Back to Journals » International Journal of General Medicine » Volume 16
Re-Emerging COVID-19: Controversy of Its Zoonotic Origin, Risks of Severity of Reinfection and Management
Authors Chala B, Tilaye T, Waktole G
Received 21 May 2023
Accepted for publication 19 August 2023
Published 20 September 2023 Volume 2023:16 Pages 4307—4319
DOI https://doi.org/10.2147/IJGM.S419789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Bayissa Chala,1 Tigist Tilaye,2 Gemechis Waktole1,3
1Department of Applied Biology, School of Applied Natural Science, Adama Science and Technology University, Adama, Ethiopia; 2Olanchiti Hospital, Oromia Health Bureau, Oromia Regional State, Ethiopia; 3Department of Biotechnology, College of Natural and Computational Science, Dambi Dollo University, Dambi Dollo, Ethiopia
Correspondence: Bayissa Chala, Email [email protected]
Abstract: The re-emergence of COVID-19 has sparked controversy around its zoonotic origin, management strategies, risks posed by the virus, and the severity of reinfection. While it is widely accepted that the virus originated from animals, the exact source and transmission pathway remain unclear. This has led to debates regarding the regulation of wildlife markets and trade, as well as the need for more robust surveillance and monitoring systems. Hence, the objective of this review is to provide a brief overview of the disease’s biology, preventative strategies, risk factors, degree of reinfection, and epidemiological profile. It offers a thorough examination of the disease’s root cause, potential zoonotic transmission, and the most recent preventive measures, like vaccines. In terms of management, there is ongoing debate about the most effective strategies to mitigate the spread of the virus. While public health measures such as social distancing and mask-wearing have been widely implemented, there are differing opinions on the effectiveness of lockdowns and restrictions on public movement. The risks posed by COVID-19 are also a topic of debate, with some arguing that the virus is relatively low-risk for the majority of the population while others highlight the potential for severe illness, particularly among vulnerable populations such as the elderly or those with underlying health conditions. Finally, the possibility of reinfection has raised concerns about the longevity of immunity following infection or vaccination. While some studies have suggested that reinfection may be possible and potentially more severe, the overall risk remains uncertain and further research is needed to fully understand the implications of reinfection.
Keywords: Coronavirus, COVID-19, pandemic, SARS-CoV-2, zoonosis
Introduction
The origin of the SARS-CoV-2 virus, which causes COVID-19, is still unclear. It is thought to have started in animals and spread to people via a process known as zoonotic transmission. Bats are considered the natural hosts of coronaviruses, so another animal species likely served as an intermediary for their transfer to humans.1 Researchers are currently studying and investigating the specific details of this animal-to-human transmission. Everybody has a different tolerance for the intensity of COVID-19. While some people might only have minor symptoms or none at all, others might get very sick.
Its severity is regulated by a person’s immune system, age, and underlying medical issues. Although uncommon, incidences of SARS-CoV-2 reinfection have been documented, indicating that it is conceivable. Reinfection with COVID-19 is typically milder compared to the initial infection. It has been proven that getting vaccinated significantly lowers the risk of severe disease and reinfection.2
To effectively manage COVID-19, a combination of approaches is employed, including testing, treatment, immunization, and public health initiatives. Public health measures often include advocating for practices such as regularly washing hands, wearing masks, maintaining distance from others, and implementing lockdowns or restrictions as needed to prevent the spread of viruses. Vaccines have been developed and are currently being widely distributed to reduce the impact of the virus. Therapy primarily focuses on providing supportive care and antiviral treatments, while testing assists in identifying and isolating individuals who are infected. Therefore, this review was intended to give new insights on the emergence, re-emergence, risk factors, controversies on the transmission and management of COVID-19. In addition to addressing public health issues, it tries to initiate scholarly discussions among scientific communities for the upcoming research projects.
History
Coronaviruses, which are a family of enveloped positive-sense single-stranded RNA viruses, were first discovered in the 1960s. The name “coronavirus” comes from the Latin word “corona”, meaning “crown” or “halo”, due to the characteristic appearance of the virion under two-dimensional transmission electron microscopy. This appearance resembles a crown or halo, hence the name. It is interesting to note that the oldest common ancestor of coronaviruses has been estimated to exist as far back as the 9th century BC, based on genetic analyses.3
Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are associated with more severe respiratory illnesses compared to the strains of COVID-19 that typically led to milder clinical symptoms. In 2002, a specific type of beta-coronavirus quickly spread throughout Guangdong, China, causing 8000 infections and 774 deaths across 37 countries. Similarly, MERS-CoV was first detected in Saudi Arabia in 2012 and led to 2494 confirmed cases and 858 fatalities.4
Severe acute respiratory syndrome was originated in Wuhan, China, when a cluster of atypical pneumonia cases surfaced in late December 2019.5 Genetic sequencing studies identified the cause as a new strain of coronavirus, initially named novel coronavirus-2019 (2019-nCoV).6 However, the International Committee on Taxonomy of Viruses officially designated it as SARS-CoV-2, and the disease caused by this virus was named COVID-19 by the World Health Organization (WHO) on February 11, 2020. Unlike other coronaviruses such as SARS-CoV and MERS-CoV, which tend to result in severe respiratory illnesses, the subtypes of COVID-19 often lead to milder clinical symptoms.7
The rapid spread of an emerging disease caused by SARS-CoV-2, known as COVID-19, occurred following the initial recorded case on December 1, 2019, in Wuhan. The disease swiftly expanded both within China and beyond its borders.8,9
Despite initial reports suggesting a rapid spread of the virus, with cases doubling every 7.5 days, the outbreak of COVID-19 has quickly reached global proportions in less than three months since its first reported case. After extensive analysis, WHO officially declared the COVID-19 epidemic as a pandemic on March 11, 2020.10 As of the beginning of September 2020, after a span of eight months since the initial outbreak on December 31, 2019, the global tally of confirmed COVID-19 cases has surpassed 27 million, along with nearly 900,000 reported fatalities. These distressing numbers reflect the ongoing wave of COVID-19 that the world is currently grappling with, as reported by WHO.
Structure and Composition
Coronaviruses are viruses with a medium size of approximately 125 nm on average. They have a helical shaped nucleocapsid, which is unusual for viruses that have RNA with a positive sense.11 These viruses generally have a spherical shape that is relatively uniform in size, despite some slight variations. They have a very simple structure, as shown in Figure 1.
Coronaviruses are viruses with a unique appearance, characterized by club-shaped spike peplomers covering their surfaces.12 Their genome size is larger than most other RNA viruses and encodes five structural proteins: spike, membrane protein, nucleocapsid protein, hemagglutinin-esterase glycoprotein, and a small envelope.4,13 The spike glycoproteins form the characteristic spikes in the coronavirus “crown” and are important for receptor binding and fusion with the host cell membrane, stimulating neutralizing antibodies, and targeting cytotoxic lymphocytes.14 The M protein plays a role in viral assembly, while the nucleocapsid protein may regulate viral RNA synthesis and interact with the M protein during virus budding.15 The hemagglutinin binds to the host cell surface, allowing the virus to initially adsorb to the membrane. The structure of SARS-CoV-2 and the spike-ACE2 complex provide valuable insights for understanding disease pathogenesis. Overall, understanding the structure and function of SARS-CoV-2 is essential in developing treatments and vaccines to combat the COVID-19 pandemic.
 |
Figure 1 Structure of Coronavirus. Note: Used under license from Shutterstock.com. |
Etiology
Coronaviruses, lethal infections, can infect both humans and animals. Similar to the common cold, they are known to be responsible for about one-third of community-acquired upper respiratory tract infections. According to recent studies, coronaviruses are responsible for 5–10% of cases of acute respiratory infections.16 Coronaviruses are a diverse group of viruses with single-stranded RNA. They classified into several families, including Roniviridae, Arteriviridae, and Coronaviridae.17 There are four genera-Alpha-CoV, Beta-CoV, Delta-CoV, and Gamma-CoV-within the coronavirus family.18,19 Beta-CoV can also be separated into five different lineages.20 As of this writing, there are seven different types of coronaviruses that have been identified to infect humans. From them, three coronaviruses known as SARS-CoV-1, MERS-CoV, and SARS-CoV-2 are particularly concerning as they can lead to severe respiratory illnesses that may even result in death. These three coronaviruses belong to the beta coronavirus group. While delta-CoV and gamma-CoV coronaviruses are thought to have derived from avian species, based on the examination of their DNA, genetic research reveals that alpha-CoV and beta-cov coronaviruses have their roots in bats and rodents.21 Recent research on the genomic characterization of SARS-CoV-2 has revealed that it shares approximately 89% similarity in terms of nucleotide sequence with a bat SARS-like coronavirus called CovZXC21,13,22 an 82% nucleotide match with the human SARS virus and 64% with MERS-CoV.23 SARS-CoV-2, the virus responsible for COVID-19, has a complete genetic sequence spanning from nucleotide 29,891 to 29,903. Its genome contains instructions for the production of 16 nonstructural proteins (nsp 1–16) in around two-thirds of the 14 open reading frames. The remaining one-third of the genome encodes nine additional proteins, alongside the four structural proteins known as spike (S), envelope (E), membrane (M), and nucleocapsid (N). Among these, the spike protein plays a crucial role in facilitating the entry of SARS-CoV into host cells.24
Transmission and Zoonotic Potential of COVID-19
Transmission
In addition to respiratory droplets, other transmission methods were seen during the SARS pandemic, including aerosols, direct contact with infected surfaces, and fecal-oral transmission.25 The early cases of COVID-19 were likely related to contact with infected animals at a seafood market in Wuhan, China, suggesting animal-to-human transmission. The primary route for COVID-19 transmission from animals to humans is believed to be zoonotic overflow. In the case of COVID-19, it is generally accepted that the virus originated in bats and that an intermediate animal host, presumably a pangolin, contributed to the virus’s transmission to humans.26 It’s important to remember that researchers are still examining and researching specific transmission methods. Regardless matter where COVID-19 originated, according to public health recommendations are always advised to stop its spread.
Later, human-to-human transmission of the virus was reported and even considered to be the main form of transmission.5,22,27 Respiratory droplets are the means of human-to-human transmission of COVID-19. Respiratory droplets from the virus can be released into the air when an infected person coughs, sneezes, talks, or breathes. When surrounding people inhale these droplets, an illness may result. The risk of transmission increases with proximity to an infected person, usually within 6 feet or 2 meters. The notion of person-to-person transmission of COVID-19 has been established further from the study of family clusters.28
On the other hand, the disease is quite new, and insufficient research has been done on its transmission dynamics. SARS-CoV-2, the virus causing COVID-19, is believed to be highly contagious, although there is limited research on this topic. It has been suggested that the virus has a basic reproduction number of 2.2, meaning that on average, each infected person can transmit the infection to two other individuals. While asymptomatic individuals can also spread the virus, the main source of infection is usually symptomatic people. Transmission commonly occurs through respiratory droplets released by coughing or sneezing.29 Close contact between individuals and closed spaces due to elevated aerosol concentrations was suggested for transmission, yet further research is required to establish the transmission dynamics of the virus.
The dynamics of SARS-CoV-2 transmission are influenced by a number of variables, such as the extent of seasonal fluctuation in transmission, the length of immunity, the degree of cross-immunity between SARS-CoV-2 and other coronaviruses, and the strength and timing of control measures. To accurately predict the dynamics of SARS-CoV-2 transmission, more research is still needed on the post-recovery immune response, environmental factors that affect transmission, and seasonal effects.30
However, given that high susceptibility is a key driver, it is still uncertain whether seasonal and geographic differences in climate may significantly influence the pandemic course. Studies suggest that the climate may have an impact on the severity of SARS-CoV-2.31 The SARS CoV-2 virus exhibits diverse patterns of transmission: Most people do not spread viruses, but some do, leading to large numbers of secondary cases in transmission clusters known as super spreading episodes.32
Although this dichotomy may be oversimplified, droplet and aerosol transmission can be difficult to distinguish in clinical settings. Droplets are typically thought of as particles larger than 5 m that fall to the ground within approximately 6 feet and aerosols as particles smaller than 5 m that can remain suspended in the air for extended periods of time.33 Respiratory transmission is the primary method by which SARS-CoV-2 spreads. It also emphasizes the significance of respiratory transmission and that ventilation plays a role in either preventing or facilitating spread.34
The relative risk of frequent transmission of SARS-CoV-2 is regarded as low in comparison to direct contact, droplet transmission, or airborne transmission because of the numerous parameters determining the efficacy of environmental transmission. However, the percentage of SARS-CoV-2 infections that are contracted through surface transmission is unknown.35 An overview of the various SARS-CoV-2 transmission routes has been provided in Figure 2.
Although the virus can spread between cats and ferrets and replicates efficiently in cats,36 there have been no verified examples of domestic pet-to-human transmission. Despite the presence of viral RNA in feces, there is currently no evidence to support the fecal-oral infection of SARS CoV-2 in people, and there is also very little evidence for vertical transmission.37 SARS-CoV-2 transmission from person to person most frequently occurs when a person is in the infectious stage of the illness or is a carrier who exhibits no symptoms or only moderate symptoms. Droplets, infected hands, skin-to-skin contact, and contact with inanimate surfaces are the primary factors of virus transmission.38
 |
Figure 2 Various SARS-CoV-2 transmission mechanisms. |
Zoonotic Potential of COVID-19
The term zoonosis refers to the transmission of diseases and infections between animals and humans in a natural manner. The zoonotic potential of COVID-19 refers to the capacity of the disease to transmit from animals to humans. Zoonotic diseases are caused by pathogenic microorganisms, such as viruses or bacteria, that are transmitted from animals to humans through contact with animal blood, feces, or saliva.39 The high infectivity and transmissibility of COVID-19 have raised concerns about its zoonotic potential and the possibility of future outbreaks. Understanding the zoonotic potential of COVID-19 is crucial for preventing future pandemics caused by similar viruses.40 This includes the need for better surveillance of animal-borne diseases and improved practices on the handling and sale of live animals for consumption.
Emerging viral diseases are illnesses that undergo a process of adapting to new hosts and vice versa. They originate in one organism and subsequently transfer to another, resulting in the development of diseases. The unanticipated effects of coronavirus infection on the general public’s health can occur when they infect humans over the species barrier. SARS-CoV-2 infection exhibits a higher transmission rate than other closely related SARS-CoV infections. In-depth knowledge about the history, entry mechanism, and structure of SARS-CoV-2 has provided valuable insights into disease pathogenesis and facilitated the development of vaccines and drugs.41
Studies have indicated that zoonotic diseases, which are newly identified or emerging pathogens, are three times more prone to be influenced by socioeconomic, environmental, and ecological factors.42 To put it another way, the bulk of recently discovered infections-roughly 66% of them-come from animals, primarily wildlife. Similarly, various viruses that affect humans have their origins in animals, suggesting the possibility of cross-species transmission.43 Pathogens that first infect animals can undergo evolutionary changes that enable them to infect humans, thus becoming novel human pathogens. Scientific studies have also suggested that certain contemporary viruses have roots in the earliest ancestors of mammals and have coevolved with humans over time.
There is ongoing debate regarding the source of human coronaviruses. According to one concept, the viruses may have animal origins and spread to people through the ingestion of contaminated food. Another hypothesis suggests that the viruses undergo mutations and evolve into new infectious agents. Recent reports from the COVID-19 outbreak in Wuhan, China, indicated that many patients who contracted the disease had connections to the Huanan Seafood Market, suggesting a possible zoonotic origin.44–46 Additional research on gene characterization indicated that alpha-cov and beta-cov have their genetic origins in bats and rodents. Conversely, delta-cov and gamma-cov are believed to have genetic origins in avian species. Similarly, genomic characterization studies revealed an 89% nucleotide similarity between SARS-CoV-2 and a bat coronavirus called SARS-like CoVZXC21.47 In addition, the virus was extracted from various animal species such as camels, masked palm civets, mice, canines, and felines.4,48 The recurring appearance and spread of CoVs pose a significant risk to public health. This indicates the potential for transmission of these new CoVs from animals to humans and between humans. The ongoing alterations in ecology and climate increase the probability of future incidents where such infections arise.49
In the context of the COVID-19 pandemic caused by the SARS-CoV-2 virus, it is most likely that the virus was transmitted to humans through direct or indirect contact with unidentified animals or their products. The exact animal reservoir for the virus is still unknown, making it premature to categorize it as a zoonotic disease. To effectively combat the pandemic, it is crucial to determine the zoonotic source of SARS-CoV-2. Various studies have shown that the virus can infect both laboratory and wild animals, indicating that different hosts may be susceptible to this novel coronavirus. These findings suggest that several mammal species could potentially serve as intermediate hosts for SARS-CoV-2.50
The imminent threat posed by horseshoe bats, which are located more than 1500 kilometers away from Yunnan Province, was discovered as a result of increased monitoring during the SARS outbreak, which generated the following seeming mystery: How did SARS-CoV-2 reach Wuhan? The wide geographic distribution of the probable reservoir hosts, including the intermediate (Rhinolophus affinis) horseshoe bat species, which are known to harbor sarbecoviruses, suggests that focusing just on Yunnan is unnecessary.51 The genetically closest bat sarbecoviruses are thought to have shared a common ancestor with SARS-CoV-2 at least 40 years ago, supporting this claim. However, knowledge originating from the scientific community is continually changing on the issue; this virus has never been found in humans before. These results show that they came from animals or animal products, even though their exact animal origin is uncertain.
Risks Factors and Severity of COVID-19 Reinfection
Risk Factors
Key COVID-19 risk factors work together and can have a variety of distinct influences on one another’s occurrences and outcomes. Despite the fact that the virus can infect people of various ages and health conditions, there are some risk factors that raise the possibility of developing a serious illness or dying from COVID-19. Therefore, it is crucial to comprehend the risk factors for severe COVID-19 both in the therapeutic setting and at the epidemiological level. The following are some of the main COVID-19 risk factors:
Host Risk Factors
Elderly persons, especially those over 65, are more likely to get serious disease from COVID-19 or perhaps pass away from it.52 People who have chronic conditions like diabetes, heart disease, lung disease, and obesity are at a higher risk of contracting COVID-19 and can experience more severe symptoms.53 People are also more at risk if their immune systems are weak due to underlying illnesses, drugs, or medical procedures.54 COVID-19 is more likely to be contracted by those who reside or work in crowded environments, such as nursing homes, jails, or meat processing facilities. Many racial and ethnic groups, particularly Black and Hispanic communities, have higher rates of hospitalization and death from COVID-19 because to underlying health disparities and social determinants of health.55 It is essential to take preventative measures, such as using a mask, often washing your hands, keeping your distance, and being immunized, in order to help halt the spread of COVID-19.
Environmental Risk Factors
Environmental risk factors for COVID-19 include exposure to the virus in indoor or poorly ventilated spaces, proximity to infected individuals, and contact with contaminated surfaces. Additionally, air pollution and poor air quality may increase the risk of COVID-19 infection and severity of symptoms.56 People living in crowded living conditions and those with poor access to basic hygiene facilities such as handwashing may also be at a higher risk of contracting the virus. Overcrowded or communal living situations, such as nursing homes, prisons, and homeless shelters, can increase the spread of COVID-19.57 People who work in certain industries, such as healthcare, food service, and transportation, are at higher risk of exposure to COVID-19. People who have lower incomes and limited access to healthcare may be at higher risk of COVID-19 due to underlying health conditions and less access to testing and treatment.58 Education is also another socio-economic risk factors because of people with lower levels of education may be less likely to have access to accurate information about COVID-19 and preventative measures.59 It’s important to address these socio-economic risk factors in order to reduce the disparities in COVID-19 outcomes among different populations. Additionally, following public health guidelines to reduce the risk of COVID-19 transmission in all settings in very crucial.
Agents Risk Factors
One of the risk factors, agents risk factor, makes some people more likely to contract COVID-19 and experience severe symptoms. COVID-19 can spread through respiratory droplets when an infected person talks, coughs or sneezes. The amount of virus in an infected individual’s respiratory secretions can affect the likelihood of transmission. The virus can mutate and lead to the emergence of new strains, which can potentially increase its transmission and severity. COVID-19 can survive on surfaces for several hours or even days, allowing it to be transmitted from contaminated surfaces.60 The virus has an incubation period of up to 14 days, which means an infected person may not show symptoms during this period and unknowingly spread the virus to others. It is important to note that these agent risk factors interact with other factors such as host susceptibility and environmental factors, which can also influence the transmission and severity of COVID-1961 The fundamental triads of analytical epidemiology for risk factors for COVID-19 are shown in Figure 3. The likelihood of severe COVID-19 infections can therefore be increased by viral agents like SARS-CoV-2. In order to lower the risk of infection and serious disease, it is crucial that people take precautions against viral exposure.
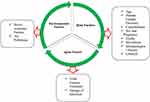 |
Figure 3 The Basic Triads of Analytical Epidemiology of COVID-19 Risk Factors. |
Severity of Reinfection
The severity of a reinfection with COVID-19 can vary based on the patient’s age, any underlying conditions, and the body’s immunological response. The second infection might not be as terrible as the first, according to some evidence, though.62 After contracting SARS-CoV-2, people’s immune systems respond to defend against the virus, most notably by generating antibodies. This immune response can provide excellent protection against infection with the same or different SARS-CoV-2 strains for some months, but over time, this defense deteriorates. Despite the presence of herd immunity resulting from natural infection, it is possible for SARS-CoV-2 to continue spreading among humans. This was demonstrated in August 2020 when the first case of COVID-19 reinfection was identified in Hong Kong. A 33-year-old man, who showed no symptoms, was found to be infected with two different strains of the virus. To put it simply, the virus can still circulate even when people have previously been infected and have developed immunity.63
Results from animal studies showed that after an initial SARS-CoV-2 infection lasting between 21 and 28 days, there was a risk of re-infection with the same or a different strain, suggesting that people may also be susceptible to re-infection.64 Although there have been confirmed occurrences of COVID-19 reinfection, the severity of illness might vary significantly. The second time around, some people get sicker but less severely while others could have worse symptoms.65 It is significant to highlight that reinfection is still very uncommon and that most COVID-19 survivors develop some degree of immunity that can act as defense against subsequent infections. Nonetheless, it’s still crucial to maintain precautions to stop the virus from spreading, such as donning masks, engaging in social isolation, and constantly washing hands.66
Prevention and Treatment
The preferred method to prevent the spread of COVID-19 is to take precautions like wearing a mask, using physical distance, routinely washing your hands, avoiding crowded places, and remaining at home when you are sick. Since COVID-19 does not presently have a known cure, treatment focuses on symptom management and avoiding complications. With over-the-counter drugs, relaxation, and fluids, mild symptoms can be treated. Hospitalization may be necessary in more serious situations for mechanical ventilation or oxygen therapy. To prevent the spread of COVID-19, it’s critical to keep educated and adhere to health experts’ recommendations.67 Here are some key points for the prevention of COVID-19:
Vaccines
Viruses have remained a significant worry since their discovery, posing a challenge in terms of prevention and treatment. One of the reasons for this challenge is their constant adaptation and change as they spread. The COVID-19 pandemic, which originated in China in December 2019, has had a profound impact on a vast number of individuals across the globe. As of July 2022, there have been more than 546 million confirmed cases and over 6.3 million reported deaths worldwide.68 Prioritizing vaccination and antiviral medication therapy should be the main goals in the fight against viral infections. Considering that the COVID-19 pandemic is still ongoing, it is crucial to create community-level protection by either vaccine or natural infections. In order to properly stop the virus’s spread, which is still causing morbidity and fatalities all over the world, this is crucial.69
As a result, many pharmaceutical corporations and university research organizations have begun developing SARS-CoV-2 vaccines based on both traditional and genetic or viral vector platforms.70 Due to recent progress in vaccine development, various national and international drug regulatory agencies have been able to grant emergency use authorizations (EUAs) for multiple COVID-19 vaccine candidates within a year of the release of the virus genome sequence, despite the typical requirement for a longer timeframe.71 There are now approximately 270 COVID-19 vaccine candidates in development, with over 90 in clinical studies. Nucleic acid vaccines (RNA and DNA), adenoviral-vectored vaccines (human and simian replication-deficient and replication-competent), whole-cell inactivated virus, subunit protein vaccines, and virus-like particles are among them.72
For use in emergencies worldwide, several vaccines have received approval from the World Health Organization. Since both SARS-CoVs bind to the same ACE2 receptors in human lung tissue, SARS-CoV-2 is believed to have evolved from the SARS-CoV seen in bats. In addition, the genomic size of each is approximately 30 kb. With SARS-like coronaviruses (beta coronaviruses) found in Chinese bats, SARS-CoV-2 shares 89% of its nucleotides.73
The forefront of vaccine research is currently focused on mRNA vaccines, specifically the ones developed by Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) for COVID-19. These mRNA vaccines have the distinction of being the first authorized immunizations for human use. Over the last two decades, extensive research has been conducted on developing mRNA vaccines against various viruses like rabies, influenza, and zika.74 The mRNA vaccines, namely lipid nanoparticle-encapsulated mRNA-based vaccines, contain genetic instructions encoding the full-length prefusion-stabilized spike protein of the SARS-CoV-2 virus. Both young and old adults showed dose-dependent neutralizing activities in response to the BNT162b2 vaccine.75 The vaccine also enhanced the immune response, specifically the activity of antibody-dependent cellular cytotoxicity, in both individuals who had not been previously exposed to the virus and those who had been infected before.76 Mostly, mRNA vaccines have several advantages, such as scalability, fast design and development, safety, and lack of infectious agent handling, and can induce humoral and cellular responses.77
One of the most recent methods for developing vaccines is the use of viral vectors. Adenoviral vector vaccines, like mRNA vaccines, have emerged as a recent advancement in the field. However, they have been utilized as a means of delivering genes in gene therapy for a long time. Adenoviral vector vaccines offer certain benefits over mRNA vaccines, such as being more cost-effective and having the ability to remain stable at different temperatures.78 Additionally, they have good safety profiles and can, with a single dose, generate strong humoral and cellular responses. The candidate is the viral vector vaccine developed by Oxford University and AstraZeneca. It was one of the first to begin clinical trials and the only one using a weakened chimpanzee adenovirus (ChAdOx1) platform to circumvent the issue of preexisting immunity against the vector considering that very few, if any, humans would have had prior exposure to a simian virus. The wild-type SARS-CoV-2 spike protein’s codon information has been engineered into the ChAdOx1 vector.79
There have been several variants that have evolved as the SARS-CoV-2 pandemic continues to spread worldwide. Currently available SARS-CoV-2 vaccines that are approved for emergency use have demonstrated some advantages in terms of offering sufficient protection against new variations.80 On July 1, 2021, Johnson & Johnson reported promising results from their research, showing that their one-dose COVID-19 vaccine provided robust and durable protection against the fast-spreading Delta variant as well as other commonly found strains of the SARS-CoV-2 virus.81 Despite the success of vaccines against recently discovered variations, the epidemic continues to claim thousands of lives. These findings call for an efficient and proactive immune response against SARS-CoV-2 strain variations that have undergone mutation and genetic drift. Controlling a pandemic such as COVID-19 also requires an understanding of the likely zoonotic origin and the implementation of various preventive measures.
Convalescent Plasma
Convalescent plasma (CP) was a readily available treatment option for COVID-19 at the start of the pandemic when neither a vaccine nor monoclonal antibodies were available. Additionally, due to this, low-resource countries all around the world have begun utilizing convalescent plasma.82 According to studies, individuals who received convalescent plasma had shorter hospital stays and lower mortality rates than those who did not receive it. The evidence suggests that convalescent plasma, obtained from individuals who have recovered from viral infections, can be used as a treatment option with low risk of severe adverse effects. Therefore, it may be beneficial to conduct studies to evaluate the effectiveness and safety of convalescent plasma transfusion in individuals infected with the SARS-CoV-2 virus.83 Additionally, COVID-19 progression was slowed down by early treatment of high-titer convalescent plasma against SARS-CoV-2 to mildly unwell older infected individuals.84
The use of convalescent plasma for COVID-19 treatment may have had an impact on the formation of SARS-CoV-2 subtypes, in contrast to its antiviral effect. This issue was developed in response to a report that suggested that CP therapy in an immunosuppressed patient with COVID-19 was responsible for the emergence of novel SARS-CoV-2 mutations. SARS-CoV-2 mutations that were immune to antibodies, such as the vaccine-resistant E484K mutation, were chosen as the virus repeatedly passed through CP.85 Convalescent plasma should therefore be administered with caution if it is necessary to treat COVID-19.
Antiviral Agents
Since its occurrence, several antiviral drug regimens, such as corticosteroids, hydroxychloroquine, and ribavirin, have been developed and proposed for the treatment of COVID-19. However, their prospective therapy for COVID-19 is not yet fully utilized due to the low quality of the data, the variability of interventions, and the indications.86 Consequently, there are no currently approved specific antiviral agents targeting SARS-CoV-2. However, there are some antiviral drugs that have been approved for emergency use in some countries, including remdesivir and lopinavir/ritonavir, that are still under investigation for possible chemotherapy.87 In one COVID-19 patient in the United States, there have been reports suggesting that remdesivir might have an antiviral effect. Currently, there are ongoing randomized controlled trials to assess the safety and effectiveness of remdesivir.88 Furthermore, there is ongoing debate regarding the use of corticosteroid treatment for lung injury caused by COVID-19.89,90 This is because it can potentially delay the clearance of the viral infection and lead to complications. Since there are currently no effective vaccines or antiviral medications available for the variants of the SARS-CoV-2 virus, it is crucial to explore alternative treatment strategies, particularly for severe cases. Nonetheless, preventive measures such as vaccination, wearing masks, and practicing social distancing continue to play a vital role in controlling the spread of COVID-19.
Concluding Remarks
The origin of the COVID-19 virus is still a topic of controversy. This review was sought to update readers’ knowledge of COVID-19, its potential zoonotic spread, Management, Risks, and Severity of Reinfection. Many scholars believe that COVID-19 can be related to animals or products made from them, even though this is not yet conclusively shown. Some of the scientists even speculate that the virus may have undergone a protracted evolutionary process to develop its current traits. Several presumptive hypotheses have been put forth on the origin of the human coronavirus. To date, there have been conflicting dilemmas regarding the origin of the human coronavirus. Some people claim that viruses underwent mutations and turned into new infectious diseases. Investigations have led some scientists to hypothesize that both humans and animals may have unknowingly consumed the virus. By evaluating the available information regarding the origin of SARS-CoV-2, we suggested that the genesis was most likely zoonotic or spread from an animal source to humans. Despite significant efforts to stop the spread of this new virus, fresh instances and different varieties have been appearing in the world, haunting people’s lives and constituting a pandemic threat even now. Re-emerging COVID-19 cases have raised concerns about the risks of severity of reinfection and the management of the disease. Studies suggest that reinfection may result in more severe symptoms or lead to long-term complications. However, more research is needed to fully understand the risks of reinfection and how to manage it effectively. An efficient and proactive immune response is required to resist the many SARS-CoV-2 strain variations that have undergone mutation and genetic drift. In this context, a number of effective immune response-stimulating vaccines, including those based on mRNA, have been available. Understanding the probable zoonotic genesis of a pandemic like COVID-19 is equally crucial, as are the need for many preventative actions. Overall, it is important to continue to monitor the situation closely, and to implement appropriate measures to control the spread of the virus. Vaccination remains a key tool in this fight, as it can help to reduce the severity of the disease, as well as the risk of transmission and reinfection.
Disclosure
The authors declare that they have no competing interests.
References
1. Machhi J, Herskovitz J, Senan AM, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15(3):359–386. doi:10.1007/s11481-020-09944-5
2. Medić S, Anastassopoulou C, Lozanov-Crvenković Z, et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: a population-level observational study. Lancet Reg Health Eur. 2022;20:100453. doi:10.1016/j.lanepe.2022.100453
3. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11):S223–S227.
4. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi:10.1016/S0140-6736(20)30251-8
5. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
6. Chen Y, Liu Q, Guo D, et al. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423.
7. Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19). Treasure Island (FL): StatPearls Publishing LLC; 2023.
8. Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
10. World Health Organization (WHO). WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva: World Health Organization; 2020.
11. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi:10.1007/978-1-4939-2438-7_1
12. Goldsmith CS, Tatti KM, Ksiazek TG, et al. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10:320–326. doi:10.3201/eid1002.030913
13. Sexton NR, Smith EC, Blanc H, et al. Homology-based identification of a mutation in the coronavirus RNA-dependent RNA polymerase that confers resistance to multiple mutagens. J Virol. 2016;90(16):7415–7428.
14. Masters PS, Kuo L, Ye R, et al. Genetic and molecular biological analysis of protein‒protein interactions in coronavirus assembly. Adv Exp Med Biol. 2006;(581):163–173. doi:10.1007/978-0-387-33012-9_29
15. Sandonís V, García-Ríos E, McConnell MJ, et al. Role of neutralizing antibodies in CMV infection: implications for new therapeutic approaches. Trends Microbiol. 2020;28(11):900–912. doi:10.1016/j.tim.2020.04.003
16. Ma J, Qi X, Chen H, et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis. 2021;72(10):e652–e654. doi:10.1093/cid/ciaa1283
17. de Groot RJ, Cowley JA, Enjuanes L, et al. Order Nidovirales. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. London: Elsevier Inc; 2011:785–795.
18. Beeraka NM, Sukocheva OA, Lukina E, et al. Development of antibody resistance in emerging mutant strains of SARS CoV‐2: impediment for COVID‐19 vaccines. Rev Med Virol. 2022;32(5):e2346. doi:10.1002/rmv.2346
19. Amoutzias GD, Nikolaidis M, Tryfonopoulou E, et al. The remarkable evolutionary plasticity of coronaviruses by mutation and recombination: insights for the COVID-19 pandemic and the future evolutionary paths of SARS-CoV-2. Viruses. 2022;14(1):78. doi:10.3390/v14010078
20. Cueno ME, Imai K. Structural comparison of the SARS CoV 2 spike protein relative to other human-infecting coronaviruses. Front Med. 2021;7:594439. doi:10.3389/fmed.2020.594439
21. Kumar N, Kaushik R, Tennakoon C, et al. Evolutionary signatures governing the codon usage bias in coronaviruses and their implications for viruses infecting various bat species. Viruses. 2021;13(9):1847. doi:10.3390/v13091847
22. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. EmergMicrobesInfect. 2020;9(1):221–236. doi:10.1080/22221751.2020.1719902
23. Benvenuto D, Giovanetti M, Ciccozzi A, et al. The 2019‐new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi:10.1002/jmv.25688
24. Wang Y, Kirkpatrick J, Zur Lage S, et al. 1H, 13C, and 15N backbone chemical-shift assignments of SARS-CoV-2 non-structural protein 1 (leader protein). Biomol NMR Assign. 2021;15(2):287–295.
25. Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi:10.1016/j.jhin.2015.08.027
26. Do Vale B, Lopes AP, Fontes MDC, et al. Bats, pangolins, minks and other animals - villains or victims of SARS-CoV-2? Vet Res Commun. 2021;45(1):1–19. doi:10.1007/s11259-021-09787-2
27. Ghinai I, McPherson TD, Hunter JC, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395(10230):1137–1144. doi:10.1016/S0140-6736(20)30607-3
28. Ye F, Xu S, Rong Z, et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020;94:133–138. doi:10.1016/j.ijid.2020.03.042
29. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12–16. doi:10.1016/j.jmii.2020.05.001
30. Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi:10.1126/science.abb5793
31. Baker RE, Yang W, Vecchi GA, et al. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315–319. doi:10.1126/science.abc2535
32. Meyerowitz EA, Richterman A, Gandhi RT, et al. Transmission of SARS-CoV-2. Ann Intern Med. 2021;174(7):1037. doi:10.7326/L21-0166
33. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441–442. doi:10.1001/jama.2020.12458
34. Rabaan AA, Al-Ahmed SH, Al-Malkey M, et al. Airborne transmission of SARS-CoV-2 is the dominant route of transmission: droplets and aerosols. Infez Med. 2021;29(1):10–19.
35. Ji S, Xiao S, Wang H, et al. Increasing contributions of airborne route in SARS-CoV-2 omicron variant transmission compared with the ancestral strain. Build Environ. 2022;221:109328. doi:10.1016/j.buildenv.2022.109328
36. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi:10.1056/NEJMc2011400
37. Falahi S, Kenarkoohi A. Transmission routes for SARS-CoV-2 infection: review of evidence. New Microbes New Infect. 2020;38:100778.
38. Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi:10.1016/j.jhin.2020.01.022
39. Rahman MT, Sobur MA, Islam MS, et al. Zoonotic diseases: etiology, impact, and control. Microorganisms. 2020;8(9):1405. doi:10.3390/microorganisms8091405
40. Petrovan SO, Aldridge DC, Bartlett H, et al. Post COVID‐19: a solution scan of options for preventing future zoonotic epidemics. Bio Rev. 2021;96(6):2694–2715. doi:10.1111/brv.12774
41. Yesudhas D, Srivastava A, Gromiha MM. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infect. 2021;49(2):199–213. doi:10.1007/s15010-020-01516-2
42. Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi:10.1038/nature06536
43. Chan JF, To KK, Tse H, et al. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi:10.1016/j.tim.2013.05.005
44. Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. doi:10.1007/s12098-020-03263-6
45. Huang X, Zhang C, Pearce R, et al. Identifying the zoonotic origin of SARS-CoV-2 by modeling the binding affinity between the spike receptor-binding domain and host ACE2. J Proteome Res. 2020;19(12):4844–4856. doi:10.1021/acs.jproteome
46. Zhonghua L, Xing B, Xue Z, et al. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. China J Epidemiol. 2020;(41):145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003
47. Hassan SA, Sheikh FN, Jamal S, et al. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12(3):e7355. doi:10.7759/cureus.7355
48. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9
49. Sharun K, Dhama K, Pawde AM, et al. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet Q. 2021;41(1):181–201. doi:10.1080/01652176.2021.1921311
50. Damas J, Hughes GM, Keough KC, et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. PNAS. 2020;117(36):22311–22322. doi:10.1073/pnas.2010146117
51. Lytras S, Hughes J, Martin D, et al. Exploring the natural origins of SARS-CoV-2 in the light of recombination. Genome Biol Evol. 2022;14(2):evac018. doi:10.1093/gbe/evac018
52. Hernandez RA, Colaner C. “This is not the hill to die on. Even if we literally could die on this hill”: examining communication ecologies of uncertainty and family communication about COVID-19. Am Behav Sci. 2021;65(7):956–975. doi:10.1177/0002764221992840
53. Chiner-Vives E, Cordovilla-Perez R, De la Rosa-Carrillo D, et al. Short and long-term impact of COVID-19 infection on previous respiratory diseases. Arch Bronconeumol. 2022;58(1):39–50. doi:10.1016/j.arbres.2022.03.011
54. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi:10.1016/j.clim.2020.108393
55. Grosicki GJ, Bunsawat K, Jeong S, et al. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in white, Black/African American, and latinx populations: social determinants of health. Prog Cardiovasc Dis. 2022;71:4–10. doi:10.1016/j.pcad.2022.04.004
56. Piscitelli P, Miani A, Setti L, et al. The role of outdoor and indoor air quality in the spread of SARS-CoV-2: overview and recommendations by the research group on COVID-19 and particulate matter (RESCOP commission). Environ Res. 2022;211:113038. doi:10.1016/j.envres.2022.113038
57. Cevik M, Marcus JL, Buckee C, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission dynamics should inform policy. Clin Infect Dis. 2021;73(2):S170–S176. doi:10.1093/cid/ciaa1442
58. Lima NN, de Souza RI, Feitosa PW, et al. People experiencing homelessness: their potential exposure to COVID-19. Psychiatry Res. 2020;288:112945. doi:10.1016/j.psychres.2020
59. Kusuma D, Pradeepa R, Khawaja KI, et al. Low uptake of COVID-19 prevention behaviors and high socioeconomic impact of lockdown measures in South Asia: evidence from a large-scale multi-country surveillance programme. SSM Popul Health. 2021;13:100751. doi:10.1016/j.ssmph.2021.100751
60. Han J, Zhang X, He S, et al. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2021;19(1):5–16. doi:10.1007/s10311-020-01101-x
61. Głuchowska K, Dzieciątkowski T, Sędzikowska A, et al. The new status of parasitic diseases in the COVID-19 pandemic-risk factors or protective agents? J Clin Med. 2021;10(11):2533. doi:10.3390/jcm10112533
62. Milne G, Hames T, Scotton C, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. 2021;9(12):1450–1466. doi:10.1016/S2213-2600(21)00407-0
63. To KK, Hung IF, Ip JD, et al. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2021;73(9):e2946–e2951. doi:10.1093/cid/ciaa1275
64. Field CJ, Heinly TA, Patel DR, et al. Immune durability and protection against SARS-CoV-2 re-infection in Syrian hamsters. Emerg Microbes Infect. 2022;11(1):1103–1114. doi:10.1080/22221751
65. Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. doi:10.1038/s41591-020-01202-8
66. Zheng L, Chen K, Ma L. Knowledge, attitudes, and practices toward COVID-19 among construction industry practitioners in China. Front Public Health. 2021;8:599769. doi:10.3389/fpubh.2020.599769
67. Nicola M, O’Neill N, Sohrabi C, et al. Evidence based management guideline for the COVID-19 pandemic-Review article. Int J Surg. 2020;(77):206–216. doi:10.1016/j.ijsu.2020.04.001
68. World Health Organization. COVID-19 Weekly Epidemiological Update, 9 March 2021. World Health Organization; 2022.
69. Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21(4):245–256. doi:10.1038/s41577-021-00522-1
70. Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immuni. 2020;52(4):583–589. doi:10.1016/j.immuni.2020.03.007
71. Kyriakidis NC, López-Cortés A, González EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of Phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi:10.1038/s41541-021-00292-w
72. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi:10.1038/s41577-021-00578-z
73. Wu F, Zhao S, Yu B, et al. Complete genome characterization of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. BioRxiv. 2020. doi:10.1101/2020.01.24.919183
74. Scorza FB, Pardi N. New kids on the block: RNA-based influenza virus vaccines. Vaccines. 2018;6(2):20. doi:10.3390/vaccines6020020
75. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi:10.1038/s41586-021-03653-6
76. Tauzin A, Nayrac M, Benlarbi M, et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29(7):1137–1150. doi:10.1016/j.chom.2021.06.001
77. Jafari A, DaneshPouya F, Niknam Z, et al. Current advances and challenges in COVID-19 vaccine development: from conventional vaccines to next-generation vaccine platforms. Mol Biol Rep. 2022;49(6):4943–4957. doi:10.1007/s11033-022-07132-7
78. Holm MR, Poland GA. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine. 2021;39(3):457–459. doi:10.1016/j.vaccine.2020.12.017
79. Mahasirimongkol S, Khunphon A, Kwangsukstid O, et al. The pilot study of immunogenicity and adverse events of a COVID-19 vaccine regimen: priming with inactivated whole SARS-CoV-2 vaccine (CoronaVac) and boosting with the adenoviral vector (ChAdOx1 nCoV-19) vaccine. Vaccines. 2022;10(4):536. doi:10.3390/vaccines10040536
80. Bian L, Gao F, Zhang J, et al. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(4):365–373. doi:10.1080/14760584.2021.1903879
81. Chmielewska AM, Czarnota A, Bieńkowska-Szewczyk K, et al. Immune response against SARS-CoV-2 variants: the role of neutralization assays. NPJ Vaccines. 2021;6(1):142. doi:10.1038/s41541-021-00404-6
82. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi:10.1172/JCI138745
83. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi:10.1007/s10096-004-1271-9
84. Fisher DL, Alin P, Malnick S. The evidence for high-titer convalescent plasma in SARS-CoV-2. SN Compr Clin Med. 2021;3(3):790–792. doi:10.1007/s42399-021-00827-1
85. Casadevall A, Henderson JP, Joyner MJ, et al. SARS-CoV-2 variants and convalescent plasma: reality, fallacies, and opportunities. J Clin Invest. 2021;131(7):e148832. doi:10.1172/JCI148832
86. Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID-19-lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020;157:104872. doi:10.1016/j.phrs.2020.104872
87. Ismaila MS, Bande F, Ishaka A, et al. Therapeutic options for COVID-19: a quick review. J Chemother. 2021;33(2):67–84. doi:10.1080/1120009X.2020.1868237
88. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi:10.1056/NEJMoa2001191
89. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi:10.1016/S0140-6736(20)30317-2
90. Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi:10.1016/S0140-6736(20)30361-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
