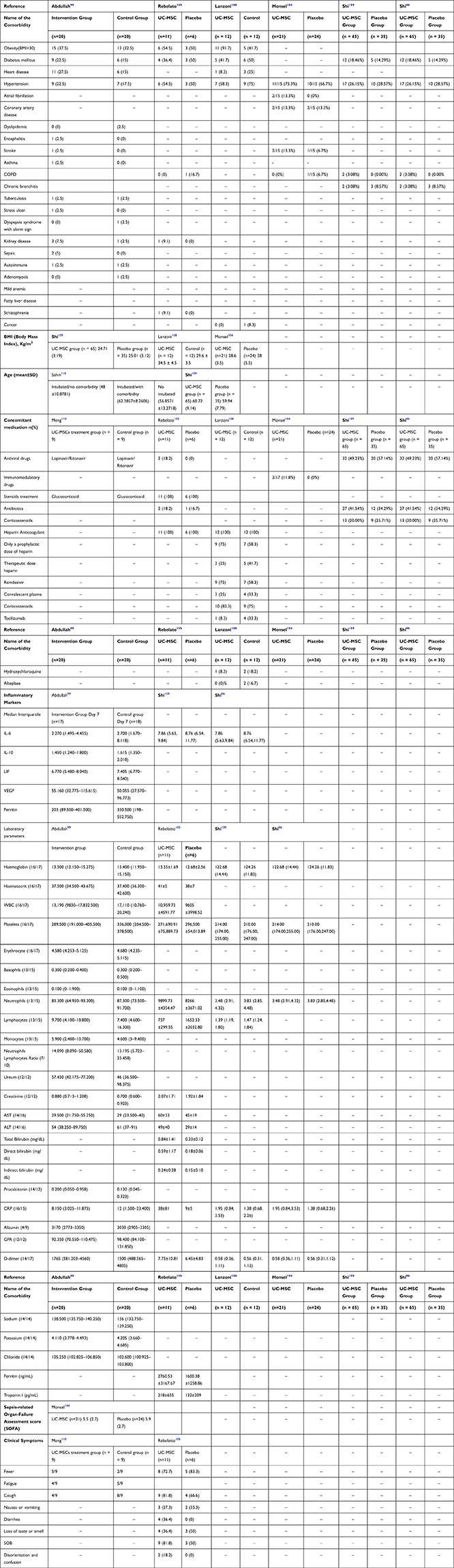 |
Table 3 Demographic Factors of MSC Applications on COVID-19 Patients |
Discussion
COVID-19, caused by SARS-CoV-2, was first identified in December 2019 as the source of a cluster of respiratory infections.4,114 There is currently no viable TX for COVID-19.14 MSC-based therapy might be a potential alternative TX for severe and serious COVID-19.12 The number of ventilator-free and organ-failure-free days in patients with ARDS was statistically lower in the MSC group than in the placebo group.75,79 MSCs have been studied in various clinical trials; however, they face hurdles such as carcinogenic risk and particular storage conditions, as well as limited evidence regarding their mode of action. The core of MSCs’ distinctive features is attributable to their paracrine activity, notably their exosomes.53
The aim of the study was “to identify the effectiveness of RCTs pooling evidence on MSC therapeutic applications during the COVID-19 epidemic”. The articles were extracted around 12,567 from the different databases from 1st December 2022 to 31st December 2022. As per the PRISMA checklist, the articles were screened, and find 14 relevant RCTs which reported on MSC applications among COVID-19 patients during the pandemic. The articles were evaluated by the CASP quality assessment tool. The included articles were published between the years 2019–2022.
The included 14 RCTs, the highest 8 studies were conducted in the year 2022 and one study was conducted in the year 2020, and 5 studies were published in the year 2021 with a pooled sample size of n = 574 (treatment group (n = 318) + control group (n = 256) in different countries). The majority of 3 studies were recorded from Indonesia, 2 studies from Iran, 2 studies from Brazil, 2 studies from Turkey, 2 studies from China, 1 study from Florida, 1 study from the UK, and 1 study from France.
The application of MSCs by the different studies among 14 RCTs was that the majority 8 studies experimented on UC-MSCs among COVID-19 patients and MSC secretome, MSCs, PL-MSCs, hIDPSC, DW-MSC infusion, and WJ-MSCs were experimented by another study. The highest studies’ interventional dosage of 1 × 106 cells/kg was used by 6 studies, 1 × 107 cells/kg was used by 4 studies, 1 × 105 cells/kg was used by one study, and 1 million cells/kg was applied by one study. The studies focused on DV, CS, LT, comorbidities, RM, CT, SOFA score, MV, BMI, AEs, IMs, and PaO2/FiO2 ratio were all recorded as study characteristics. There is a promising result on pooled evidence of RCTs on MSC applications among COVID-19 patients and may be used in the management of severe infectious disease.
Limitations of the Study
The studies included in this SR were RCT studies published on MSC applications among COVID-19 patients, studies limited to the English language, from the year 2019–2022, and full-text articles.
Conclusion
COVID-19, caused by SARS-CoV-2, was discovered in December 2019 and has since expanded globally. ARDS is a catastrophic consequence of the new COVID-19 pandemic, and it is directly connected to high levels of inflammatory cytokines. MSCs offer a viable therapy against this illness due to their IDM properties. Clinical evidence presented on MSC’s therapeutic applications during the COVID-19 pandemic has proven to be a promising therapy for COVID-19 patient recovery with no side consequences, and it may be applied as a routine TX for the prevention of challenging disorders. The study results were explained the good progress of the patient condition during the COVID-19 pandemic times with MSC application with RCT trials. These methods are dramatically brought the progressed effective results among patients with COVID-19.
Acknowledgment
College of Health Sciences, Arsi University, Asella, Ethiopia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled Phase 2 trial. Signal Transduct Target Ther. 2021;6:58.
2. World Health Organization. Coronavirus Disease (COVID-19) Situation Reports. World Health Organization; 2022. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost JTH. 2020;18:844–847.
4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
5. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422.
6. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473.
7. Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182:1077–1092.
8. Lal A, Erondu NA, Heymann DL, Gitahi G, Yates R. Fragmented health systems in COVID-19: rectifying the misalignment between global health security and universal health coverage. Lancet Lond Engl. 2021;397:61–67.
9. Rubin EJ, Baden LR, Morrissey S. Audio interview: studying potential covid-19 therapies. N Engl J Med. 2020;382:e72.
10. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523.
11. Yang Q, Zhou Y, Ai J, et al. Collaborated effort against SARS‐CoV‐2 outbreak in China. Clin Transl Med. 2020;10:13–16.
12. Xu X, Jiang W, Chen L, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11:e297.
13. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433.
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
15. Sun Y, Koh V, Marimuthu K, et al. Epidemiological and Clinical Predictors of COVID-19. Clin Infect Dis off Publ Infect Dis Soc Am. 2020;71:786–792.
16. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (covid-19): a review. JAMA. 2020;324:782–793.
17. Xu X-W, Wu -X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
18. Xu M, Wang D, Wang H, et al. COVID-19 diagnostic testing: technology perspective. Clin Transl Med. 2020;10:e158.
19. Hossein-Khannazer N, Shpichka A, Shokoohian B, et al. Novel therapeutic approaches for treatment of COVID-19. J Mol Med Berl Ger. 2020;98:789–803.
20. Ramezankhani R, Solhi R, Memarnejadian A, et al. Therapeutic modalities and novel approaches in regenerative medicine for COVID-19. Int J Antimicrob Agents. 2020;56:106208.
21. Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11:707–722.
22. Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk H-D, Reinke P. MSC Therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091.
23. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469.
24. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149–150.
25. Atala A, Henn A, Lundberg M, et al. Regen med therapeutic opportunities for fighting COVID‐19. Stem Cells Transl Med. 2020;10:5–13.
26. Group RC. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704.
27. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578.
28. Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511.
29. Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5:eabd7114.
30. Song J-W, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11:3410.
31. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current State of the Science. Immunity. 2020;52:910–941.
32. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059.
33. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470.
34. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818.
35. FDA. Pfizer-Biontech COVID-19 Vaccines. FDA; 2023.
36. FDA. Moderna COVID-19 Vaccines. FDA; 2023.
37. FDA. Janssen COVID-19 Vaccine. FDA; 2023.
38. FDA. Novavax COVID-19 Vaccine, Adjuvanted. FDA; 2022.
39. Beigel JH. What is the role of remdesivir in patients with COVID-19? Curr Opin Crit Care. 2021;27:487–492.
40. Bose S, Adapa S, Aeddula NR, et al. Medical management of COVID-19: evidence and experience. J Clin Med Res. 2020;12:329–343.
41. Rogers CJ, Harman RJ, Bunnell BA, et al. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18:1–19.
42. Chen Y, Zhang Q, Peng W, et al. Efficacy and safety of mesenchymal stem cells for the treatment of patients infected with COVID-19: a systematic review and meta-analysis protocol. BMJ Open. 2020;10:e042085.
43. Bamba C, Singh SP, Choudhury S. Can mesenchymal stem cell therapy be the interim management of COVID-19? Drug Discov Ther. 2020;14:139–142.
44. Shahsavari A, Weeratunga P, Ovchinnikov DA, Whitworth DJ. Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell-derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells. Sci Rep. 2021;11:3486.
45. Damayanti RH, Rusdiana T, Wathoni N. Mesenchymal stem cell secretome for dermatology application: a review. Clin Cosmet Investig Dermatol. 2021;14:1401–1412.
46. Atluri S. Safety and effectiveness of intravascular mesenchymal stem cells to treat organ failure and possible application in COVID-19 Complications. Pain Physician. 2020;4:S391–S420.
47. Singh B, Mal G, Verma V, et al. Stem cell therapies and benefaction of somatic cell nuclear transfer cloning in COVID-19 era. Stem Cell Res Ther. 2021;12:1–16.
48. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886.
49. Kolanko E, Mazurski A, Czekaj P. Potential therapeutic application of mesenchymal stem cells in COVID-19 complications. Med Pr. 2021;72:693–700.
50. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716.
51. Farkhad NK, Mahmoudi A, Mahdipour E. How similar are human mesenchymal stem cells derived from different origins? A review of comparative studies. Curr Stem Cell Res Ther. 2021;16:980–993.
52. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88.
53. Yousefi Dehbidi M, Goodarzi N, Azhdari MH, Doroudian M. Mesenchymal stem cells and their derived exosomes to combat Covid–19. Rev Med Virol. 2022;32:e2281.
54. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells Dayt Ohio. 2017;35:851–858.
55. Park WB, Kim SY, Lee SH, Kim H-W, Park J-S, Hyun JK. The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 2010;11:119.
56. Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52.
57. Zhu Y-G, Shi -M-M, Monsel A, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. 2022;13:220.
58. Kaffash Farkhad N, Sedaghat A, Reihani H, et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful Phase 1, control-placebo group, clinical trial. Stem Cell Res Ther. 2022;13:283.
59. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024.
60. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–1451.
61. Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704.
62. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736.
63. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396.
64. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848.
65. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833.
66. Caplan H, Olson SD, Kumar A, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645.
67. Moll G, Ankrum JA, Kamhieh-Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25:149–163.
68. Le Blanc K, Davies LC. MSCs-cells with many sides. Cytotherapy. 2018;20:273–278.
69. Mastrolia I, Foppiani EM, Murgia A, et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med. 2019;8:1135–1148.
70. Harrell CR, Sadikot R, Pascual J, et al. Mesenchymal stem cell-based therapy of inflammatory lung diseases: current understanding and future perspectives. Stem Cells Int. 2019;2019:25.
71. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6.
72. Yang J, Petitjean SJL, Koehler M, et al. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. 2020;11:4541.
73. Schäfer R, Spohn G, Bechtel M, et al. Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem Cell Rep. 2021;16:419–427.
74. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228.
75. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32.
76. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) Infection: a hint for COVID-19 treatment. Engineering. 2020;6:1153–1161.
77. Chan MCW, Kuok DIT, Leung CYH, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113:3621–3626.
78. WHO Working. Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197.
79. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162.
80. Simonson OE, Mougiakakos D, Heldring N, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4:1199–1213.
81. Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39.
82. Tang L, Jiang Y, Zhu M, et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14:664–673.
83. Adas G, Cukurova Z, Yasar KK, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:09636897211024942.
84. Tsuchiya A, Takeuchi S, Iwasawa T, et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40:14.
85. Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361.
86. Monsel A, Hauw-Berlemont C, Mebarki M, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care Lond Engl. 2022;26:48.
87. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
88. Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5:172.
89. Laroye C, Gibot S, Huselstein C, Bensoussan D. Mesenchymal stromal cells for sepsis and septic shock: lessons for treatment of COVID-19. STEM CELLS Transl Med. 2020;9:1488–1494.
90. Liau LL, Ruszymah BHI, Ng MH, Law JX. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr Res Transl Med. 2020;68:5–16.
91. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16:427–433.
92. Hashemian S-MR, Aliannejad R, Zarrabi M, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:91.
93. Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673.
94. Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 2017;19:1351–1382.
95. Patel AN, Vargas V, Revello P, Bull DA. Mesenchymal stem cell population isolated from the subepithelial layer of umbilical cord tissue. Cell Transplant. 2013;22:513–519.
96. Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front Immunol. 2014;5:205.
97. Wu X, Luo Y, Chen J, et al. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245–1257.
98. Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10:1.
99. Fathi-Kazerooni M, Tavoosidana G, Taghizadeh-Jahed M, et al. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy. 2017;19:1474–1490.
100. Bozorgmehr M, Gurung S, Darzi S, et al. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 2020;8:8.
101. Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365(9471):1647–1656.
102. Can A, Coskun H. The rationale of using mesenchymal stem cells in patients with COVID-19-related acute respiratory distress syndrome: what to expect. Stem Cells Transl Med. 2020;9:1287–1302.
103. Wu M, Zhang R, Zou Q, et al. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci Rep. 2018;8:5014.
104. Yang Q, Liu Q, Xu H, Lu H, Liu S, Li H. Imaging of coronavirus disease 2019: a Chinese expert consensus statement. Eur J Radiol. 2020;127:109008.
105. McKenzie JM, Bossuyt JE, Boutron PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
106. Abdullah M, Pawitan JA, Irawan C, et al. Effectiveness and safety profile of mesenchymal stem cell secretome as a treatment for severe cases of COVID-19: a randomized controlled trial. F1000Research. 2022;11:143.
107. Aghayan HR, Salimian F, Abedini A, et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (Phase I clinical trial): safety profile assessment. Stem Cell Res Ther. 2022;13:365.
108. Araldi RP, Prezoto BC, Gonzaga V, et al. Advanced cell therapy with low tissue factor loaded product NestaCell® does not confer thrombogenic risk for critically ill COVID-19 heparin-treated patients. Biomed Pharmacother. 2022;149:112920.
109. Karyana M, Djaharuddin I, Rif’ati L, et al. Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther. 2022;13:134.
110. Rebelatto CLK, Senegaglia AC, Franck CL, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13:122.
111. Sahin AS, Kaya E, Turgut G, Dolay K, Kocatas A. Mesenchymal stem cell therapy in COVID-19 pneumonia: a prospective, randomized clinical research. Turkiye Klin Tip Bilim Derg. 2022;2022:5–13.
112. Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–1287.
113. Shi L, Yuan X, Yao W, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789.
114. Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
