Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Relative Safety and Efficacy of Two Doses of Tandospirone Citrate for Generalized Anxiety Disorder: A Multicenter Randomized Controlled Trial
Authors Li Q, Zhang H, Lin G, Shi S, Zhang Y, Ji J, Yang L, Yao J, Wu W
Received 11 March 2022
Accepted for publication 1 August 2022
Published 8 August 2022 Volume 2022:18 Pages 1653—1664
DOI https://doi.org/10.2147/NDT.S366048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Qingwei Li,1 Haiyin Zhang,2 Guozhen Lin,3 Shenxun Shi,4 Yingli Zhang,5 Jianlin Ji,6 Lipeng Yang,7 Jun Yao,1 Wenyuan Wu1
1Department of Psychiatry, Tongji Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Clinical Psychology, Shanghai Mental Health Center, Shanghai, People’s Republic of China; 3Department of Psychiatry, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 4Psychiatry Department, Fudan University Affiliated Huashan Hospital, Shanghai, People’s Republic of China; 5Department of Depressive Disorders, Shenzhen Kangning Hospital, Shenzhen Mental Health Center, Shenzhen, People’s Republic of China; 6Department of Psychological Medicine, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 7Department of Neurology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China
Correspondence: Wenyuan Wu, Department of Psychiatry, Tongji Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China, Email [email protected]
Purpose: To determine the relative safety and efficacy of different doses of tandospirone in treating generalized anxiety disorder (GAD).
Patients and Methods: This parallel randomized controlled trial enrolled patients with GAD from eight centers in China. The patients were randomly assigned to 60 mg/day or 30 mg/day tandospirone groups. The primary endpoint was the overall response rate after receiving 6-week treatment. The secondary endpoints included significant response rate, clinical recovery rate, change in the Hamilton Anxiety Scale (HAMA) total score, HAMA subscale score, Hamilton Depression Scale-17 (HAMD-17), Clinical Global Impression-Severity Scale (CGI-S) score, and Impression-Improvement scale (CGI-I) score.
Results: No significant difference was found in the overall response rate between the two groups (65.7% vs 58.4%, p = 0.213). A higher significant response rate and change in the HAMA total score were found in the 60 mg/day group. The reduction in the CGI-S score and percentage of patients with a CGI-I score of ≤ 2 were higher in 60 mg/day group. The reduction in HAMA somatic anxiety factor, cardiovascular symptom factor, gastrointestinal symptom factor, and HAMD-17 score were more significant in the 60 mg/day group. The incidence of total adverse events was higher in the 60 mg/day group than in the 30 mg/day group. No significant difference was found in the proportion of withdrawal due to adverse events.
Conclusion: Both 60 mg/day and 30 mg/day tandospirone show good efficacy in treating patients with GAD. High doses of tandospirone may have advantages in relieving the somatic symptoms but also present disadvantages due to their high level.
Trial Registration: The trial registration no. was NCT01614041.
Keywords: generalized anxiety disorder, tandospirone, randomized controlled trial, safety, efficacy
Introduction
Generalized anxiety disorder (GAD) is a subtype of anxiety disorder characterized by psychological and somatic symptoms,1,2 which severely affect the health and quality of life. GAD is common in China.3 Based on a large-sample survey of mental disorders in a Chinese population, anxiety disorders were the most prevalent, both in the 12-month period before the interview (weighted prevalence 5.0%, 4.2–5.8) and over the lifetime (7.6%, 6.3–8.8).4,5 In recent years, anxiety treatment has become a focus of research and clinical practice.
The first-line pharmacotherapies, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs), have some limitations, such as partial response,6–8 risk of agitation and anxiety at the initiation of treatment,9 delayed treatment effect, and high incidence of sexual dysfunction.10–12 These limitations result in unsatisfactory compliance, as well as less than 8.5% of patients receiving anxiolytic treatment in China.13 A total of 46% of patients with GAD discontinued their treatment after 3.7 months for various reasons. Drug intolerance was one reason for the smaller proportion of GAD patients receiving systematic treatment.14
Tandospirone citrate is a partial agonist of the 5-hydroxytryptamine type 1A (5-HT1A) receptor,15,16 which selectively binds to the 5-HT1A receptor in the brain. The anti-anxiety effect is exerted by inhibiting the activities of the 5-HT1A system via selectively stimulating the 5-HT1A receptor widely distributed in these sites. The action sites are primarily in the limbic system, including the hippocampus and amygdala, and the raphe nucleus projecting 5-HT1A nerves. Tandospirone has been reported to significantly reduce anxiety symptoms and to be well tolerated in previous studies.17–19
Rat experiments demonstrated that the anti-anxiety effect of tandospirone significantly correlated with its concentration in the plasma and brain after 0.5 h injection, without increases in corresponding side effects.20 It was speculated that a higher dose of tandospirone would achieve adequate blood concentrations and reduce anxiety more effectively. A clinical study on GAD and mixed anxiety–depression demonstrated that the higher dose of tandospirone had a good anti-anxiety effect without severe side effects.21 However, this study had a small sample size, with only 23 cases included. Large-scale studies are warranted to evaluate the benefits of different doses of tandospirone. Therefore, this multicenter, parallel-group, randomized controlled trial was designed to explore the safety and efficacy of different doses of tandospirone on GAD with the hypothesis that higher doses of tandospirone might have better anxiolytic efficacy and comparable safety profile.
Methods
Study Design and Participants
This multicenter, parallel-group, randomized controlled trial enrolled patients with GAD from eight hospitals in China between January 2012 and September 2018.
The inclusion criteria were as follows: (1) inpatients or outpatients; (2) meet the criteria for GAD the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition;22 (3) age 18–65 years; (4) Hamilton Anxiety Scale (HAMA) score ≥17 at screening and at baseline; and (5) discontinuation of SSRIs, SNRIs, and noradrenaline and specific serotonergic antidepressant (NaSSA) over 2 weeks before the study.
The exclusion criteria were as follows: (1) with severe suicidal tendencies; (2) score of the sixth item in HAMA scale ≥3; (3) Hamilton Depression Scale (HAMD) score ≥21 at screening; (4) baseline HAMA score reduction rate ≥25% compared with screening; (5) anxiety disorder secondary to other mental or physical diseases; (6) lactation, pregnancy, or chances of becoming pregnant during the trial; (7) diseases related to the heart, liver, kidney, endocrine system, blood, and lungs, other diseases, or abnormal thyroid-stimulating hormone level in the blood; (8) history of epilepsy, except for childhood febrile convulsions; or (9) current use of benzodiazepines to treat anxiety.
This study was approved by the ethics committee of the Tongji Hospital of Tongji University [Approval No. (Tong) Lun Shen No. (117)]. Written informed consent was signed by all patients. The trial has been registered in clinical trail.gov (NCT01614041).
Intervention
Patients were randomized 1:1 into 60 mg/day and 30 mg/day groups using the stratified block randomization. Patients with GAD were given tandospirone 20 mg three times per day (60 mg/day group) or tandospirone 10 mg three times per day (30 mg/day group) for six consecutive weeks and followed up at baseline and week 1, 2, 4, and 6.
Efficacy Assessments
The primary endpoint was the overall response rate in week 6 (defined as a decrease in the HAMA total score of ≥50% compared to baseline). The secondary endpoints included significant response rate (defined as a decrease in the HAMA total score of ≥75% compared to baseline), clinical recovery rate defined as a HAMA score of ≤7) in week 6, the change in HAMA total score, HAMA subscale score (somatic anxiety factor, psychic anxiety factor, fear symptom factor, insomnia symptom factor, memory symptom factor, cardiovascular symptom factor, and gastrointestinal symptom factor), HAMD-17 score, Clinical Global Impression-Severity Scale (CGI-S) score, the Clinical Global Impression-Improvement scale (CGI-I) score, the percentage of patients with a CGI-I score of ≤2, and the 12-Item Short-Form Health Survey (SF-12) score from baseline till week 6.
The somatic anxiety factor score was calculated from items 7, 8, 9, 10, 11, 12 and 13 of the HAMA scale. The psychic anxiety factor score was calculated based on items 1, 2, 3, 4, 5, 6 and 14 of the HAMA scale.23,24 The fear symptom factor score, insomnia symptom factor score, memory symptom factor score, cardiovascular symptom factor score and gastrointestinal symptom factor score were the score of item 3, item 4, item 5, item 9 and item 11 of the HAMA scale, respectively.
Safety Assessment
Adverse events during the treatment were collected for safety evaluation. The frequency of adverse events and adverse events leading to withdrawal were determined. The incidence of adverse events was calculated.
Calculation of Sample Size
According to previous findings,19 the overall response rate of tandospirone in the low-dose group was estimated to be 67% after 6 weeks of treatment. It was estimated that the high-dose group would be 15% better than the low-dose group in the present study. Based on the ratio of 1:1, two-sided α = 0.05 and β = 0.2 (power = 80%), the sample size of each group was calculated as 133 patients. Considering loss to follow-up, 150 patients were enrolled in each group, and a total of 300 patients were needed.
Statistical Analysis
SAS 9.4 (SAS Institute Inc., NC, USA) and SPSS 20.0 software (IBM Corp., NY, USA) were used for statistical analysis. Continuous data were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed continuous data were expressed as means ± standard deviation. Categorical data were expressed as n (%). Continuous data with normal distribution were tested using the Student’s t-test. Categorical data were analyzed using the chi-square test or Fisher’s exact test. Logistic analysis was used to adjust the baseline variates. All statistical tests were two-sided, with p < 0.05 indicating a statistically significant difference.
Full analysis set (FAS) included qualified patients and those lost to follow-up but did not include excluded patients. For the primary endpoint, last observation carried forward (LOCF) methods was used for missing data according to the intention-to-treat analysis. Safety set (SS) included patients who received at least one treatment with safety records. The endpoints were analyzed using FAS. The adverse events were analyzed using SS.
Results
Clinical and Demographic Characteristics
A total of 280 patients were screened at baseline, and 274 were randomly assigned to the study treatments, with 137 patients in the 60 mg/day group and 137 patients in the 30 mg/day group, respectively. Further, 129 and 132 patients in the 60 mg/day and 30 mg/day groups completed the study, respectively (Figure 1).
 |
Figure 1 Flow chart of the study. |
The baseline demographic and clinical characteristics are summarized in Table 1. No significant difference between the two groups was found in demographic data including age, sex, education level, body mass index, and clinical data, such as HAMA total score. HAMA somatic anxiety factor score, HAMA cardiovascular symptom factor score, HAMA gastrointestinal symptom factor score, and HAMD-17 score were higher in the 60 mg/day group than in the 30 mg/day group (all p < 0.05).
 |
Table 1 Baseline Characteristics of Patients with Generalized Anxiety Disorder |
Efficacy
Proportions of Response, Significant Response, and Clinical Recovery
The overall response rate (65.7% vs 58.4%, p = 0.213) and clinical recovery rate (41.6% vs 39.4%, p = 0.712) in week 6 were comparable in two groups. After adjusting for the somatic anxiety factor, cardiovascular symptom factor, gastrointestinal symptom factor, and HAMD-17 score of baseline, no statistical differences in the overall response rate were detected between the two groups (Supplementary Table 1). The significant response rate (34.3% vs 22.6%, p = 0.032) in week 6 was higher in the 60 mg/day group than in the 30 mg/day group (Figure 2 and Supplementary Table 2).
Hamilton Anxiety Scale
Compared to baseline, the reduction in the HAMA total score was 17.84 ± 6.89 and 15.77 ± 5.74 in the 60 mg/day group and the 30 mg/day group, respectively, which is higher in 60 mg/day group (p = 0.007). Compared to baseline, the reduction in the HAMA somatic anxiety factor score was 8.93 ± 3.88 in the 60 mg/day group versus 7.38 ± 3.28 in the 30 mg/day group (p < 0.01). The reduction in the cardiovascular symptom factor score was 1.35 ± 0.81 and 1.03 ± 0.80 in the 60 mg/day group and the 30 mg/day group, respectively (p = 0.001). The reduction in the gastrointestinal symptom factor score was 1.25 ± 0.80 and 1.01 ± 0.79 in the 60 mg/day group and the 30 mg/day group, respectively (p = 0.013). The reduction in the HAMA psychic anxiety factor score was 8.91 ± 3.51 in the 60 mg/day group versus 8.39 ± 3.21 in the 30 mg/day group (p = 0.197). The reduction in the fear symptom factor score was 1.26 ± 0.88 and 1.26 ± 0.81 in the 60 mg/day group and the 30 mg/day group, respectively (p = 0.943). The reduction in the insomnia symptom factor score was 1.39 ± 0.86 and 1.31 ± 0.80 in the 60 mg/day group and the 30 mg/day group, respectively (p = 0.468). The reduction in the memory symptom factor score was 1.31 ± 0.85 and 1.12 ± 0.83 in the 60 mg/day group and the 30 mg/day group, respectively (p = 0.063) (Figure 3 and Supplementary Table 2).
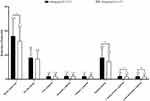 |
Figure 3 Comparison of the change from baseline of the HAMA total score and HAMA subscale scores between two groups. Abbreviation: HAMA, Hamilton Anxiety Scale. Note: *p < 0.05. |
Hamilton Depression Scale
HAMD-17 analysis revealed that after 6 weeks of treatment, the total HAMD score change was 7.95 ± 3.55 in the 60 mg/day group and 6.68 ± 3.10 in the 30 mg/day group, with statistically significant difference (p = 0.002) (Figure 4 and Supplementary Table 2).
 |
Figure 4 Comparison of the change from baseline in the HAMD-17 score between two groups. Abbreviation: HAMD, Hamilton Depression Scale. Note: *p < 0.05. |
Clinical Global Impression Scale
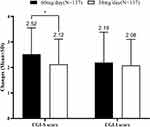
At the end of week 6, the change in the CGI-S score from baseline was significantly different between the two groups (2.52 ± 1.03 in the 60 mg/day group vs 2.12 ± 0.99 in the 30 mg/day group, p = 0.002), no difference was found between the two groups in the change in CGI-I score (2.19 ± 1.19 vs 2.08 ± 1.02, p=0.423) (Figure 5 and Supplementary Table 2). The percentage of patients with a CGI-I score of ≤2 was higher in the 60 mg/day group than in the 30 mg/day group (96.9% vs 89.5%, p = 0.017). No difference was found between the two groups in the percentage of patients with a CGI-S score of ≤2(47.7% vs 44.4%, p=0.588) (Figure 2 and Supplementary Table 2).
12-Item Short-Form Health Survey
No difference in the SF-12 physical component summary score change (–27.36 ± 23.56 vs –25.86 ± 22.06, p = 0.301) or the mental component summary total score change (–20.91 ± 14.59 vs –18.42 ± 14.96, p = 0.173) was observed between the two groups. No significant difference was observed in the score change of other items (Supplementary Table 2).
Safety
A total of 32 (23.4%) and 16 (11.7%) patients experienced adverse events in the 60 mg/day and 30 mg/day groups, respectively (p = 0.011). No patient developed serious adverse events.
Adverse events mainly occurred in the nervous system, with 18 patients (13.1%) in the 60 mg/day group and 3 patients (2.2%) in the 30 mg/day group developed. The most common adverse event in the nervous system was dizziness. Twelve patients (8.8%) in the 60 mg/day group experienced dizziness, which lasted for a median of 10 days (range 1 to 25 days) and all were relieved spontaneously without dose reduction. One patient in the 30 mg/day group (0.7%) had dizziness, which was recovered after 5 days. The incidence of dizziness was statistically significantly different between the two groups (Table 2).
 |
Table 2 Treatment-Emergent Adverse Reactions in Patients with Generalized Anxiety Disorder |
Eighteen (13.1%) patients in the 60 mg/day group and five (3.6%) patients in the 30 mg/day group developed adverse events in the gastrointestinal system. Common adverse events included abdominal discomfort and nausea. No significant difference in incidence was found between the two groups.
Three participants in the 60 mg/day group withdrew due to adverse events, including epigastric discomfort, dizziness, and early awakening. One patient in the 30 mg/day group withdrew because of the feeling of burnout. No statistically significant difference was found in the percentage of patients who withdrew due to adverse events between the two groups.
Discussion
This multicenter randomized clinical trial compared the efficacy and safety of 60 mg/day and 30 mg/day tandospirone in patients with GAD. The main finding was that both 60 mg/day and 30 mg/day of tandospirone showed promising efficacy, with an overall response rate of 65.7% and 58.4%, respectively. In the 60 mg/day group, patients showed more improvement in the change in the HAMA total score from baseline, and a higher proportion of patients achieved a significant response. Also, more improvement was found in HAMD score, CGI-S score, and percentage of patients with a CGI-I score of ≤2 was higher in the 60 mg/day group compared with the 30 mg/day tandospirone group.
The present study was the first to systematically assess the safety and efficacy of 60 mg/day of tandospirone in treating GAD with a large sample size. Both 30 mg/day and 60 mg/day tandospirone effectively improved anxiety symptoms in patients with GAD. A meta-analysis showed that the response rate for common anxiety medications for anxiety disorder ranged from 54.5% to 67.7%.25 The efficacy of 20 mg and 40 mg paroxetine in treating GAD was compared. It showed that the response rate was 62% and 68%, and the remission rate was 30% and 36%, respectively, using a remission standard of HAMA ≤7.26 The present study showed that the significant response rate of 60 mg/day tandospirone treatment was similar to those of 40 mg/day paroxetine. In addition, the response of 10–20 mg/day escitalopram and sertraline for GAD was 68%27 and 63%28 or 59.2%,29 respectively. Based on these results, the high dose might be more effective, in keeping with the idea that GAD required a higher therapeutic dose.
The present study suggested that 60 mg/day tandospirone might improve the somatic anxiety symptoms more effectively compared with 30 mg/day tandospirone. Moreover, 60 mg/day tandospirone could more significantly improve cardiovascular (–1.35 ± 0.81 vs –1.03 ± 0.80, p = 0.001) and gastrointestinal symptoms (–1.25 ± 0.80 vs –1.01 ± 0.79, p = 0.013). This might be due to the fact that cardiovascular and gastrointestinal symptoms are important clinical manifestations in GAD patients, as is somatic anxiety. In addition to the improvement in anxiety symptoms, the cardiovascular and digestive symptoms are significantly alleviated. Previous studies on the efficacy and safety of other drugs for somatic symptoms suggest that safer and more effective GAD treatments for somatic symptoms are greatly needed. SSRIs such as sertraline are more effective than placebo for treating psychic anxiety.29 Venlafaxine, which has a similar efficacy for somatic anxiety and psychiatric anxiety symptoms,30 has relatively poor tolerability. Benzodiazepines have better efficacy for somatic anxiety than for psychiatric anxiety.31 Multiple consensuses and guidelines list benzodiazepines as second- or even third-line anxiolytics because of their side effects and addiction risks.32
Patients with GAD often present depressive symptoms, and for such patients, the relief of depressive symptoms is also important.33 Previous studies have suggested that the addition of tandospirone was able to improve the outcomes in patients with major depressive disorder18 and vascular depression.34 In this study, we also demonstrated that tandospirone significantly improved the HAMD score, regardless of the doses. Besides, significant difference was found in HAMD scores between the two groups after treatment, indicating that a higher dose of tandospirone may be more effective for treating depressive symptoms of GAD patients.
The present study found that a higher proportion of adverse events occurred in the high-dose group than in the low-dose group (23.4% vs 11.7%, respectively). However, a higher incidence of adverse events did not result in a higher withdrawal rate, which suggests that 60 mg/day is tolerable for patients with GAD. A placebo-controlled study on the use of duloxetine to treat GAD reported at least one adverse event in the duloxetine and placebo groups, with incidences of 60.2% and 44.1%, respectively.35 The adverse events were similar to those of other antidepressants that act on the 5-HT system to treat anxiety disorders and depression, mostly manifested as dizziness and gastrointestinal issues.18 Among the adverse events, dizziness was significantly increased in the high-dose group.
The present study had some limitations. First, patients are followed up for six weeks, which is not a very long process and symptoms of GAD fluctuated over time during this period. However, the effectiveness of anti-anxiety drugs is often estimated by patients’ responses in the first 2 to 4 weeks. The response in week 6 correlated with the long-term efficacy. Second, the study enrollment process was slow due to the difficulty of enrolling enough subjects, but continuous clinical monitoring ensured the quality of study. Third, even though patients with higher doses may show more benefits from relief of somatic symptoms, higher doses may also be associated with a higher proportion of adverse events and higher medical costs. The effectiveness and safety, as well as the cost-effectiveness, of different doses of tandospirone in GAD needed to be explored in the large-scale real-world study.
Conclusions
Both 60 mg/day and 30 mg/day tandospirone may show promising efficacy in patients with GAD. A higher dose may have more advantages in relieving somatic symptoms and depressive symptoms, along with a relatively higher proportion of adverse events was found in the higher dose group.
Data Sharing Statement
The datasets used and/or analyzed in the present study are available from the corresponding authors on reasonable requests.
Ethics Approval and Consent to Participate
This study was approved and supervised by the ethics committee of the Tongji Hospital of Tongji University (Approval No. 117). The written informed consent form was signed by all patients. The trial registration no. was NCT01614041. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for Publication
Written informed consent form was signed by all patients.
Acknowledgments
This study was supported by the Psychosomatic Medicine Project of Key Developing Disciplines of Shanghai Municipal Health Commission (2019ZB0202), the Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (GWV-10.2-XD29), and the Projects of Shanghai Health and Family Planning Commission (201740007). We also wish to thank Sumitomo Pharma (Suzhou) Co., Ltd. for funding support (No. DSPC-SED-1101).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Psychosomatic Medicine Project of Key Developing Disciplines of Shanghai Municipal Health Commission (2019ZB0202), the Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (GWV-10.2-XD29), and the Projects of Shanghai Health and Family Planning Commission (201740007). We also wish to thank Sumitomo Pharma (Suzhou) Co., Ltd. for funding support (No. DSPC-SED-1101). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Disclosure
The authors confirm that there are no conflicts of interest.
References
1. Stein MB, Sareen J, Solomon CG. CLINICAL PRACTICE. Generalized anxiety disorder. N Engl J Med. 2015;373(21):2059–2068. doi:10.1056/NEJMcp1502514
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington: DC: American Psychiatric Association; 2013.
3. Guo X, Meng Z, Huang G, et al. Meta-analysis of the prevalence of anxiety disorders in mainland China from 2000 to 2015. Sci Rep. 2016;6:28033. doi:10.1038/srep28033
4. Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi:10.1016/j.psychres.2020.112954
5. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
6. Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin Pharmacother. 2015;16(11):1669–1681. doi:10.1517/14656566.2015.1059424
7. Kapczinski FLM, Souza JS, Schmitt R. Antidepressants for generalized anxiety disorder. Cochrane Database Syst Rev. 2003;68:CD003592–CD003592.
8. Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Arch Intern Med. 2004;164(11):1197–1204. doi:10.1001/archinte.164.11.1197
9. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222–228. doi:10.4088/PCC.v10n0307
10. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63(4):357–366. doi:10.4088/JCP.v63n0414
11. Werneke U, Northey S, Bhugra D. Antidepressants and sexual dysfunction. Acta Psychiatr Scand. 2006;114(6):384–397. doi:10.1111/j.1600-0447.2006.00890.x
12. Moll JL, Brown CS. The use of monoamine pharmacological agents in the treatment of sexual dysfunction: evidence in the literature. J Sex Med. 2011;8(4):956–970. doi:10.1111/j.1743-6109.2010.02190.x
13. Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. doi:10.1016/S0140-6736(09)60660-7
14. Chollet J, Saragoussi D, Clay E, Francois C. A clinical research practice datalink analysis of antidepressant treatment patterns and health care costs in generalized anxiety disorder. Value Health. 2013;16(8):1133–1139. doi:10.1016/j.jval.2013.09.001
15. Huang X, Yang J, Yang S, et al. Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms. Oncotarget. 2017;8(60):102705–102720. doi:10.18632/oncotarget.22170
16. Hamik A, Oksenberg D, Fischette C, Peroutka SJ. Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol Psychiatry. 1990;28(2):99–109. doi:10.1016/0006-3223(90)90627-E
17. Liao Z, Zhao X, Li T, et al. EEG power spectral analysis reveals tandospirone improves anxiety symptoms in patients with Alzheimer’s disease: a prospective cohort study. Ann Transl Med. 2021;9(1):64. doi:10.21037/atm-20-6647
18. Lin J, Su Y, Wang C, et al. Effects of tandospirone augmentation in major depressive disorder patients with high anxiety: a multicenter, randomized, parallel-controlled, open-label study. J Psychiatr Res. 2018;99:104–110. doi:10.1016/j.jpsychires.2018.01.020
19. Wu W, Li C, Fang F, et al. Effectiveness of tandospirone in treatment of patients with general anxiety disorder and its impact on quality of life: a multicenter open study. Chin J New Drug Clin Rem. 2006;4:282–285.
20. Shimizu H, Tatsuno T, Tanaka H, Hirose A, Araki Y, Nakamura M. Serotonergic mechanisms in anxiolytic effect of tandospirone in the Vogel conflict test. Jpn J Pharmacol. 1992;59(1):105–112. doi:10.1254/jjp.59.105
21. Nishitsuji K, To H, Murakami Y, et al. Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression: results of a comparatively high dosage trial. Clin Drug Investig. 2004;24(2):121–126. doi:10.2165/00044011-200424020-00007
22. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington: DC: American Psychiatric Association; 2000.
23. Mingyuan Z. Handbook of Psychiatric Scales. Hunan: Hunan Science and Technology Press; 1998.
24. Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–68. doi:10.1016/0165-0327(88)90072-9
25. Bereza BG, Machado M, Ravindran AV, Einarson TR. Evidence-based review of clinical outcomes of guideline-recommended pharmacotherapies for generalized anxiety disorder. Can J Psychiatry. 2012;57(8):470–478. doi:10.1177/070674371205700805
26. Rickels K, Zaninelli R, McCafferty J, Bellew K, Iyengar M, Sheehan D. Paroxetine treatment of generalized anxiety disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2003;160(4):749–756. doi:10.1176/appi.ajp.160.4.749
27. Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress Anxiety. 2004;19(4):234–240. doi:10.1002/da.10146
28. Allgulander C, Dahl AA, Austin C, et al. Efficacy of sertraline in a 12-week trial for generalized anxiety disorder. Am J Psychiatry. 2004;161(9):1642–1649. doi:10.1176/appi.ajp.161.9.1642
29. Brawman-Mintzer O, Knapp RG, Rynn M, Carter RE, Rickels K. Sertraline treatment for generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(6):874–881. doi:10.4088/JCP.v67n0603
30. Meoni P, Hackett D, Lader M. Pooled analysis of venlafaxine XR efficacy on somatic and psychic symptoms of anxiety in patients with generalized anxiety disorder. Depress Anxiety. 2004;19(2):127–132. doi:10.1002/da.10141
31. Balon R, Starcevic V. Role of benzodiazepines in anxiety disorders. Adv Exp Med Biol. 2020;1191:367–388.
32. Craske MG, Stein MB. Anxiety. Lancet. 2016;388(10063):3048–3059. doi:10.1016/S0140-6736(16)30381-6
33. Hettema JM. The nosologic relationship between generalized anxiety disorder and major depression. Depress Anxiety. 2008;25(4):300–316. doi:10.1002/da.20491
34. Chen H, Lin Q, Lin T, et al. A controlled study of the efficacy and safety of tandospirone citrate combined with escitalopram in the treatment of vascular depression: a pilot randomized controlled trial at a single-center in China. J Psychiatr Res. 2019;114:133–140. doi:10.1016/j.jpsychires.2019.04.024
35. Wu WY, Wang G, Ball SG, Desaiah D, Ang QQ. Duloxetine versus placebo in the treatment of patients with generalized anxiety disorder in China. Chin Med J. 2011;124(20):3260–3268.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.