Back to Journals » Pragmatic and Observational Research » Volume 13
A Phase IV Study on Safety, Tolerability and Efficacy of Dolutegravir, Lamivudine, and Tenofovir Disoproxil Fumarate in Treatment Naïve Adult Indian Patients Living with HIV-1
Authors Dravid A , Morkar D, Prasad D, Ramapuram JT, Patel KV, Naik KS, Bhrusundi M, Kulkarni M, Hegde S, Anuradha S, Nageswaramma S, Madan S, Jayaprakash T, Kulkarni V
Received 23 February 2022
Accepted for publication 25 July 2022
Published 10 August 2022 Volume 2022:13 Pages 75—84
DOI https://doi.org/10.2147/POR.S361907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Ameet Dravid,1 Dnyanesh Morkar,2 Dwijendra Prasad,3 John T Ramapuram,4 Kartik Vikrambhai Patel,5 K Sunil Naik,6 Milind Bhrusundi,7 Milind Kulkarni,8 Sanjeev Hegde,9 S Anuradha,10 Siddabathuni Nageswaramma,11 Surabhi Madan,12 Thammisetty Jayaprakash,13 Vinay Kulkarni14
1Department of Infectious Diseases and Clinical Research, Poona Hospital and Research Centre, Pune, Maharashtra, India; 2Department of Medicine, KLE’s Dr Prabhakar Kore Hospital and MRC, Belagavi, Karnataka, India; 3Department of General Medicine, People Tree Hospital 2, Bangalore, Karnataka, India; 4Department of Medicine, Kasturba Medical College Hospital, Mangalore, Karnataka, India; 5Department of Medicine, Kanoria Hospital and Research Centre, Gandhinagar, Gujarat, India; 6Department of General Medicine, Rajiv Gandhi Institute of Medical Sciences and RIMS Government General Hospital, Srikakulam, Andhra Pradesh, India; 7Department of Medicine, Lata Mangeshkar Multi Specialty Hospital, Nagpur, Maharashtra, India; 8Department of Medicine, Sahyadri Super Specialty Hospital, Pune, Maharashtra, India; 9Department of Global Clinical Operations, Viatris, Bengaluru, Karnataka, India; 10Department of Medicine, Maulana Azad Medical College and Associated Lok Nayak, New Delhi, India; 11Department of Dermatology, Guntur Government General Hospital, Guntur, Andhra Pradesh, India; 12Department of Clinical Research, Care Institute of Medical Sciences (CIMS) Hospital, Ahmedabad, Gujarat, India; 13Department of Pulmonology, Anu Hospitals, Vijayawada, Andhra Pradesh, India; 14Department of Dermatology, LMMF’s Deenanath Mangeshkar Hospital and Research Center, Pune, Maharashtra, India
Correspondence: Sanjeev Hegde, Department of Global Clinical Operations, Viatris, Bengaluru, Karnataka, India, Tel +91-80-66728587, Email [email protected]
Purpose: WHO recommends dolutegravir (DTG) based regimens as first-line treatment for HIV-1 infection. However, few studies have been conducted in Indian population. Hence, our study evaluated the safety, tolerability, and efficacy of DTG 50 mg with Tenofovir and Lamivudine (300/300mg) fixed dose combination in treatment naïve adult Indian patients.
Methods: This was an open label, multicenter, prospective, interventional, phase IV study conducted across 14 sites between February 2019 and July 2020. 24 weeks was the treatment duration for each subject. The primary end point was to assess the incidence of adverse events (AEs) and secondary end points were to assess the proportion of patients achieving plasma HIV-1 RNA levels < 50 copies/mL at week 24 and change in CD4+ cell count from the baseline. Safety analysis was conducted using Safety Analysis Set and efficacy analysis was carried out using Full Analysis Set and Per protocol set.
Results: A total of 288 patients were screened; 250 were enrolled; and 229 completed the study. 389 AEs were reported from 58% of patients. Of these, 61 were related to study treatment. One event of decreased creatinine clearance led to study discontinuation. One serious event of pyrexia was reported, which was unrelated to the study drug. The most common AEs were headache (18%), pyrexia (14%), vomiting (6.4%) and upper respiratory tract infections (6%). No deaths were reported. At week 24, 86.8% of the patients achieved plasma HIV-1 RNA levels < 50 copies/mL and the mean CD4 cell count increased from 350.2 (SD, 239.73) at baseline to 494.6 (SD, 261.40) with an average increase of 143.2 (SD, 226.14) cells.
Conclusion: This study demonstrated the safety and efficacy of DTG based regimen in treatment naïve HIV-1 patients in Indian population and support use of DTG as first-line treatment regimen.
Keywords: HIV-1, dolutegravir, safety, efficacy, CD4 cell count, viral load, adverse events
Introduction
Human Immunodeficiency Virus Type 1 (HIV-1) remains a global health problem with an estimated 37.6 million people globally living with HIV-1 in 2020.1 Most of these cases were reported from low and middle-income countries (LMIC). In India, about 2.3 million people were living with HIV, with 0.22% prevalence in 2020.2
World Health Organization (WHO) 2016 guidelines recommend non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV) based regimens as first-line antiretroviral therapy (ART).3 However, in many LMIC, there was increasing evidence of pretreatment HIV drug resistance (PDR) to NNRTIs.4 This led to low rates of virological suppression, acquisition of new mutations and high rates of ART discontinuation, especially with EFV.5–8 In response to this, WHO issued new guidelines recommending countries to move away from NNRTI regimens to dolutegravir (DTG) based regimens, in the regions with resistance ≥10%.9,10
DTG is a potent second generation integrase strand transferase inhibitor (INSTI), with high rates of viral suppression, low rates of treatment discontinuation and high genetic barrier to develop HIV drug resistance in treatment naïve patients living with HIV-1.11–14 Furthermore, the drug shows rare severe side effects and has low rates of drug-drug interactions.15
In 2017, the generic DTG was introduced in India. All major guidelines16–19 and network meta-analysis (NMA)20–22 recommend DTG based regimens as preferred first-line ART in treatment naïve HIV patients. Also, DTG based regimens are now the standard of care in HIV treatment as per WHO guidelines.16 The National AIDS control organization recommend DTG 50 mg, in combination with Tenofovir (300mg) and Lamivudine (300mg) as preferred first line regimen for all PLHIV (age >10 years and weight >30kg).23 However, there was limited data on exposure of Indian patients to DTG based regimens. So, the Indian regulatory agency, DCGI approved the product in India conditionally based on the global trials. However, the marketing authorization holder (Mylan) was required to conduct a phase IV study in India to characterize the safety and efficacy of DTG based regimens in Indian population. Hence, the study was designed as single arm, pragmatic clinical trial, designed to see if the DTG regimen is safe and efficacious when used as per the label. Therefore, the results of this study has maximum applicability and generalizability to evaluate if DTG regimen is working in the same way that is reported in preregistration clinical trials conducted by the originator.
The current study was planned to evaluate the safety, tolerability, and efficacy of once-daily DTG when given along with Tenofovir (TDF) and Lamivudine (3TC), in treatment naïve patients living with HIV-1.
Materials and Methods
Study Design
This was a Phase IV, Open-label, Multicenter, Prospective, Interventional Study, conducted in 250 HIV-1 positive patients in 14 sites across India from Feb 2019 to July 2020. The total duration of the study was approximately 28 weeks with a screening period of up to 3 weeks, study treatment period of 24 weeks and a safety follow-up period of 1 week (week 25) (Figure 1).
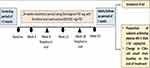 |
Figure 1 Overall schema of study design. |
The study was carried out in accordance with the Declaration of Helsinki, ICH GCP guidelines (2016), New Drugs and Clinical trial Rules (2019) and National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017).
Study Population
Participants meeting the following inclusion criteria were enrolled in the study: Age ≥18 years, weight >40kg, confirmed and documented HIV-1 infection, patients receiving ART therapy for the first time (treatment naïve).
Exclusion criteria include the following: Pregnant or nursing women, evidence of uncontrolled opportunistic infections or malignancy, absolute neutrophil count less than 500 cells per μL, estimated glomerular filtration (eGFR) rates <50 mL/min/1.73 m2, known hypersensitivity to any of the study drug. A full list of inclusion and exclusion criteria is provided in the Supplementary File 1.
Study Procedures
Following the provision of informed consent, all the eligible patients at baseline (Day 1) received one tablet each of DTG (50 mg) and TDF and 3TC (300/300 mg) fixed dose combination once daily for 24 weeks.
The efficacy assessment was performed at week 24. Safety monitoring was performed throughout the study and included telephonic follow-up done at week 8, week 18 and week 25 to assess treatment compliance and for recording any adverse events (AEs). A subject diary was provided at baseline visit, and the physician reviewed it for drug compliance, at every site visit (week 4, 12 and 24).
Blood and urine samples were collected from the eligible patients at screening, week 12 and week 24 (end of treatment) for assessing renal and liver function tests, complete blood count, lipid profile and urine analysis. HIV-1 viral load and CD4 counts were performed at screening and week 24, serum pregnancy test at screening and week 24 (for women of childbearing potential), physical examination, weight and vitals were monitored at screening and all study visits.
Study Assessments and Endpoint
Safety was assessed by monitoring AEs, vital signs, 12-lead ECGs, physical examinations, and clinical laboratory test results. Any abnormality in these assessments from the baseline values were recorded as AEs or serious AEs (SAEs). Measuring the incidence of AEs during the study was the primary end point.
Efficacy was assessed using HIV-1 viral load and CD4 cell count. The proportion of patients achieving plasma HIV-1 RNA < 50 copies/mL at the end of treatment and change in CD4+ cell count from baseline to the end of treatment were the secondary endpoints assessed. Full details of the assessment are provided in Supplementary File 2.
Statistical Analysis
A sample size of 250 was chosen for this study based on the primary endpoint of incidence of AEs reported. When the sample size is 250, the 95% two-sided confidence interval (CI) will provide a precision of at least 6.2% for the occurrence of any AE of interest, where the precision of estimation is defined as half width of 95% CI. Additionally, a sample size of 242 per arm was also used in the FDA registration study to evaluate the efficacy of dolutegravir in treatment naïve patients living with HIV-1.26
Statistical processing was performed using Statistical Analysis System (SAS®). Descriptive statistics consist of summary statistics (number of observations, mean, standard error (SE), standard deviation (SD), minimum, median, and maximum) for continuous data and frequency counts and percentages for categorical data. The safety analyses were conducted using the Safety Analysis Set (SAF) which included all subjects who received at least one dose of the study treatment. AEs were tabulated according to the current version (version 22.1 or later) of the Medical Dictionary for Regulatory Activities (MedDRA) using Preferred Term (PT) within MedDRA System Organ Class (SOC). Frequencies and percentages were used to summarize treatment-emergent AEs (TEAEs), treatment-related AEs, AEs leading to discontinuation, and SAEs. Efficacy analysis was performed on Full Analysis Set (FAS) and repeated based on Per Protocol Set (PPS; all patients who received study treatment, had achieved both the end points, and had no major protocol deviations) as a supportive analysis. In FAS, patients with missing plasma HIV-1 RNA viral load were considered as non-responders. Hence, FAS with complete cases was performed excluding the patients with missing data. The proportion of subjects achieving plasma HIV-1 viral load less than 50 copies per mL with Clopper–Pearson 95% CI were presented at the end of the study treatment visit. In addition, log10 transformed value of change from baseline plasma HIV-1 viral load has been summarized descriptively. For CD4 cell counts, actual and change from screening cell counts have been summarized descriptively by visits. The 95% CI was constructed by using t-test statistics for the treatment group.
Results
A total of 288 patients were screened, of which 250 treatment naïve patients living with HIV-1 were enrolled; 38 patients did not meet inclusion/exclusion criteria. Of the 250 enrolled patients, 229 (91.6%) completed the study. Twenty-one (8.4%) patients failed to complete the study due to consent withdrawal [12 (4.8%) patients], lost to follow up [8 (3.2%) patients] and SAE/AE [1 (0.4%) patient]. Approximately 90% of the patients reported 80–125% compliance to the study treatment.
SAF included all enrolled 250 (100.0%) patients, whereas FAS included 227 (90.8%) patients as 23 (9.2%) patients did not have both HIV-1 RNA load, and CD4 cell counts at post-baseline visits, hence these patients were excluded from FAS. PPS included 211 (84.4%) patients, as 29 (11.6%) patients had missing HIV-1 RNA load and CD4 cell count, while 7 (2.8%) and 3 (1.2%) patients reported major protocol deviation and treatment non-compliance respectively and hence excluded from PP set (Figure 2).
 |
Figure 2 Disposition of subjects. |
Demographic Characteristics
Of 250 enrolled patients, 145 (58.0%) were male, while 105 (42.0%) were female. The study cohort was Asian. The median (inter quartile range [IQR]) age and BMI of the cohort were 38.5 (18–70) years, and 22.2 (14.43–36.74) kg/m2 respectively. The median (IQR) HIV-1 viral load (HIV RNA copies/mL) was 24,621.2 (10–5,500,145), and the mean CD4 cell counts (cells per cubic millimeter) (N = 249) was 350.3 ± 237.84 (Table 1).
 |
Table 1 Summary of Demographics (All Enrolled Patients) |
Safety Evaluation
A total of 389 TEAEs were reported by 144 (57.6%) patients. Of these, 61 TEAEs reported from 36 (14.4%) patients were related to the study treatment; one event “decreased creatinine renal clearance” led to study medication discontinuation and early termination; one event of “Pyrexia” in 1 (0.4%) patient was a TESAE, which was not related to the study drug and patient recovered and completed the study. Further based on severity, of the total 250 enrolled patients, 110 (44.0%) patients had mild AEs, 33 (13.2%) patients had moderate AEs and 1 (0.4%, anemia) patient had a severe AE (Table 2).
 |
Table 2 Summary of Overall Treatment Emergent Adverse Events - Safety Set |
The most frequent AE reported was “headache” by 45 (18%) patients, followed by 40 events of “pyrexia” reported by 35 (14%) patients. Twenty-three events of “vomiting” were reported by 16 (6.4%) patients, 22 events of “upper respiratory tract infection” were reported by 15 (6%) patients. Additionally, 10 events of “rash” were reported by 8 (3.2%) patients, and 7 events of insomnia were reported by 5 (2%) patients (Table 3). No deaths were reported throughout the study.
 |
Table 3 Summary of Treatment Emergent Adverse Events by SOC and PT [> 2% Patients] - Safety Set |
Efficacy Evaluation
The percentage of patients achieving plasma HIV-1 RNA < 50 copies/mL at week 24 was 86.8% (FAS), 89.1% (FAS complete cases), and 88.6% (PPS) (Figure 3). When the missing data from FAS was imputed with log10 transformed value of 1, the mean ± SD plasma HIV-1 viral load showed 3.8 ± 1.77 and 1.3 ± 1.03 at baseline and at week 24, respectively, with a change from the base line value of 2.4 (SD, 1.95). Similar results were recorded for the PP set. The CD4 cell count showed marked improvements with an increase from 350.2 (SD, 239.73) at baseline to 494.6 (SD, 261.40) at week 24 with an average increase of 143.2 (SD, 226.14) cells. The proportion of patients with virological response were similar across FAS and PP [134.9 (SD-214.96) cells] sets for the CD4 cell count.
 |
Figure 3 Forest plot for the proportion and confidence interval (95% CI) for efficacy responders. |
Discussion
In this study, the safety, tolerability and efficacy of DTG (50 mg once daily) given along with TDF and 3TC, in treatment naïve adult Indian patients living with HIV-1 was evaluated.
We found that majority of the AEs reported were of mild or moderate severity; however, one severe AE was reported in the form of Anemia. A single SAE (0.4%, pyrexia) was reported in one patient, which was comparable to FLAMINGO study.24 The event was unrelated to the study drug, resolved within three days and the patient continued and completed the study. One TEAE (0.4%) of “decreased creatinine clearance” led to drug discontinuation of a single patient. This is in line with other studies24–27 signifying lower or comparable proportions of discontinuation with DTG compared to EVF [3% vs 11%], bictegravir [2% vs 2%], darunavir [2% vs 4%] and raltegravir [2% vs 2%]. Some of the common AEs observed in this study include headache, pyrexia, vomiting, upper respiratory tract infection and rash. These observations were in line with other published literature,24,27–29 reference safety information and product label. Very few events of sleep disturbance and no events of weight gain were seen, though such reports have been widely observed previously.12,30–34 No clinically significant abnormalities in vital signs, laboratory assessments and physical examination from baseline were reported during the study period. This is consistent with a DTG study conducted previously in the Indian population.35
Efficacy analysis showed that 86.8% of patients achieved efficacy endpoint of HIV-1 RNA load < 50 copies per mL by week 24. Results were consistent with FAS and PPS. Our findings are similar to Phase 3 reports of FLAMINGO (90%) and SPRING-2 studies (88%); however, different combinations of ARV drugs were studied in these trials [FLAMINGO: DTG 50 mg or Darunavir 800mg + Ritonavir 100mg vs Tenofovir-Emtricitabine or Abacavir-Lamivudine; SPRING-2: DTG 50 mg or Raltegravir 400mg vs Tenofovir–Emtricitabine or Abacavir–Lamivudine] with end points measured at week 48 and week 96 as against to week 24 in our study.24,27 Furthermore, the immunological response to the treatment was observed by a mean increase in CD4+ count by 143.2 cells from baseline to week 24 as seen in previous studies24,27 gives the advantage for the physician to use DTG based regimens as the first option of treatment. These observations can be attributed to a high percentage of treatment compliance (96.6%).
A recent NMA evaluating the safety and efficacy of DTG over low dose EFV concluded that DTG has significantly higher odds of viral suppression rates (OR: 1.64; 95% CI: 1.35–1.96 at 48 weeks), more effective in increasing the CD4 cell count, lower rates of drug resistance11 and less neuropsychiatric AEs.11,36 In another study37 comparing EFV and DTG clinical outcomes, DTG had a better survival rate at 2 years (90.2% vs 86.7%) and 5 years (83.0% vs 76.7%), higher life expectancy (24.8 years vs 22.0 years and better compliance (99.2% vs 97.9%).
National AIDS control organization (NACO) of India is focused on achieving the ‘End of AIDS’ by 2030. To achieve this, it has adopted Joint United Nations Programme on HIV and AIDS (UNAIDS) 90–90-90 plan where 90% of people living with HIV/AIDS (PLHA) know their HIV status; 90% who know their status are on ART; and 90% on ART have suppressed viral load.38 However, India has not achieved the target despite good improvement in the year 2019–2020 compared to year 2018–2019. Currently, 76% of PLHA know their HIV status, 84% are on ARV and 84% have suppressed viral load.39 Considering the roll out of safer, efficacious, and cost saving37 DTG generic regimen,40 India is focused and committed to achieve its target by 2030. Furthermore, implementing DTG as first-line anti-retroviral regimen (instead of EFV, current first-line regimen) will reduce transmission rates and deaths related to HIV/AIDS. Nonetheless, the study has few limitations. This was a single arm open label study. Also, the study was not powered to detect rare AEs with very low incidence. We also excluded pregnant women and hence, we cannot comment on the safety of DTG in pregnancy.
Conclusion
This study demonstrated the safety, efficacy, and tolerability of Dolutegravir 50 mg administered along with fixed dose combination of Tenofovir and Lamivudine among treatment naïve patients living with HIV-1. Our study results support using DTG as the first-line standard of care for treatment naïve patients living with HIV-1 in Indian population.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. However, data sharing will be governed by the company confidentiality policies of Mylan Laboratories Limited, India.
Ethics Approval and Consent to Participate
The study protocol, informed consent and any other written information regarding this study was submitted and approved by the respective independent ethics committees (IEC) and/or institutional review boards of the 14 investigation sites. The details of the IEC and/or institutional review boards is provided in the Supplementary File 3. A written informed consent in compliance with regulatory authority regulations was obtained from each subject before entering the study or performing any unusual or non-routine procedure that involves risk to the subject.
Consent for Publication
This publication contains aggregate data from the clinical trial. No specific subject level information has been included.
Acknowledgments
The authors would like to acknowledge JSS Medical Research Asia Pacific Private Limited, for providing study monitoring and clinical project management. The authors would like to thank Dr. Ravi Shankar and Dr. Sanjay Hadigal for making significant contribution for the study conceptualization and oversight, Dr. Sumit Sant for monitoring the safety of patients involved in the study, Dr. Rajesh Nachankar, Dr. Nageswari and Sharfaa T for study execution and acquisition of data and Unmesh G for data analysis and interpretation. The authors further thank all of them for critically reviewing the manuscript draft. The authors would like to thank Venkata Satya Sai M and Aswin Kumar A for writing support and editorial assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Mylan Laboratories Limited (now Viatris) was the sponsor for this study and was responsible for oversight of the trial.
Disclosure
AD has received financial grants for conducting clinical research from Cipla, Mylan, Emcure and MSD pharma private limited and received fees for being speaker at pharma sponsored seminars and webinars organized by Cipla, Mylan, Emcure, MSD, Genex and Aurobindo pharma private limited. SH is an employee of Viatris, Bangalore, India. All other authors have no competing interests to declare in this work.
References
1. Rashid A, Li K, Feng Y, et al. HIV-1 genetic diversity a challenge for AIDS vaccine development: a retrospective bibliometric analysis. Hum Vaccin Immunother. 2022;18(1):2014733. doi:10.1080/21645515.2021.2014733
2. National AIDS Control Organisation & ICMR-National Institute of Medical Statistics. India HIV estimates 2020: technical brief; 2020. Available from: naco.gov.in.
3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 9789241549684_eng.pdf; 2016. Available from: who.int.
4. Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi:10.1016/S1473-3099(17)30702-8
5. Bertagnolio S, Hermans L, Jordan MR, et al. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor-containing antiretroviral therapy: a systematic review and meta-analysis. J Infect Dis. 2021;224(3):377–388. doi:10.1093/infdis/jiaa683
6. Rutstein SE, Chen JS, Nelson JAE, Phiri S, Miller WC, Hosseinipour MC. High rates of transmitted NNRTI resistance among persons with acute HIV infection in Malawi: implications for first-line dolutegravir scale-up. AIDS Res Ther. 2019;16(1):5. doi:10.1186/s12981-019-0220-8
7. Kantor R, Smeaton L, Vardhanabhuti S, et al. Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis. 2015;60(10):1541–1549. doi:10.1093/cid/civ102
8. Hamers RL, Schuurman R, Sigaloff KC, et al. PharmAccess African Studies to Evaluate Resistance (PASER) Investigators. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12(4):307–317. doi:10.1016/S1473-3099(11)70255-9
9. World Health Organization. Guidelines on the public health response to pretreatment HIV drug resistance. Geneva: World Health Organization. 9789241550055-eng.pdf; 2017. Available from: who.int.
10. World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland: World Health Organization; 2019. Available from: WHO-CDS-HIV-19.15-eng.pdf.
11. Kanters S, Vitoria M, Zoratti M, Doherty M, Penazzato M, Rangaraj A. Comparative efficacy, tolerability and safety of dolutegravir and efavirenz 400mg among antiretroviral therapies for first-line HIV treatment: a systematic literature review and network meta-analysis. EClinicalMedicine. 2020;28:2589–5370.
12. Calmy A, Tovar Sanchez T, Kouanfack C, et al. New antiretroviral and monitoring strategies in HIV-infected adults in low-income countries (NAMSAL) ANRS 12313 Study Group. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020;7(10):e677–e687. doi:10.1016/S2352-3018(20)30238-1
13. Weng YW, Chen IT, Tsai HC, et al. Trend of HIV transmitted drug resistance before and after implementation of HAART regimen restriction in the treatment of HIV-1 infected patients in southern Taiwan. BMC Infect Dis. 2019;23(1):741. doi:10.1186/s12879-019-4389-1
14. Cahn P. Candidates for inclusion in a universal antiretroviral regimen: dolutegravir. Curr Opin HIV AIDS. 2017;12(4):318–323. doi:10.1097/COH.0000000000000388
15. Sculier D, Wandeler G, Yerly S, et al. Efficacy and safety of dolutegravir plus emtricitabine versus standard ART for the maintenance of HIV-1 suppression: 48-week results of the factorial, randomized, non-inferiority SIMPL’HIV trial. PLoS Med. 2020;10(11):e1003421. doi:10.1371/journal.pmed.1003421
16. World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. Available from: who.int.
17. Ryom L, Cotter A, De Miguel R, et al. Update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21(10):617–624. doi:10.1111/hiv.12878
18. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services; 2021.
19. Ahmed N, Angus B, Boffito M, et al. BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy. treatment-guidelines-2016-interim-update.pdf; 2015. Available from. bhiva.org.
20. Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis. 2021;21(1):222. doi:10.1186/s12879-021-05850-0
21. Snedecor SJ, Radford M, Kratochvil D, Grove R, Punekar YS. Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis. BMC Infect Dis. 2019;19(1):484. doi:10.1186/s12879-019-3975-6
22. Kanters S, Vitoria M, Doherty M, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016;3(11):e510–e520. doi:10.1016/S2352-3018(16)30091-1
23. National Guidelines for HIV care and treatment. National AIDS control organization. National_Guidelines_for_HIV_Care_and_Treatment_2021.pdf; 2021. Available from. naco.gov.in.
24. Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. doi:10.1016/S0140-6736(14)60084-2
25. Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515–519. doi:10.1097/QAI.0000000000000790
26. Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–e372. doi:10.1016/S2352-3018(19)30080-3
27. Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. doi:10.1016/S0140-6736(12)61853-4
28. Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6(9):e576–e587. doi:10.1016/S2352-3018(19)30149-3
29. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–849. doi:10.1016/S0140-6736(17)33095-7
30. Calza L, Colangeli V, Borderi M, et al. Simplification to dual therapy containing lamivudine and raltegravir or dolutegravir in HIV-infected patients on virologically suppressive antiretroviral therapy. J Antimicrob Chemother. 2020;75(11):3327–3333. doi:10.1093/jac/dkaa319
31. Todd S, Rafferty P, Walker E, et al. Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. Int J STD AIDS. 2017;28(11):1074–1081. doi:10.1177/0956462416688127
32. Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a Phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4(12):e547–e554. doi:10.1016/S2352-3018(17)30152-2
33. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi:10.1056/NEJMoa1902824
34. Namsal ANRS, Kouanfack C, Mpoudi-Etame M, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826.
35. Kumarasamy N, Prabhu S, Chandrasekaran E, et al. Safety, tolerability, and efficacy of generic dolutegravir-containing antiretroviral therapy regimens among South Indian human immunodeficiency virus-infected patients. Clin Infect Dis. 2019;68(6):1048–1051. doi:10.1093/cid/ciy763
36. Fernández-Bargiela N, Rotea-Salvo S, Margusino-Framiñán L, et al. Discontinuation due to neuropsychiatric adverse events with efavirenz- and dolutegravir-based antiretroviral therapy: a comparative real-life study. Eur J Hosp Pharm. 2020;29:207–211.
37. Zheng A, Kumarasamy N, Huang M, Paltiel AD, Mayer KH, Rewari BB. The cost-effectiveness and budgetary impact of a dolutegravir-based regimen as first-line treatment of HIV infection in India. J Int AIDS Soc. 2018;21(3):e25085. doi:10.1002/jia2.25085
38. The Joint United Nations Programme on HIV/AIDS. Ending AIDS: progress Towards the 90-90-90 Targets. Geneva: joint United Nations Programme on HIV/AIDS. Global_AIDS_update_2017_en.pdf; 2017. Available from: unaids.org.
39. National AIDS Control Organization (2020). Sankalak: status of National AIDS Response (Second edition, 2020). New Delhi: NACO, Ministry of Health and Family Welfare, Government of India. Sankalak Status of National AIDS Response, Second Edition (2020); 2020. Available from: pdf.naco.gov.in.
40. National AIDS control organization. National technical guidelines on anti-retroviral treatment; 2018. NACO-National Technical Guidelines on ART_October.2018.1.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
