Back to Journals » Journal of Inflammation Research » Volume 16
Distinct Features of Vascular Diseases in COVID-19
Authors Ceasovschih A , Sorodoc V, Shor A, Haliga RE, Roth L, Lionte C , Onofrei Aursulesei V , Sirbu O, Culis N, Shapieva A, Tahir Khokhar MA, Statescu C, Sascau RA, Coman AE, Stoica A, Grigorescu ED, Banach M, Thomopoulos C, Sorodoc L
Received 18 April 2023
Accepted for publication 28 June 2023
Published 6 July 2023 Volume 2023:16 Pages 2783—2800
DOI https://doi.org/10.2147/JIR.S417691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Alexandr Ceasovschih,1,2,* Victorita Sorodoc,1,2 Annabelle Shor,1,* Raluca Ecaterina Haliga,1,2,* Lynn Roth,3 Catalina Lionte,1,2 Viviana Onofrei Aursulesei,4,* Oana Sirbu,1,2,* Nicolae Culis,5 Albina Shapieva,6 Mohammed AR Tahir Khokhar,1 Cristian Statescu,7 Radu A Sascau,7 Adorata Elena Coman,1 Alexandra Stoica,1,2 Elena-Daniela Grigorescu,1 Maciej Banach,8 Costas Thomopoulos,9 Laurentiu Sorodoc1,2
1Faculty of Medicine, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, 700115, Romania; 2 2nd Internal Medicine Department, Sf. Spiridon Clinical Emergency Hospital, Iasi, 700111, Romania; 3Laboratory of Physiopharmacology, Department of Pharmaceutical Sciences, University of Antwerp, Wilrijk, 2610, Belgium; 4Department of Cardiology, Clinical Emergency Hospital “Sfantul Spiridon”, Iasi, 700106, Romania; 5Nottingham University Hospitals NHS Trust, Queen’s Medical Center, Nottingham, NG72UH, UK; 6Cardiac Electrophysiology Department, Petrovsky National Research Center of Surgery, Moscow, 119991, Russia; 7Department of Cardiology, Cardiovascular Diseases Institute “Prof. Dr. George I.M. Georgescu”, Iasi, 700503, Romania; 8Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Lodz, 93338, Poland; 9Department of Cardiology, Elena Venizelou General Hospital, Athens, GR-11522, Greece
*These authors contributed equally to this work
Correspondence: Victorita Sorodoc, 2nd Internal Medicine Department, Sf. Spiridon Clinical Emergency Hospital, Independentei 1 Street, Iasi, 700111, Romania, Tel +40232 240 822, Email [email protected] Lynn Roth, Laboratory of Physiopharmacology, Department of Pharmaceutical Sciences, University of Antwerp, Wilrijk, 2610, Belgium, Email [email protected]
Abstract: The Coronavirus Disease 2019 (COVID-19) pandemic was declared in early 2020 after several unexplained pneumonia cases were first reported in Wuhan, China, and subsequently in other parts of the world. Commonly, the disease comprises several clinical features, including high temperature, dry cough, shortness of breath, and hypoxia, associated with findings of interstitial pneumonia on chest X-ray and computer tomography. Nevertheless, severe forms of acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) are not limited to the respiratory tract but also may be extended to other systems, including the cardiovascular system. The bi-directional relationship between atherosclerosis and COVID-19 is accompanied by poor prognosis. The immune response hyperactivation due to SARS-CoV-2 infection causes an increased secretion of cytokines, endothelial dysfunction, and arterial stiffness, which promotes the development of atherosclerosis. Also, due to the COVID-19 pandemic, access to healthcare amenities was reduced, resulting in increased morbidity and mortality in patients at risk. Furthermore, as lockdown measures were largely adopted worldwide, the sedentary lifestyle and the increased consumption of processed nutrients or unhealthy food increased, and in the consequence, we might observe even 70% of overweight and obese population. Altogether, with the relatively low ratio of vaccinated people in many countries, and important health debt appeared, which is now and will be for next decade a large healthcare challenge. However, the experience gained in the COVID-19 pandemic and the new methods of patients’ approaching have helped the medical system to overcome this crisis and will hopefully help in the case of new possible epidemics.
Keywords: vascular diseases, COVID-19, SARS-CoV-2, endothelial dysfunction, atherosclerosis, arterial stiffness, vaccines
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic was declared in early 2020 after several unexplained pneumonia cases were first reported in Wuhan, China, and subsequently in other parts of the world. Commonly, the disease comprises several clinical features, including high temperature, dry cough, shortness of breath, and hypoxia, associated with findings of interstitial pneumonia on chest X-ray and computer tomography.1–3 This disease requires specific treatment, which directly depends on the COVID-19 severity.4–6 Nevertheless, severe forms of acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) are not limited to the respiratory tract but also may be extended to other systems, including the cardiovascular system.7–11
Therefore, the aim of the current review is to summarize the effects of SARS-CoV-2 infection on the initiation and progression of atherosclerotic diseases and the pathophysio-logical mechanisms involved.
Pathophysiological Mechanisms That Drive COVID-19 Related Vascular Diseases
Endothelial Dysfunction
The vascular endothelium consists of endothelial cells that act as a semipermeable barrier by forming the inner cellular lining of blood vessels. The endothelium is essential for maintaining vascular health and hemostasis, as it inhibits clot formation and restores vascular homeostasis.12 It synthesizes nitric oxide (among other vasoactive substances), which protects against atherosclerosis, causes vasodilation by acting on vascular smooth muscles, and regulates thrombus formation and fibrinolysis.13,14
Endothelium that fails to function appropriately is associated with the vast majority of cardiovascular conditions and is characterized by the loss of vascular integrity, a pro-thrombotic and proinflammatory phenotype and reduced vasodilation.15–17 Factors that promote endothelial dysfunction include smoking, metabolic disorders, chronic illnesses, and disturbed blood flow.18,19
In clinically severe infections, endothelial dysfunction can be elicited directly through signaling effects of the virus or indirectly by maladaptation of the immuno-logical response leading to inappropriate endothelial activation. This particular state of endothelial dysfunction caused by viral infections predisposes to coagulopathies and it is characterized by vascular leakage. The excess of free radicals disrupts the semipermeable barrier reducing nitric oxide release, which in turn promotes vasoconstriction and allows penetration of toxins into the underlying tissues. Previous coronavirus infections have been documented to cause indirect endothelial dysfunction through elevated proinflammatory mediators leading to coagulopathies. Likewise, COVID-19 appears to reproduce a similar prothrombotic effect.12
Autopsy reports indicate that the histologic pattern in the peripheral lung was diffuse alveolar damage with perivascular T-cell infiltration. The lungs of patients with COVID-19 also showed distinctive vascular features, consisting of severe endothelial injury associated with the presence of intracellular viruses and disrupted cell membranes. Histologic analysis of pulmonary vessels in patients with COVID-19 showed widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were 9 times as prevalent in patients with COVID-19 as in patients with influenza.20
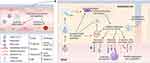
It is presumed that the primary entry and infection mechanism of SARS-CoV-2 is via angiotensin-converting enzyme 2 (ACE2) receptors, which are expressed in the respiratory and vascular endothelium, among other cells.21 The injury of endothelial cells, and subsequent endothelial dysfunction, is initiated by the attachment of the pathogen to the ACE2 receptor.22 SARS-CoV-2 can affect any organ expressing ACE2 receptors and downregulates its expression on the cell surface.23 The primary role of ACE2 is cardioprotective by converting angiotensin I and angiotensin II into angiotensin 1–9 and angiotensin 1–7, respectively. Loss of ACE2 on vascular endothelium can therefore exacerbate inflammation, thrombosis, and endothelial dysfunction.24,25 Damage to the endothelial cells might also be directly triggered by the infection, resulting in reduced nitric oxide (NO) availability, oxidative stress and inflammation (Figure 1).26
In addition, COVID-19 induces a specific proinflammatory state, called a “cytokine storm”. This is an excessive release of cytokines that has been observed in severe forms of the disease, further promoting vascular inflammation, endothelial dysfunction, coagulation disorders, and cardiovascular complications. However, it is still under debate whether the inflammatory-associated changes are a direct effect of COVID-19 or a consequence of the “cytokine storm”.27,28
Among COVID-19 patients, reduced levels of NO are observed. The underlying pathological manifestations in severe clinical cases are endothelial dysfunction, coagulopathy, inflammation, and vascular leakage. Increased platelet activation, high levels of fibrinogen and D-dimers in the plasma have also been observed in severe cases of COVID-19. Especially, elevated D-dimer levels are associated with a poor prognosis.29 The marked increase in D-dimer levels and thrombocytopenia may suggest a similar coagulation derangement between COVID-19 and disseminated intravascular coagulation. However, some coagulation parameters in COVID-19 differ from those observed in disseminated intravascular coagulation. Indeed, elevated fibrinogen levels and factor VIII activity suggest no high consumption of clotting factors in COVID-19.30
The endothelial glycocalyx (eGC) is another important factor in the pathogenesis of COVID-19 endothelial dysfunction. This complex network structure, of a thickness up to 3 μm, consists of proteoglycans, heparan sulfate, chondroitin sulfate and highly sulfated glycosaminoglycans. The eGC damage plays a key role in the membrane permeability during infections, as it increases leukocyte recruitment, barrier disruption and the development of end-organ complications, especially in the lungs and kidneys. This process involves endo-β-glucuronidases, which destroy the glycocalyx on the cell surface by acting on the glycosaminoglycans hyaluronan (HA) and heparan sulfate chains. Thus, the “cytokine storm” in COVID-19 leads to abnormal degradation of the endothelial glycocalyx, further resulting in endothelial dysfunction determined by the action of HA fragments.31,32
Arterial Stiffness
Causes and Consequences of Arterial Stiffness
Elastic properties of the aorta provide the Windkessel effect, thus maintaining a stable blood flow to distal organs throughout the cardiac cycle.33 Ageing and other cardiovascular risk factors, including hypertension, metabolic diseases, chronic kidney disease, smoking, and sleep disturbances, contribute to the progressive stiffening of the aorta. Previous reviews have extensively described the pathophysiological mechanisms involved in this process. They are related to 1) dysfunction of different types of vascular cells (eg, endothelial cells, vascular smooth muscle cells), 2) changes in extracellular matrix composition (eg, elastin fragmentation, enhanced collagen production), and 3) altered shear stress, and 4) increased inflammation and oxidative stress.34–37 Thus, an increased arterial stiffness deteriorates the mechanics of the aorta. Moreover, an increase in systolic and pulse pressure (PP) can further aggravate arterial stiffening and contribute to 1) heart failure development and 2) damage of the distal microcirculation.33,36,38
A reliable measure of arterial stiffness might be the assessment of pulse wave velocity (PWV). PWV represents the speed of the pressure wave traveling along the arterial system, which is increased in stiffer arteries. Carrying carotid-femoral pulse wave velocity (cfPWV) is the gold standard because it predicts cardiovascular events.39–42 Although cfPWV reflects the large elastic artery stiffness, brachial-ankle pulse wave velocity (baPWV) is partly determined by peripheral artery stiffness. Other less frequently used techniques include the direct measurement of arterial distensibility and calculation of stiffness-related parameters such as augmentation index (based on pulse wave reflection) and ambulatory arterial stiffness index.43
Overall, progressive stiffening of the aorta contributes to cardiovascular disease development and is considered an independent predictor of mortality.39,42 Due to these major implications for cardiovascular health, it is pivotal to demonstrate and understand the acute and long-term consequences of COVID-19 infection on arterial stiffness.
Effect of COVID-19 Infection on Arterial Stiffness
During the acute phase of the SARS-CoV-2 infection, increases in measures of arterial stiffness have been described.44 For instance, a higher cfPWV and baPWV were detected in a study comparing twenty-two acutely ill patients with and without COVID-19.45 Ratchford et al showed that eleven young adults had a greater cfPWV 3–4 weeks following SARS-CoV-2 infection than healthy controls. The same study reported a significantly lower brachial artery flow-mediated dilation (FMD), suggesting an impaired vascular function.46 Szeghy et al reported higher carotid stiffness and Young’s modulus in fifteen young adults who recovered from COVID-19 compared with healthy control adults. In addition, a greater aortic augmentation index was observed within the same patient population, which suggests aortic stiffening.47 Interestingly, the severity of the infection seemed to be positively correlated with arterial stiffness. Moderate and severe COVID-19 patients had significantly elevated arterial stiffness compared to mild COVID-19 patients as measured by cfPWV and aortic augmentation index.48 Thus, during the acute phase of COVID-19, arterial stiffness seems to be increased, although these results need to be interpreted with caution due to the limited number of included patients.
Since arterial stiffness is a major risk factor for cardiovascular disease development, it is important to determine whether the acute increase in stiffness during COVID-19 infection persists over a longer period of time. For this reason, several studies aimed to determine the long-term consequences of COVID-19 on vascular health.
In young adults, the reported acute effects on arterial stiffness and vascular function seem transient since no difference in cfPWV was observed in sixteen subjects beyond 4 weeks after diagnosis. However, peripheral vascular function remained impaired in patients who were still symptomatic.49 Furthermore, in a 6-month follow-up study, cfPWV significantly declined after 4 months and eventually reached levels of the healthy control group.46,50 The changes in cfPWV were correlated to mean arterial pressure fluctuations.50 Given that PWV is highly dependent on blood pressure (BP) levels, PWV values will increase with BP rise.51 Therefore, it might be possible that the initial increase in cfPWV during the acute phase of SARS-CoV-2 infection, and the subsequent decline over time, is related to BP changes rather than vascular wall structural changes. Indeed, cfPWV values did not change over time when corrected for mean arterial pressure.50 These findings were confirmed by Nandadeva et al, who showed that ambulatory BP and cfPWV were inversely related with time since the COVID-19 diagnosis (ranging from 3 to 22 weeks) in young adults. However, the correlation between cfPWV and time after diagnosis was still significant after adjustment for BP changes.52
Within a broader patient population, the effects of an initial SARS-CoV-2 infection on arterial stiffness parameters seem to persist for a longer period than reported for young adults. For instance, fifty patients with a positive SARS-CoV-2 PCR test in the last 3 to 6 months showed a decreased aortic strain and aortic distensibility combined with an increased PP and aortic stiffness index.53 In a longitudinal study by Zanoli et al, forty-one COVID-19 patients showed a higher aortic PWV approximately 5 months after the onset of infection. This measure of arterial stiffness significantly decreased but remained higher than in control individuals 1-year post-COVID-19.54 The persistence of arterial stiffness was also confirmed by Lambadiari et al, who observed greater cfPWV, central PP, and systolic BP among seventy COVID-19 patients 4 months after diagnosis.55 Even after 12 months, central systolic BP remained high, and cfPWV slightly decreased, reaching values between the 4 months studied patients and the control group.56 In addition, endothelial and vascular dysfunctions were observed. Interestingly, a 10-fold increase in malondialdehyde (lipid peroxidation marker) was seen in the COVID-19 group 4 months after diagnosis, suggesting an association between COVID-19-related oxidative stress and impaired vascular function.55 Even after 1 year of follow-up, endothelial and vascular impairment remained present. However, a significant reduction in malondialdehyde was observed, and levels were still 2-fold higher than healthy controls.56 In contrast, another study reported no increase in cfPWV and other cardiovascular risk factors (eg, BP, BMI) in patients 6 months following SARS-CoV-2 infection (n = 101).57 Since increases in arterial stiffness were transient,54,56 conflicting findings may be related to different timeframes after infection, ranging between 4 and 8 months.57 Overall, several studies have reported the effects of SARS-CoV-2 infection on arterial stiffness-related parameters, which gradually decline over time but persist in some patient populations until 1 year after infection.
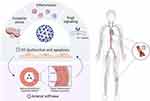
One of the main mechanisms for the effects of SARS-CoV-2 infection on arterial stiffness is endothelial dysfunction, resulting from increased vascular inflammation and oxidative stress. A dysfunctional endothelium is associated with vasoconstriction, which increases arterial stiffness partly because of the reduced nitric oxide availability.34 However, increased stiffness can also induce further endothelial damage, resulting in a vicious cycle that can lead to long-term consequences (Figure 2).34 Besides endothelial dysfunction, vascular inflammation and oxidative stress are also known to induce the synthesis and activation of matrix metalloproteinases (MMPs). Several studies have reported increased serum or plasma levels of MMP-9 during the acute phase of COVID-19,58–60 which were normalized 3 months after diagnosis.61 However, tissue inhibitor of metalloproteinases 2 (TIMP-2) levels, an inhibitor of MMP-9 activity, remained unchanged.61 Also, increased circulating levels of MMP-2 and MMP-3 have been documented.59,62,63 Because MMPs can promote arterial stiffness by degrading elastin and increasing collagen deposition, changes in extracellular matrix composition by this mechanism should not be neglected.64
Although acute and chronic effects of COVID-19 on arterial stiffness parameters have been described, many uncertainties remain. For instance, sex-specific effects on arterial stiffness and vascular function could not be investigated with sufficient statistical power due to the small number of included participants in current studies. Also, the influence of psychosocial factors remains elusive. However, the ongoing CARTESIAN study, a longitudinal and multicentre trial with more than 2500 participants would shed light on controversial or purely studied problems.65 In addition, unraveling the pathophysiological mechanisms underlying the effects of COVID-19 on arterial stiffness would be desirable in future research to develop adequate therapeutic strategies to reduce cardiovascular complications. In conclusion, endothelial dysfunction and vascular stiffness seem to be the main causal factors for the initiation and progression of atherosclerotic disease by SARS-CoV-2 infection (Figure 2).
Atherosclerotic Diseases and COVID-19
Atherosclerosis, consisting of plaque development in the arterial wall, is a chronic inflammatory disease.66,67 Activation of different pathophysiological pathways contribute to a self-maintaining inflammatory state,68–70 including 1) cytokine secretion by leukocytes, endothelial cells, platelets, or mast cells,71 and 2) subendothelial infiltration and deposition of lipids and their oxidation.72 Eventually, vulnerable atherosclerotic plaques can rupture, leading to thrombotic occlusion and complications such as a myocardial infarction or stroke, depending on the artery involved. Severe stenosis of arteries, in the absence of rupture, might also be sufficient to restrict blood flow and induce ischemic complications.73
In the process of SARS-CoV-2 penetration into host cells, one of the substantial roles is played by cholesterol. It is important to note that high cholesterol content at the lipid rafts is significant in increasing viral infectivity. The lipid rafts role is both in the interaction between the ACE2 receptors and S proteins, as well as in process of SARS-CoV-2 endocytosis contribution. The cholesterol presence in the cell membrane and on the viral envelope creates more favorable conditions for SARS-CoV-2 replication; furthermore, it interacts with the coronavirus N-terminal fusion peptide, which in turn ensures the virus penetration into the host cell. In the presence of dyslipidemia, a high ACE2 receptors number in lipid rafts of cells contributes to the SARS-CoV-2 penetration into them. In this process, the cholesterol influence on the SARS-CoV-2 S spike configuration also plays an important role, contributing to an increase in affinity for ACE2 receptors. Thus, hypercholesterolemia is associated with a higher risk of severe COVID-19 outcomes. Moreover, type 2 diabetes mellitus and obesity highly worsen the prognosis of patients with COVID-19.74,75
COVID-19 may further promote atherogeneses in several ways. Firstly, the exaggerated inflammatory and immune response creates a “cytokine storm”, leading to atherosclerotic plaque progression. Experimental studies demonstrated a positive relationship between high concentrations of pro-inflammatory cytokines (TNF-alpha, IL-6, or IL-1β) and plaque instability or rupture. IL-1β induces an increased expression of additional cytokines, such as IL-6, TNF-alpha, IL-8, or chemokines (eg, MCP-1), with pro-inflammatory effects. It also increases lipid uptake by macrophages and enhances vascular inflammation and plaque instability. In addition, the complement system contributes to the exacerbation of inflammatory responses and atherosclerotic plaque progression. Other pathogenic pathways are closely correlated with atherosclerosis progression and instability, like CD14++ CD16+ cells activated by atherosclerosis.68 Secondly, endothelial dysfunction caused by SARS-CoV-2 infection may also activate the release of prothrombotic factors such as von Willebrand and factor VIII, which favors thromboembolic events. Furthermore, the expression of adhesion molecules is upregulated, resulting in leukocyte recruitment and enhanced vascular inflammation. All these mechanisms are reciprocally reinforced, leading to the perpetuation of proinflammatory and prothrombotic states. In addition, due to impaired nitric oxide synthesis, arterial stiffening might occur. All these aspects can contribute to atherosclerotic plaque instability, including a higher risk of rupture and acute vascular events.71,73
Conversely, pre-existing atherosclerosis can also affect COVID-19 severity. Inflammatory signaling pathways activated in atherosclerosis trigger cytokine and chemokine release, contributing to a pro-inflammatory state, coagulation disorders, and the consecutive activation of innate and adaptive immunity. This inflammatory state of the arterial wall represents the ideal hosting area for SARS-CoV-2 replication. A study using HEK293T cells showed that loading these cells with cholesterol, resulted in an increased endocytosis and infectivity of SARS-CoV-2. A possible explanation could be that cholesterol traffics ACE2 to the entry site of the virus, thereby facilitating endocytosis. Thus, high cellular cholesterol levels, as observed in patients with atherosclerosis, promote viral replication, resulting in a more severe clinical presentation of COVID-19.76 Moreover, the increased SARS-CoV-2 infectivity favors immune system overactivation, further promoting atherogenesis and creating a vicious cycle.68 Also, in humans, the link between cardiovascular disease (CVD) and COVID-19 has been investigated. A systematic review of 72 studies including 3470 COVID-19 patients showed the presence of CVD and hypertension in 8.3% and 13.3%, respectively.77 These comorbidities have been found to worsen the prognosis,78 with morbidity being the highest (10.5%) for patients with pre-existing cardiovascular conditions.79
Atherosclerosis is the main underlying pathophysiological mechanism causing coronary artery disease (CAD) and peripheral artery disease (PAD). It the context of CAD, plaque has accumulated in coronary arteries, which supply blood to the heart. If peripheral arteries (mainly in the legs) are affected by atherosclerosis, this is referred to as PAD. Both conditions have different symptoms and complications, and can be affected by COVID-19, as discussed below.
Coronary Artery Disease
Coronary artery disease (CAD) is the leading cause of death worldwide. More than 80% of sudden deaths are caused by atherosclerotic CAD.80 Modifiable risk factors associated with CAD include diabetes mellitus, hypertension, smoking, hyperlipidemia, obesity, and psychosocial stress.81,82 In addition, family history is recognized as a non-modifiable risk factor for CAD.83
Due to the COVID-19 pandemic, many scheduled appointments and preventive consultations had to be canceled or postponed. Therefore, many CAD patients were exposed to an increased risk of coronary events.83,84 Clinical data showed that although hospital admissions for acute myocardial infarction decreased since the COVID-19 outbreak, the cases were more severe. Indeed, higher troponin levels, increased mortality rate, major cardiac complications, and delays from symptoms onset to first medical contact were noticed. The delay in seeking medical treatment was associated with the inertia of 1) health services to treat morbidities unrelated to COVID-19 and 2) patients to visit medical settings because of the potential exposure to SARS-CoV-2 infection.85 In addition, it has been reported that patients with severe COVID-19 have a proportionally higher incidence of CAD and are more likely to die while hospitalized than patients without COVID-19.1,86,87 Some studies proposed the association between inflammation and changes in hemodynamics during COVID-19 with an increased risk of plaque rupture in CAD patients.79,88–90 Others emphasized the pro-coagulatory COVID-19 effect.91 Similar findings were obtained by postmortem examinations of COVID-19 patients.92 Furthermore, a sedentary lifestyle during the COVID-19 pandemic, because of lockdowns and social distancing rules, contributed to CAD development.93 The above-mentioned emerging problems emphasize the need for detailed and rigorous cardiovascular assessment and treatment in patients with COVID-19.
Pathophysiology
There are at least five pathophysiological mechanisms identified that affect the myocardium in COVID-19 patients: (1) sepsis; (2) lung injury; (3) respiratory failure as a result of severe physiological strain;94 (4) type I myocardial infarction secondary to the rupture of atherosclerotic plaque and (5) type II myocardial infarction due to a mismatch between oxygen demand and supply.95 The former could be caused by several COVID-19 related mechanisms, including reduced myocardial perfusion due to local coronary atherosclerosis, endothelial dysfunction within the coronary microcirculation, high levels of angiotensin II causing severe systemic hypertension and vasoconstriction leading to hypoxia due to acute respiratory distress syndrome (ARDS) or pulmonary vascular thrombosis. In addition, severe ‘cytokine storms’ (eg, IL-6, IL-7, IL-22, IL-17) have been described during the SARS-CoV-2 infection.96–98 Particularly, higher plasma concentrations of IL-6 were detected in severe COVID-19 patients and linked to increased mortality.86 Several studies have established the link between “cytokine storms” and myocardial damage.99–101 Interestingly, inflammatory cytokine expression is increased in the epicardial adipose tissue of patients with CAD.102,103
Diagnosis
Current guidelines for chronic coronary syndrome diagnosis recommend starting with an assessment of the CAD pretest probability, which is based upon patient history, examination, and basic tests such as an electrocardiogram. Patients with a pretest probability score >85% require no further noninvasive testing to be diagnosed with CAD. Instead, they can undergo invasive coronary angiography to gauge the risk level and to develop an appropriate management plan. Patients with a score <15% are considered low risk, and the diagnosis of CAD can be safely excluded. However, a coronary computed tomography angiography can still provide useful information to exclude CAD.104 Patients with COVID-19 might experience different cardiac events, such as arrhythmias, myocardial damage, and cardiac arrest, complicating CAD diagnosis.1,105 Moreover, cardiac manifestations can be the initial symptoms in some patients with COVID-19.106
Several biomarkers are associated with myocardial injury during COVID-19 infection.107,108 Myocardial injuries associated with COVID-19 result in elevated levels of high-sensitivity cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP).109 High cTnI levels correlate with the severity of the disease.110 Furthermore, elevated levels of cTnI correlate with viral load, suggesting it induces cardiac injury, likely through plaque rupture or intracoronary thrombus formation.1,111 Similarly, a positive correlation between cTnI elevation and C-reactive protein or NT-proBNP suggests a close relationship between CAD and the extent of inflammation or ventricular impairment.86,88 Alternatively, many patients with COVID-19 have detectable levels of cTnI and no evidence of CAD as measured by coronary angiography.112 Thus, the diagnosis of cardiac injury cannot be solely based on cardiac biomarkers and needs to consider other examinations, such as an electrocardiogram and imaging techniques.113 Moreover, it was hypothesized that the up‐regulation of microRNAs (eg, miR‐21, miR‐155, miR‐208a, and miR‐499) in COVID‐19 survivors might better refine myocardial damage compared to markers like high-sensitive troponin. MicroRNAs can be useful in evaluating the long‐term cardiovascular consequences of COVID-19.114–116
Treatment
The current CAD management mainly relies on educating the general population on modifiable risk factors for atherosclerosis and how to prevent the disease through lifestyle changes. Unfortunately, quarantine and other social distancing measures during the COVID-19 pandemic harmed the quality of care and disease outcome in CAD patients due to a more sedentary lifestyle, reduced physical activity, postponed medical visits and reduced adherence to drug treatment.117
For CAD patients, drug treatment includes antiplatelet agents, statins, β-blockers, and angiotensin-converting enzyme inhibitors. To prevent thrombotic complications, treatment protocols in COVID-19 and CAD overlap to some extent. For example, hospitalized adults with COVID‐19 may receive thromboprophylaxis based on venous thromboembolism risk scores and D-dimer levels. The recommended prophylaxis is daily treatment with low‐molecular‐weight heparins.118,119 Although endothelial dysfunction and increased platelet reactivity are common features of CAD and COVID-19 infection, current data is inconsistent regarding the antiplatelet agents benefits in the coronavirus infectious disease progression. In fact, prior chronic treatment with aspirin was found to induce a possible increased risk of hospitalization rates in COVID-19 patients, without any impact on intermediate COVID-19 outcomes (intensive care unit admission, need for intubation) or the all-cause mortality.120
Statin treatment showed a beneficial effect on the cardiac vasculature in patients with COVID-19.121 Interference with main pathogenic viral mechanisms (endothelial activation, release of proinflammatory cytokines, regulation of surface cell expression of ACE2) is considered a key determinant in interpreting the anti-thrombotic and anti-inflammatory effects associated with the administration of statins.122 In addition, statin use was shown to be associated with reduction of the risk of intensive care unit admission and lower mortality in COVID-19 patients.123–125 Another study has shown that older adults on statin therapy have a higher chance of developing asymptomatic COVID-19 as opposed to a symptomatic form of the disease.126,127 Although extensive research pleads in favor of statin administration (in both pre-hospital and in-hospital settings) in COVID-19 patients, several uncharted findings are yet to be defined (a proven causal relationship between the use of statins and the beneficial effects, type of statins that provide better protection, role of drug interaction, impact of pre-existing comorbidities).125,128
Many controversies surrounded the negative impact that ACE2 inhibitors or angiotensin receptor blockers (ARBs) might have on SARS-CoV-2 infections, primarily because these agents increase the expression of ACE2 receptors, promoting SARS-CoV-2 entry into the cell.129 Nevertheless, this theory was wrong, and RAS blockers showed a protective effect through different mechanisms.130
Analyzing data of the Lean European Open Survey on SARS-CoV-2 (LEOSS) registry including 1946 COVID-19 patients with cardiovascular comorbidities revealed that ARBs intake was associated with a significantly lower incidence in overall mortality compared to patients treated with ACE inhibitors or patients who received neither an ACE inhibitor nor an ARB. The protective effects of ARBs seem to involve the limitation of inflammatory disease progression and thrombogenic activation, both variables representing in fact potentially aggravating conditions for the COVID-19 infection evolution.131,132
Potential benefits of beta blockers in COVID-19 treatment should be related with the antagonist effect over the increased circulating catecholamine levels (by both endogenous and possible iatrogenic mechanism – nebulizers with beta 2 agonists and/or intravenous norepinephrine in septic shock associated with SARS-CoV-2 infection), as well as providing cytokine blockade and effective management of sympathetic storm. In addition, beta blockers may be responsible for down-regulating the expression of ACE2 surface receptors, thus limiting the attachment and viral entry into the host cells.133,134
Treatment in COVID-19 patients aims to suppress viral replication and reduce the excessive immune response. Anti-inflammatory therapies are found to reduce vascular impairment in COVID-19 patients. Some studies have described reduced endothelial impairment with the administration of tumor necrosis factor-alpha inhibitors135 or anti-IL-6 receptor antibodies.136 Antiviral therapies have been used extensively during this pandemic and have been shown to reduce viral load and improve symptoms significantly.137–139
In addition, some studies have shown positive outcomes in improving vascular function through lifestyle changes and therapies. Recommendations to maintain a healthy lifestyle and to manage chronic medical conditions are highlighted in the guideline regarding the management of cardiovascular disease in association with COVID-19. It is admitted that CAD is a condition which may lead to a more severe course of SARS-CoV-2 infection.140 Actually, the efficacy regarding reducing cardiovascular events in patients diagnosed with CAD was observed in research in which intervention was focused on multiple lifestyle factors: dietary pattern (increased fruit and vegetable intake, decrease fat intake), physical activity, overweight, smoking, alcohol consumption, perceived stress, sleep quality and adherence to medications.141,142 The assessment of the association between lifestyle risk factors (physical inactivity, smoking, overweight/obesity) and incidence of COVID-19-cases hospitalized in a UK population-based cohort study concluded that patients with unhealthy behaviours had 4-fold higher risk for severe infection with SARS-CoV-2.143 Based on positive result regarding the improvement in lifestyle habits in patients with CAD, in COVID-19 era, the lifestyle-related interventions should be priority for the secondary CAD prevention.144
Peripheral Artery Disease
Peripheral artery disease (PAD) is a common circulatory problem that affects more than 22 million individuals worldwide, particularly the elderly.145 It is a chronic arterial occlusive disease, mainly caused by the progression of atherosclerosis, and most frequently affects the lower limbs. However, it can affect any extra-coronary or cerebral artery.146,147 Smoking, obesity, diabetes mellitus, hypertension and a sedentary lifestyle represent the pivotal modifiable risk factors for PAD.22 The clinical presentation of PAD varies from no symptoms to intermittent claudication or critical limb ischemia. It also affects the functional status, leading to reduced quality of life.146,147 The COVID-19 pandemic, the lockdowns, and social distancing together or separately 1) limited PAD diagnosis, 2) delayed its treatment, and 3) increased the prevalence of the disease.93,148,149
The relationship between PAD and COVID-19 and whether its co-prevalence leads to worse outcomes is still under investigation. Nevertheless, PAD patients with COVID-19 have a 40% greater rate of major adverse cardiovascular events compared to PAD patients without COVID-19.150 Furthermore, acute limb ischemia can be a complication for hospitalized patients with severe COVID-19 and patients with mild symptoms of COVID-19. In addition, acute limb ischemia can occur with ischemic symptoms in other vascular beds and in connection with vaccine-induced immune thrombocytopenia and thrombosis (VITT).151,152 Finally, COVID-19 patients with hypertension and diabetes mellitus are more vulnerable to lower extremity complications resulting in advanced PAD (gangrene or ulcerations).153
Pathophysiology
As described above, SARS-CoV-2 can infect cells by attaching to the ACE2 receptor. This infection causes inflammation, endothelial dysfunction, and promotes a prothrombotic state. In addition, endothelial dysfunction leads to NO deficiency, accompanied by increased oxidative stress, which is known to worsen PAD progression. Finally, platelets and coagulation factors can be activated by hypoxia in PAD patients, resulting in a hypercoagulable state. Inhibition of endogenous anticoagulant proteins and an increase in plasminogen activator inhibitor I and tissue factor were involved.148,154,155
Diagnosis
The diagnosis of PAD is based upon 1) medical history (particularly suggestive symptoms for PAD), 2) physical examination, 3) ankle-brachial index (ie, <0.9 indicates obstructive disease and >1.4 exhibits arterial stiffness), and 4) toe-brachial index in elderly, diabetic or chronic kidney disease patients, as well as in patients with chronic limb-threatening ischemia, as ankle-brachial index can be falsely elevated in these patient groups. Also, imaging techniques can be used, such as duplex ultrasound (screening and diagnostic tool) or a computed tomography angiography.146,156
The following circulatory biomarkers are related to disease progression and can be evaluated in PAD patients during follow-up: C-reactive protein, IL-6, fibrinogen, D-dimers, neopterin, and pentraxin-3.157,158 Importantly, different clinical studies have also reported increases in some of these biomarkers in COVID-19 patients, such as C-reactive protein, IL-6, fibrinogen, and D-dimer. These measures have also been tested as prognostic markers of future thrombotic events.159 Moreover, studies in COVID-19 patients who experienced claudication symptoms showed an increased PAD diagnosis with computed tomography angiography and elevated clot risk.22
Treatment
The primary therapeutic goal for symptomatic and asymptomatic PAD patients is to minimize cardiovascular risk and improve quality of life. For example, improving functional status is an additional objective in symptomatic patients. In patients with critical limb ischemia, the aim is to reduce mortality and to prevent limb amputation and restore mobility.154,160–162 Lifestyle modifications by increasing physical activity, adopting healthy eating habits, and managing comorbidities, form the first-line therapy.163 In symptomatic patients, structured exercise therapy can reduce claudication.154 Physical exercise can be performed at home and may benefit PAD patients in the COVID-19 era.145,164 COVID-19 patients with comorbidities should be screened for pre-existing PAD. Additionally, as it seems to reduce mortality, COVID-19 patients with high D-dimers should receive anticoagulation therapy (low molecular weight heparin) for clot prevention.159 Statins reduce cardiovascular events and mortality in symptomatic and asymptomatic PAD patients. As reported above, their use is associated with lower clinical symptoms and mortality in COVID-19 patients.123,126 The standard approach to control comorbidities should be applied according to the guidelines.146,154,165
COVID-19 Vaccines
Vaccines are an important tool in fighting against COVID-19 and are especially effective in prevention of severe forms of the disease and of complications. Acknowledging this perspective, several vaccines were approved (eg, Pfizer-BioNtech, Moderna, Jenssen, Oxford-AstraZeneca, Sinopharm BBIBP-CorV, etc.) demonstrating significant efficacy and safety without any major side effects.166–168
Minor side effects (eg, local reaction at the injection site, headache, muscle pain) and clinically important adverse effects (eg, myocarditis, pericarditis, and thrombotic events) were reported following the COVID-19 vaccine. In addition, acute coronary syndromes, hypertension, and arrhythmias also have been reported as very rare cardiovascular side effects, without finally confirmed association.169
Acute Coronary Syndromes
A study performed in Denmark and Norway found that one dose of Astra Zeneca vaccine was linked with excess event rate of 0.6 per 100 000 vaccinations for acute myocardial infarction but without statistical significance.170 An analysis of VigiBase database of World Health Organization demonstrated 13 patients (0.27% of all cardiovascular complications) with angina pectoris, 32 (0.66%) patients with myocardial infarction and 16 (0.33%) patients with acute myocardial infarction.171
It should be emphasized that a proportion of patients receiving a COVID-19 vaccination will be prone to develop, by chance, acute myocardial infarction due to the baseline risk of acute CAD worldwide.170 In a Phase 3 trial (randomized observer-blinded, placebo-controlled) conducted in over 99 centers in the United States with over 30 thousand volunteers, the proportion of myocardial infarction was similar between patients that received or not the mRNA-1273 vaccine (3 cases of myocardial infarction in placebo group and 5 cases in vaccine).172 A retrospective study of 231 037 patients (168 310 fully vaccinated and 62,727 non-vaccinated) suffering from COVID-19 reveal that fully vaccinated patients had a lower probability of acute myocardial infarction (HR 0.48) compared with non-vaccinated people after a follow up of 84 days.173 These results are important given the result that showed an increased risk of myocardial infarction after infection with SARS-CoV-2.174 A recent large study intended to assess the short-term risk of severe cardiovascular events (MI, pulmonary embolism, stroke) after the COVID-19 vaccine was performed in France on 46.5 million adults younger than 75 years. No association was found between Moderna or Pfizer–BioNTech vaccine and MI. However, the first dose of the Oxford-AstraZeneca vaccine was associated with MI in the second week after the vaccination. An association between the Janssen vaccine and MI in the second week after a single dose could not be ruled out.175
Although no association between the COVID-19 vaccine and MI can be determined, multiple putative pathophysiological mechanisms for the association of and vaccination have been proposed:
- A Kounis syndrome development representing a discrete form of infarction based on exaggerated immune system activation.176,177
- Vaccine-induced thrombotic thrombocytopenia. It is assumed that the mechanism behind these changes is an immune-mediated mechanism, similar to heparin-induced thrombocytopenia.178
- Demand-supply mismatch mediated by the stress of getting the vaccine, especially in the elderly or individuals with comorbidities.169
- Myocarditis.169
Other possible vaccination adverse effects were Takotsubo cardiomyopathy and myocardial infarction with non-obstructive coronary arteries (MINOCA). A systematic review of case reports described four cases of Takotsubo cardiomyopathy with symptoms developing between 15 minutes to 4 days after vaccination and a case of MINOCA, which occurred 2 hours after vaccination with the first dose of BNT162b2 vaccine.179
Based on the available data the relationship between myocardial infarction and vaccination is doubtful, and no causal effect between vaccination and MI was proven.169 It is worth also to strongly emphasize that those not finally proved adverse effects cannot underweight the large benefit of vaccination to prevent CVD complications in COVID-19 patients.
Myocarditis and Pericarditis
After vaccination with various vaccine (measles, mumps, rubella, varicella zoster virus, yellow fever, or smallpox) rare cases of myocarditis have been reported. Similarly, rare cases of myopericarditis have been reported after the second dose of COVID-19 vaccine.180
A study performed in Israel compared 884,000 vaccinated cases with 884,000 unvaccinated subjects (controls). Also, about 173,000 positive cases for COVID-19 were compared with the same number of negative subjects. The conclusion was that COVID-19 disease among unvaccinated people is responsible for much greater increase in cases of myocarditis and pericarditis compared with vaccinated people (the odds ratio was 1.27 following Pfizer vaccine administration as opposed to 5.39 for patients positive with SARS-CoV-2).181 The cases of myocarditis associated with vaccination were concentrated in the first 5–7 days after the second dose, the highest incidence being found in males with age between 16 and 39.182 The analysis of Vaccine Adverse Event Reporting System (VAERS database) which included 354,100,845 total vaccinations with Pfizer-BioNTech or Moderna found 1626 cases of myocarditis (0.8 per 100,000) diagnosis establish according to “Centre for Disease Control” criteria. The peak incidence of myocarditis occurred between 15 and 17 years old for Pfizer and between 18 and 23 years for Moderna vaccine.183
A meta-analysis of 22 studies with a total of 405,272,721 vaccine doses showed that the incidence of myocarditis and pericarditis tends to be lower after COVID-19 vaccines compared with non-COVID-19 vaccines (eg, smallpox vaccine) with an incidence of 1.6 vs 5.6 cases/100,000 but without statistical significance. Also, the study found a higher incidence of myocarditis and pericarditis after mRNA vaccines than with non-mRNA vaccines and a progressively higher incidence of myocarditis with decreasing age.184
There are a few potential mechanisms proposed for pathophysiology of COVID-19 vaccine-related myocarditis and myopericarditis: (1) in patients with preexisting dysregulated pathways vaccination may trigger an immune-mediated adverse response; (2) patients with susceptibility to myocarditis have elevated heart-reactive autoantibodies that could attack cardiac myocyte after vaccination.185
Diagnosis of myocarditis is made clinically (patients presents with chest pain, dyspnea, tachipnea, palpitations, rarely headache, cough) associated with ECG changes (diffuse ST-segment elevation, nonspecific ST changes, T-wave inversions), elevated cardiac markers (troponin, BNP, NT-proBNP) echocardiography (left ventricular systolic dysfunction, pericardial effusion, left ventricular dilatation) and cMRI (impaired regional and global ventricular function, and myocardial edema ± late gadolinium enhancement based on T2-weighted imaging or T2 mapping).185,186
There are very few reports of myocardial biopsy in patients with myocarditis and have shown inflammatory infiltrates within the myocardium associated with myocyte damage/necrosis of nonischemic origin. Different histologic patterns of myocarditis are represented by lymphocytic myocarditis, eosinophilic myocarditis, or healing myocarditis.186
The treatment for myocarditis is supportive and includes treatment for symptoms, treatment for arrhythmias and adequate perfusion. Nonsteroidal anti-inflammatory drugs and colchicine are indicated, and glucocorticoids and immunoglobulins are given to the patients with poor response to reduce the immune reaction.187,188
Reported cases have shown a benign course of the disease and favorable short-term outcomes with only 2 deaths reported.185 The long-term implications of this side effect for the patients are unknown but this small risk is outweighed by the benefit of vaccination against COVID-19.180
The benefits of vaccination over non-vaccination in terms of prevented hospitalizations and serious COVID-19 complications remain undisputable. In patients that develop these cardiovascular side effects of the vaccine, the complications are effectively treated, and quick recovery of the patients are reported. Compared with the total number of vaccinated people, the incidence of cardiovascular adverse effects remains very rare, thus confirming the fact that the benefits of vaccination outweigh the risks.189,190
Conclusions
The bi-directional relationship between atherosclerosis and COVID-19 is accompanied by poor prognosis. The immune response hyperactivation due to SARS-CoV-2 infection causes an increased secretion of cytokines (so-called “cytokine storm”), endothelial dysfunction, and arterial stiffness, which promotes the development of atherosclerosis. Also, due to the COVID-19 pandemic, access to healthcare amenities was reduced, resulting in increased morbidity and mortality in patients at risk. Furthermore, as lockdown measures were largely adopted worldwide, the sedentary lifestyle and the increased consumption of processed nutrients or unhealthy food increased. However, the experience gained in the COVID-19 pandemic and the new methods of patients’ approaching have helped the medical system to overcome this crisis and will certainly help in the case of new possible epidemics.
Disclosure
Annabelle Shor, Raluca Ecaterina Haliga, Viviana Onofrei Aursulesei and Oana Sirbu have the same contribution as the first author for this study. The authors report no conflicts of interest in this work.
References
1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi:10.1001/jama.2020.1585
2. Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi:10.1016/j.jinf.2020.02.017
3. Ulinici M, Covantev S, Wingfield-Digby J, et al. Screening, diagnostic and prognostic tests for COVID-19: a comprehensive review. Life. 2021;11:561. doi:10.3390/life11060561
4. Saber-Ayad M, Saleh MA, Abu-Gharbieh E. The rationale for potential pharmacotherapy of COVID-19. Pharmaceuticals. 2020;13:96. doi:10.3390/ph13050096
5. Bonnesen B, Jensen JUS, Jeschke KN, et al. Management of COVID-19-associated acute respiratory failure with alternatives to invasive mechanical ventilation: high-flow oxygen, continuous positive airway pressure, and noninvasive ventilation. Diagnostics. 2021;11:2259. doi:10.3390/diagnostics11122259
6. Stoichitoiu LE, Pinte L, Ceasovschih A, et al. In-hospital antibiotic use for COVID-19: facts and rationales assessed through a mixed-methods study. J Clin Med. 2022;11(11):3194. doi:10.3390/jcm11113194
7. Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi:10.1038/s41569-020-0360-5
8. Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of Covid-19: a rapid review. Emerg Microbes Infect. 2020;9:2190–2199. doi:10.1080/22221751.2020.1825914
9. Monteil V, Kwon H, Prado P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi:10.1016/j.cell.2020.04.004
10. Hohberger B, Ganslmayer M, Lucio M, et al. Retinal microcirculation as a correlate of a systemic capillary impairment after severe acute respiratory syndrome coronavirus 2 infection. Front Med. 2021;8:676554. doi:10.3389/fmed.2021.676554
11. Osiaevi I, Schulze A, Evers G, et al. Persistent capillary rarefication in long COVID syndrome. Angiogenesis. 2023;26(1):53–61. doi:10.1007/s10456-022-09850-9
12. Gavriilaki E, Anyfanti P, Gavriilaki M, et al. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep. 2020;22(9):63. doi:10.1007/s11906-020-01078-6
13. Nakano H, Shiina K, Tomiyama H. Cardiovascular outcomes in the acute phase of COVID-19. Int J Mol Sci. 2021;22(8):4071. doi:10.3390/ijms22084071
14. Vlacil AK, Bänfer S, Jacob R, et al. Polystyrene microplastic particles induce endothelial activation. PLoS One. 2021;16(11):e0260181. doi:10.1371/journal.pone.0260181
15. Endemann DH, Schiffrin EL. Endothelial Dysfunction. J Am Soc Nephrol. 2004;15(8):1983–1992. doi:10.1097/01.ASN.0000132474.50966.DA
16. Kotlyarov S, Kotlyarova A. The importance of the plasma membrane in atherogenesis. Membranes. 2022;12:1036. doi:10.3390/membranes12111036
17. Tripska K, Igreja Sá IC, Vasinova M, et al. Monoclonal anti-endoglin antibody TRC105 (carotuximab) prevents hypercholesterolemia and hyperglycemia-induced endothelial dysfunction in human aortic endothelial cells. Front Med. 2022;9:845918. doi:10.3389/fmed.2022.845918
18. Alexander Y, Osto E, Schmidt-Trucksäss A, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc Res. 2020;117(1):29–42.
19. Kotlyarov S. Analysis of differentially expressed genes and signaling pathways involved in atherosclerosis and chronic obstructive pulmonary disease. Biomol Concepts. 2022;13(1):34–54. doi:10.1515/bmc-2022-0001
20. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
21. Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9(7):1652. doi:10.3390/cells9071652
22. Shankar A, Varadan B, Ethiraj D, et al. Systemic arterio-venous thrombosis in COVID-19: a pictorial review. World J Radiol. 2021;13(1):19–28. doi:10.4329/wjr.v13.i1.19
23. Sanders MJ, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi:10.1001/jama.2020.6019
24. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:1084–1090. doi:10.1152/ajpheart.00217.2020
25. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi:10.1016/j.cell.2020.02.052
26. Khomich OA, Kochetkov SN, Bartosch B, Barr JJ, Bollyky PL. Redox biology of respiratory viral infections. Viruses. 2018;11:10. doi:10.3390/v11010010
27. Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;10(11):1648. doi:10.3389/fimmu.2020.01648
28. Coman AE, Ceasovschih A, Petroaie AD, et al. The significance of low magnesium levels in COVID-19 patients. Medicina. 2023;59(2):279. doi:10.3390/medicina59020279
29. Jin Y, Ji W, Yang H, et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. doi:10.1038/s41392-020-00454-7
30. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi:10.1111/jth.14850
31. Rovas A, Osiaevi I, Buscher K, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi:10.1007/s10456-020-09753-7
32. Queisser KA, Mellema RA, Middleton EA, et al. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight. 2021;6(17):e147472. doi:10.1172/jci.insight.147472
33. Laurent S, Boutouyrie P. Arterial stiffness and hypertension in the elderly. Front Cardiovasc Med. 2020;7. doi:10.3389/fcvm.2020.544302
34. Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–848. doi:10.1161/CIRCRESAHA.118.312563
35. Lacolley P, Regnault V, Laurent S. Mechanisms of arterial stiffening: from mechanotransduction to epigenetics. Arterioscler Thromb Vasc Biol. 2020;40:1055–1062. doi:10.1161/ATVBAHA.119.313129
36. Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37:1–11. doi:10.1161/ATVBAHA.116.308563
37. Tsioufis C, Dimitriadis K. Sympathetic system-related artery stiffness. Hypertension. 2019;73(5):975–976. doi:10.1161/HYPERTENSIONAHA.119.12571
38. Zota IM, Stătescu C, Sascău RA, et al. Arterial stiffness assessment using the arteriograph in patients with moderate–severe OSA and metabolic syndrome—a pilot study. J Clin Med. 2021;10:4238.
39. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
40. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi:10.1161/CIRCULATIONAHA.109.886655
41. Niiranen TJ, Kalesan B, Hamburg NM, et al. Relative contributions of arterial stiffness and hypertension to cardiovascular disease: the Framingham Heart Study. J Am Heart Assoc. 2016;5. doi:10.1161/JAHA.116.004271
42. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi:10.1016/j.jacc.2009.10.061
43. Wang XK, Keith JC, Struthers AD, et al. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26:214–223. doi:10.1111/j.1755-5922.2008.00051.x
44. Zota IM, Stătescu C, Sascău RA, et al. Acute and long-term consequences of COVID-19 on arterial stiffness—a narrative review. Life. 2022;12:781. doi:10.3390/life12060781
45. Schnaubelt S, Oppenauer J, Tihanyi D, et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J Intern Med. 2021;290:437–443. doi:10.1111/joim.13275
46. Ratchford SM, Stickford JL, Province VM, et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol. 2021;320:404–410.
47. Szeghy RE, Province VM, Stute NL, et al. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol. 2022;107:694–707. doi:10.1113/EP089481
48. Kumar N, Kumar S, Kumar A, et al. The COSEVAST study outcome: evidence of COVID-19 severity proportionate to surge in arterial stiffness. Indian J Crit Care Med. 2021;25:1113–1119. doi:10.5005/jp-journals-10071-24000
49. Nandadeva D, Young BE, Stephens BY, et al. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol. 2021;321:479–484.
50. Szeghy RE, Stute NL, Province VM, et al. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J Appl Physiol. 2022;132:1297–1309. doi:10.1152/japplphysiol.00793.2021
51. Spronck B, Heusinkveld MH, Vanmolkot FH, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens. 2015;33:330–338. doi:10.1097/HJH.0000000000000407
52. Nandadeva D, Skow RJ, Grotle AK, et al. Impact of COVID-19 on ambulatory blood pressure in young adults: a cross-sectional analysis investigating time since diagnosis. J Appl Physiol. 2022;133:183–190. doi:10.1152/japplphysiol.00216.2022
53. Küçük U, Gazi E, Duygu A, et al. Evaluation of aortic elasticity parameters in survivors of COVID-19 using echocardiography imaging. Med Princ Pract. 2022;31:276–283. doi:10.1159/000522626
54. Zanoli L, Gaudio A, Mikhailidis DP, et al. Vascular dysfunction of COVID-19 is partially reverted in the long-term. Circ Res. 2022;130:1276–1285. doi:10.1161/CIRCRESAHA.121.320460
55. Lambadiari V, Mitrakou A, Kountouri A, et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail. 2021;23:1916–1926. doi:10.1002/ejhf.2326
56. Ikonomidis I, Lambadiari V, Mitrakou A, et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur J Heart Fail. 2022;24:727–729. doi:10.1002/ejhf.2451
57. Van der Sluijs KM, Bakker EA, Schuijt TJ, et al. Long-term cardiovascular health status and physical functioning of nonhospitalized patients with COVID-19 compared with non-COVID-19 controls. Am J Physiol Heart Circ Physiol. 2023;324:47–56.
58. Abers MS, Delmonte OM, Ricotta EE, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;2021:6.
59. Gelzo M, Cacciapuoti S, Pinchera B, et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci Rep. 2022;12:1212. doi:10.1038/s41598-021-04677-8
60. Ueland T, Holter JC, Holten AR, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect. 2020;81:41–43. doi:10.1016/j.jinf.2020.06.061
61. Lerum TV, Maltzahn NN, Aukrust P, et al. Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Sci Rep. 2021;11:23205. doi:10.1038/s41598-021-02547-x
62. Shi S, Su M, Shen G, et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J Med Virol. 2021;93:528–532. doi:10.1002/jmv.26235
63. Springall R, Gonzalez-Florez J, Garcia-Avila C, et al. Elevated levels of soluble CD147 are associated with hyperinflammation and disease severity in COVID-19: a proof-of-concept clinical study. Arch Immunol Ther Exp (Warsz). 2022;70:18. doi:10.1007/s00005-022-00657-6
64. Wang M, Kim SH, Monticone RE, et al. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension. 2015;65:698–703. doi:10.1161/HYPERTENSIONAHA.114.03618
65. Bruno RM, Spronck B, Hametner B, et al. Covid-19 effects on arterial stiffness and vascular ageing: CARTESIAN study rationale and protocol. Artery Res. 2020;27:59. doi:10.2991/artres.k.201124.001
66. Esobi I, Lasode M, Anyanwu C, et al. Nutritional impact of COVID-19 and its implications on atherosclerosis. World. 2020;8(1):16–21.
67. Kotlyarov S. Diversity of lipid function in atherogenesis: a focus on endothelial mechanobiology. Int J Mol Sci. 2021;22:11545. doi:10.3390/ijms222111545
68. Vinciguerra M, Romiti S, Fattouch K, et al. Atherosclerosis as pathogenetic substrate for Sars-Cov2 cytokine storm. J Clin Med. 2020;9:2095. doi:10.3390/jcm9072095
69. Kotlyarov S. Immune function of endothelial cells: evolutionary aspects, molecular biology and role in atherogenesis. Int J Mol Sci. 2022;23:9770. doi:10.3390/ijms23179770
70. Sukhorukov VN, Khotina VA, Chegodaev YS, Ivanova E, Sobenin IA, Orekhov AN. Lipid metabolism in macrophages: focus on atherosclerosis. Biomedicines. 2020;8(8):262. doi:10.3390/biomedicines8080262
71. Sagris M, Theofilis P, Antonopoulos AS, et al. Inflammatory mechanisms in COVID-19 and atherosclerosis: current pharmaceutical perspectives. Int J Mol Sci. 2021;22:6607. doi:10.3390/ijms22126607
72. Kotlyarov S, Kotlyarova A. Involvement of fatty acids and their metabolites in the development of inflammation in atherosclerosis. Int J Mol Sci. 2022;23:1308. doi:10.3390/ijms23031308
73. Grzegorowska O, Lorkowski J. Possible correlations between atherosclerosis, acute coronary syndromes and COVID-19. J Clin Med. 2020;9:3746. doi:10.3390/jcm9113746
74. Surma S, Banach M, Lewek J. COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis. 2021;20(1):141. doi:10.1186/s12944-021-01563-0
75. Radenkovic D, Chawla S, Pirro M, et al. Cholesterol in relation to COVID-19: should we care about It? J Clin Med. 2020;9:1909. doi:10.3390/jcm9061909
76. Wang H, Yuan Z, Pavel MA, et al. Cholesterol and COVID19 lethality in elderly. bioRxiv. 2020;10:2020–2025.
77. Luo W, Yu H, Gou J, et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints. 2020;2020:2020020407.
78. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi:10.1007/s00392-020-01626-9
79. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi:10.1016/j.jacc.2020.03.031
80. Wang Q. Molecular genetics of coronary artery disease. Curr Opin Cardiol. 2005;20(3):182–188. doi:10.1097/01.hco.0000160373.77190.f1
81. Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18(6):331–344. doi:10.1038/nrg.2016.160
82. Aursulesei Onofrei V, Ceasovschih A, Anghel RC, et al. Subendocardial viability ratio predictive value for cardiovascular risk in hypertensive patients. Medicina. 2023;59(1):24. doi:10.3390/medicina59010024
83. Zahmatkeshan N, Khademian Z, Zarshenas L, et al. Experience of adherence to treatment among patients with coronary artery disease during the COVID-19 pandemic: a qualitative study. Health Promot Perspect. 2021;11(4):467–475. doi:10.34172/hpp.2021.59
84. Anghel L, Tudurachi BS, Leonte A, et al. The challenge of high coronary thrombotic events in patients with ST-segment elevation myocardial infarction and COVID-19. J Clin Med. 2022;11:6542. doi:10.3390/jcm11216542
85. Maehl N, Bleckwenn M, Riedel-Heller SG, et al. The impact of the COVID-19 pandemic on avoidance of health care, symptom severity, and mental well-being in patients with coronary artery disease. Front Med. 2021;8:760265. doi:10.3389/fmed.2021.760265
86. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):
87. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):
88. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi:10.1001/jamacardio.2020.0950
89. Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. doi:10.1016/S1473-3099(09)70331-7
90. Timpau AS, Miftode RS, Leca D, et al. A real Pandora’s box in pandemic times: a narrative review on the acute cardiac injury due to COVID-19. Life. 2022;12:1085. doi:10.3390/life12071085
91. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi:10.1182/blood.2020006000
92. De Michele S, Sun Y, Yilmaz MM. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154(6):748–760. doi:10.1093/ajcp/aqaa156
93. De Donato G, Pasqui E, Alba G, et al. The Limitations of social behaviour imposed by Covid-19 impacted the perception and the evolution of peripheral arterial disease negatively. Ann Vasc Surg. 2021;73:107–113. doi:10.1016/j.avsg.2021.02.003
94. Bermejo-Martin JF, Almansa R, Torres A, et al. Covid-19 as a cardiovascular disease: the potential role of chronic endothelial dysfunction. Cardiovasc Res. 2020;116:132–133. doi:10.1093/cvr/cvaa140
95. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in Covid-19. Lancet. 2020;395:1417–1418. doi:10.1016/S0140-6736(20)30937-5
96. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi:10.1111/j.1365-2249.2004.02415.x
97. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi:10.1093/cvr/cvaa106
98. Duca ȘT, Chetran A, Miftode RȘ, et al. Myocardial ischemia in patients with COVID-19 infection: between pathophysiological mechanisms and electrocardiographic findings. Life. 2022;12:1015. doi:10.3390/life12071015
99. Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82(964):140–144. doi:10.1136/pgmj.2005.037515
100. Mahallawi WH, Khabour OF, Zhang Q, et al. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi:10.1016/j.cyto.2018.01.025
101. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi:10.1016/j.chom.2016.01.007
102. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. JAHA. 2014;3(2):000582. doi:10.1161/JAHA.113.000582
103. Goeller M, Achenbach S, Marwan M, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12(1):67–73. doi:10.1016/j.jcct.2017.11.007
104. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427–437. doi:10.2147/VHRM.S106838
105. Araiza-Garaygordobil D, Montalto C, Martinez-Amezcua P, et al. Impact of the COVID-19 pandemic on hospitalizations for acute coronary syndromes: a multinational study. QJM. 2021;114(9):642–647. doi:10.1093/qjmed/hcab013
106. Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi:10.1097/CM9.0000000000000744
107. Kermali M, Khalsa RK, Pillai K, et al. The role of biomarkers in diagnosis of COVID-19 – a systematic review. Life Sci. 2020;254:117788. doi:10.1016/j.lfs.2020.117788
108. Mukhitdinova O, Alyavi BA, Ubaydullaeva ZZ, et al. Changes of blood D-dimer level after COVID-19 in patients with coronary heart disease. Eur Heart J Acute Cardiovasc Care. 2022;11(Suppl 1):
109. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi:10.1016/S0140-6736(20)30183-5
110. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–391. doi:10.1016/j.pcad.2020.03.001
111. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;27:201017.
112. Clerkin KJ, Fried JA, Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi:10.1161/CIRCULATIONAHA.120.046941
113. Wei ZY, Qian HY. Myocardial injury in patients with COVID-19 pneumonia. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:006.
114. Garg A, Seeliger B, Derda AA, et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur J Heart Fail. 2021;23(3):468–475. doi:10.1002/ejhf.2096
115. Miyashita Y, Yoshida T, Takagi Y, et al. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. NPJ Vaccines. 2022;7(1):16. doi:10.1038/s41541-022-00439-3
116. Dingsdag SA, Clay OK, Quintero GA. COVID-19 severity, miR-21 targets, and common human genetic variation. Letter regarding the article ‘Circulating cardiovascular microRNAs in critically ill COVID-19 patients’. Eur J Heart Fail. 2021;23(11):1986–1987. doi:10.1002/ejhf.2317
117. Silva AB, Siqueira S, de Assis Soares WR, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. doi:10.3390/v13040700
118. Ortega-Paz L, Capodanno D, Montalescot G, et al. Coronavirus disease 2019-associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021;10(3):019650. doi:10.1161/JAHA.120.019650
119. Kotlyarov S, Kotlyarova A. Molecular pharmacology of inflammation resolution in atherosclerosis. Int J Mol Sci. 2022;23:4808. doi:10.3390/ijms23094808
120. Morrison FJ, Su M, Turchin A. COVID-19 outcomes in patients taking cardioprotective medications. PLoS One. 2022;17(10):e0275787. doi:10.1371/journal.pone.0275787
121. Ganjali S, Bianconi V, Penson PE, et al. Commentary: statins, COVID-19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113:154375. doi:10.1016/j.metabol.2020.154375
122. Vahedian-Azimi A, Mohammadi SM, Heidari Beni F, et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci. 2021;17(3):579–595. doi:10.5114/aoms/132950
123. Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabol. 2020;32(2):176–187. doi:10.1016/j.cmet.2020.06.015
124. Barkas F, Milionis H, Anastasiou G, et al. Statins and PCSK9 inhibitors: what is their role in coronavirus disease 2019? Med Hypotheses. 2021;146:110452. doi:10.1016/j.mehy.2020.110452
125. Vahedian-Azimi A, Mohammadi SM, Banach M, et al. Improved COVID-19 outcomes following statin therapy: an updated systematic review and meta-analysis. Biomed Res Int. 2021;2021:1901772. doi:10.1155/2021/1901772
126. De Spiegeleer A, Bronselaer A, Teo JT, et al. The effect of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21:909–914. doi:10.1016/j.jamda.2020.06.018
127. Mannarino MR, Bianconi V, Cosentini E, et al. Thyroid-stimulating hormone predicts total cholesterol and low-density lipoprotein cholesterol reduction during the acute phase of COVID-19. J Clin Med. 2022;11:3347. doi:10.3390/jcm11123347
128. Kouhpeikar H, Khosaravizade Tabasi H, Khazir Z, et al. Statin use in COVID-19 hospitalized patients and outcomes: a retrospective study. Front Cardiovasc Med. 2022;9:820260. doi:10.3389/fcvm.2022.820260
129. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi:10.1016/S2213-2600(20)30116-8
130. Caputo I, Caroccia B, Frasson I, et al. Angiotensin II promotes SARS-CoV-2 Infection via upregulation of ACE2 in human bronchial cells. Int J Mol Sci. 2022;23(9):5125. doi:10.3390/ijms23095125
131. Cremer S, Pilgram L, Berkowitsch A, et al. LEOSS study group. Angiotensin II receptor blocker intake associates with reduced markers of inflammatory activation and decreased mortality in patients with cardiovascular comorbidities and COVID-19 disease. PLoS One. 2021;16(10):e0258684. doi:10.1371/journal.pone.0258684
132. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. doi:10.1161/CIRCRESAHA.120.317134
133. Al-Kuraishy HM, Al-Gareeb AI, Mostafa-Hedeab G, et al. Effects of β-blockers on the sympathetic and cytokines storms in Covid-19. Front Immunol. 2021;12:749291. doi:10.3389/fimmu.2021.749291
134. Alsagaff MY, Mulia EPB. Hypertension and COVID-19: potential use of beta-blockers and a call for randomized evidence. Indian Heart J. 2021;73(6):757–759. doi:10.1016/j.ihj.2021.10.011
135. Hurlimann D, Forster A, Noll G, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumathoid arthritis. Circulation. 2002;106:2184–2187. doi:10.1161/01.CIR.0000037521.71373.44
136. Ascierto PA, Fox B, Urba W, et al. Insights from immuno-oncology: the Society for immunotherapy of cancer statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer. 2020;8:e000878. doi:10.1136/jitc-2020-000878
137. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi:10.1038/s41422-020-0282-0
138. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi:10.1056/NEJMoa2001282
139. Barkas F, Styla CP, Bechlioulis A, et al. Sinus bradycardia associated with remdesivir treatment in COVID-19: a case report and literature review. J Cardiovasc Dev Dis. 2021;8:18. doi:10.3390/jcdd8020018
140. Baigent C, Windecker S, Andreini D, et al. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2—care pathways, treatment, and follow-up. Eur Heart J. 2021;43:1059–1103.
141. Govil SR, Weidner G, Merritt-Worden T, et al. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: the multisite cardiac lifestyle intervention program. Am J Public Health. 2009;99:1263–1270. doi:10.2105/AJPH.2007.132852
142. Jankowski P, Kosior DA, Sowa P, et al. Secondary prevention of coronary artery disease in Poland. results from the Polaspire Survey. Cardiol J. 2020;27(5):533–540. doi:10.5603/CJ.a2020.0072
143. Hamer M, Kivimäki M, Gale CR, et al. Lifestyle risk factors, inflammatory mechanisms, and covid-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi:10.1016/j.bbi.2020.05.059
144. Gaudel P, Neupane S, Koivisto AM, et al. Effects of intervention on lifestyle changes among coronary artery disease patients: a 6‐month follow‐up study. Nurs Open. 2022;9:2024–2036. doi:10.1002/nop2.1212
145. Lamberti N, Straudi S, Manfredini R, et al. Don’t stop walking: the in-home rehabilitation program for peripheral artery disease patients during the COVID-19 pandemic. Intern Emerg Med. 2021;16(5):1307–1315. doi:10.1007/s11739-020-02598-4
146. Aboyans V, Ricco J, Bartelink M, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi:10.1093/eurheartj/ehx095
147. Hageman D, Fokkenrood HJP, van den Houten LNM, et al. Supervised exercise therapy versus home‐based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;2018:4.
148. Panzavolta C, Zalunardo B, Irsara S, et al. Peripheral artery disease, the ‘lost syndrome’ during lockdown for COVID‑19: a report of three cases. Med Int. 2021;1(5):15. doi:10.3892/mi.2021.15
149. Stabile E, Piccolo R, Franzese M, et al. A cross-sectional study evaluating hospitalization rates for chronic limb threatening ischemia during the COVID-19 outbreak in Campania, Italy. Vasc Med. 2021;26(2):174–179. doi:10.1177/1358863X20977678
150. Smolderen KG, Lee M, Arora T, et al. Peripheral artery disease and COVID-19 outcomes: insights from the Yale DOM-CovX Registry. Curr Probl Cardiol. 2021;47:101007. doi:10.1016/j.cpcardiol.2021.101007
151. Etkin Y, Conway AM, Silpe J, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Sur. 2021;70:290–294. doi:10.1016/j.avsg.2020.08.085
152. Indes JE, Koleilat I, Hatch AN, et al. Early experience with arterial thromboembolic complications in patients with COVID-19. J Vasc Surg. 2021;73(2):381–389. doi:10.1016/j.jvs.2020.07.089
153. Rastogi A, Dogra H, Jude EB. COVID-19 and peripheral arterial complications in people with diabetes and hypertension: a systematic review. Diabetes Metab Syndr. 2021;15(5):102204. doi:10.1016/j.dsx.2021.102204
154. Laksono S, Siregar RH, Kusharsamita H. Chronic limb ischemia manifestation in COVID-19 infection: awareness and treatment in primary care. Univ Med. 2021;40(2):163–172. doi:10.18051/UnivMed.2021.v40.166-175
155. Goudarzi E, Yousefimoghaddam F, Ramandi A, et al. COVID-19 and peripheral artery thrombosis: a mini review. Curr Probl Cardiol. 2021;2021:100992.
156. Tran B. Assessment and management of peripheral arterial disease: what every cardiologist should know. Heart. 2021;107(22):1835–1843. doi:10.1136/heartjnl-2019-316164
157. Saenz-Pipao G, Martinez-Aguilar E, Orbe J, et al. The role of circulating biomarkers in peripheral arterial disease. Int J Mol Sci. 2021;22(7):3601. doi:10.3390/ijms22073601
158. Signorelli S, Anzaldi M, Libra M, et al. Plasma levels of inflammatory biomarkers in peripheral arterial disease. Angiology. 2016;67:870–874. doi:10.1177/0003319716633339
159. Eshaq AM, Almofadhli AA, Aljarba NK, et al. Acute limb ischemia as a concomitant manifestation of COVID-19. Cureus. 2022;14(1). doi:10.7759/cureus.21032
160. McCann AB, Jaff MR. Treatment strategies for peripheral artery disease. Expert Opin Pharmacother. 2009;10(10):1571–1586. doi:10.1517/14656560902988502
161. Aursulesei Onofrei V, Ceasovschih A, Marcu DTM, et al. Mortality risk assessment in peripheral arterial disease—the burden of cardiovascular risk factors over the years: a single center’s experience. Diagnostics. 2022;12:2499. doi:10.3390/diagnostics12102499
162. Bianconi V, Mannarino MR, Figorilli F, et al. Low brachial artery flow-mediated dilation predicts worse prognosis in hospitalized patients with COVID-19. J Clin Med. 2021;10:5456. doi:10.3390/jcm10225456
163. Ritti-Dias RM, Correia MA, Carvalho JF, et al. Impact of the COVID-19 pandemic on health lifestyle in patients with peripheral artery disease: a cross-sectional study. J Vasc Nurs. 2022;40(1):54–58. doi:10.1016/j.jvn.2022.01.001
164. Anghel R, Adam CA, Marcu DTM, et al. Cardiac rehabilitation in peripheral artery disease in a tertiary center-impact on arterial stiffness and functional status after 6 months. Life. 2022;12(4):601. doi:10.3390/life12040601
165. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi:10.1093/eurheartj/ehab484
166. Akinrinmade AO, Obitulata-Ugwu VO, Obijiofor NB, et al. COVID-19 and acute coronary syndrome: a literature review. Cureus. 2022;14(9):e29747. doi:10.7759/cureus.29747
167. Padureanu V, Bogdan M, Subtirelu MS, et al. Perceptions of COVID-19 vaccination among healthcare professionals in Romania. Rev Med Chir Soc. 2020;124(3):454–460.
168. Ulinici M, Suljič A, Poggianella M, et al. Characterisation of the antibody response in sinopharm (BBIBP-CorV) recipients and COVID-19 Convalescent Sera from the Republic of Moldova. Vaccines. 2023;11:637.
169. Zafar U, Zafar H, Ahmed MS, et al. Link between COVID-19 vaccines and myocardial infarction. World J Clin Cases. 2022;10(28):10109–10119. doi:10.12998/wjcc.v10.i28.10109
170. Ho JS, Sia CH, Ngiam JN, et al. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med J. 2021;2021:1.
171. Jeet Kaur R, Dutta S, Charan J, et al. Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med. 2021;14(3):909–927. doi:10.2147/IJGM.S299531
172. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389
173. Kim YE, Huh K, Park YJ, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. 2022;328(9):887–889. doi:10.1001/jama.2022.12992
174. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi:10.1038/s41591-022-01689-3
175. Botton J, Jabagi MJ, Bertrand M, et al. Risk for myocardial infarction, stroke, and pulmonary embolism following COVID-19 vaccines in adults younger than 75 years in France. Ann Intern Med. 2022;175(9):1250–1257. doi:10.7326/M22-0988
176. Maadarani O, Bitar Z, Elzoueiry M, et al. Myocardial infarction post COVID-19 vaccine - coincidence, Kounis syndrome or other explanation - time will tell. JRSM Open. 2021;12(8):20542704211025259. doi:10.1177/20542704211025259
177. Balta A, Ceasovschih A, Șorodoc V, et al. Broad electrocardiogram syndromes spectrum: from common emergencies to particular electrical heart disorders. J Pers Med. 2022;12:1754. doi:10.3390/jpm12111754
178. Bilotta C, Perrone G, Adelfio V, et al. COVID-19 vaccine-related thrombosis: a systematic review and exploratory analysis. Front Immunol. 2021;12:729251. doi:10.3389/fimmu.2021.729251
179. Fazlollahi A, Zahmatyar M, Noori M, et al. Cardiac complications following mRNA COVID-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2022;32(4):e2318. doi:10.1002/rmv.2318
180. Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2022;19(5):332–341. doi:10.1038/s41569-021-00631-3
181. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi:10.1056/NEJMoa2110475
182. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi:10.1056/NEJMoa2109730
183. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi:10.1001/jama.2021.24110
184. Ling RR, Ramanathan K, Tan FL, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10:679–688. doi:10.1016/S2213-2600(22)00059-5
185. Chin SE, Bhavsar SM, Corson A, et al. Cardiac complications associated with COVID-19, MIS-C, and mRNA COVID-19 vaccination. Pediatr Cardiol. 2022;43(3):483–488. doi:10.1007/s00246-022-02851-x
186. Marschner CA, Shaw KE, Tijmes FS, et al. Myocarditis following COVID-19 vaccination. Cardiol Clin. 2022;40(3):375–388. doi:10.1016/j.ccl.2022.05.002
187. Li YE, Wang S, Reiter RJ, et al. Clinical cardiovascular emergencies and the cellular basis of COVID-19 vaccination: from dream to reality? Int J Infect Dis. 2022;124:1–10. doi:10.1016/j.ijid.2022.08.026
188. Parsamanesh N, Karami-Zarandi M, Banach M, et al. Effects of statins on myocarditis: a review of underlying molecular mechanisms. Prog Cardiovasc Dis. 2021;67:53–64. doi:10.1016/j.pcad.2021.02.008
189. Hana D, Patel K, Roman S, et al. Clinical cardiovascular adverse events reported post-COVID-19 vaccination: are they a real risk? Curr Probl Cardiol. 2022;47(3):101077. doi:10.1016/j.cpcardiol.2021.101077
190. Liu R, Pan J, Zhang C, et al. Cardiovascular complications of COVID-19 vaccines. Front Cardiovasc Med. 2022;9:840929. doi:10.3389/fcvm.2022.840929
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.