Back to Journals » Cancer Management and Research » Volume 14
The Efficacy and Safety of Anlotinib in Extensive-Stage Small Cell Lung Cancer: A Multicenter Real-World Study
Authors Zheng HR, Jiang AM , Gao H, Liu N , Zheng XQ , Fu X, Zhang R, Ruan ZP , Tian T , Liang X, Yao Y
Received 31 March 2022
Accepted for publication 19 July 2022
Published 2 August 2022 Volume 2022:14 Pages 2273—2287
DOI https://doi.org/10.2147/CMAR.S364125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Hao-Ran Zheng,1,2,* Ai-Min Jiang,1,* Huan Gao,1 Na Liu,1 Xiao-Qiang Zheng,1 Xiao Fu,1 Rui Zhang,1 Zhi-Ping Ruan,1 Tao Tian,1 Xuan Liang,1 Yu Yao1
1Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2Department of Medical Oncology, Xi’an No.3 Hospital, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuan Liang; Yu Yao, Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, No. 277 Yanta West Road, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel +86-29-85324600, Fax +86-29-85324086, Email [email protected]; [email protected]
Purpose: Anlotinib, an antiangiogenic multi-target tyrosine kinase inhibitor (TKI), has shown favorable anticancer efficacy and acceptable safety in treating extensive-stage small cell lung cancer (ES-SCLC) in some clinical studies. This research aimed to explore the real-world efficacy and safety of anlotinib in ES-SCLC.
Methods: Pathologically confirmed ES-SCLC patients receiving anlotinib were enrolled for this retrospective study. The primary endpoint of this study was progression-free survival (PFS), and secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and adverse reactions.
Results: In total, 202 patients were included in this study. The median PFS of all patients was 4.8 months [95% confidence interval (CI): 3.9– 5.7], and the median OS was 7.6 months (95% CI 6.5– 8.7). Respectively, the overall ORR and DCR were 30.2% and 87.1%. The univariate and multivariate Cox regression analyses revealed that patients with Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≤ 1, plus chemotherapy or immunotherapy, plus radiotherapy, and post-medication hypertension might have longer PFS and OS. The PFS and OS were significantly prolonged in combination group than that in monotherapy group [PFS 6.0 vs 3.6 months, hazards ratio (HR)=0.49, 95% CI 0.34– 0.70, P < 0.001; OS 9.2 vs 4.8 months, HR = 0.48, 95% CI 0.32– 0.72, P < 0.001]. The main treatment-related adverse reactions were generally tolerated. The incidence of adverse reactions in combination group was higher than that in monotherapy group (75.0% vs 52.6%, P = 0.001). The most common adverse reaction was hypertension, followed by hand-foot syndrome and fatigue, regardless of monotherapy or combination group.
Conclusion: Anlotinib is effective and well tolerated in patients with ES-SCLC in the real-world. The clinical efficacy of anlotinib combined with chemotherapy or immunotherapy is better than that of monotherapy. Further investigations are needed for prospective studies with larger sample size.
Keywords: anlotinib, small cell lung cancer, real-world, efficacy, safety
Introduction
Lung cancer is the most frequent cause of tumor death worldwide. Small cell lung cancer (SCLC) is a highly aggressive and deadly malignant tumor, accounting for approximately 10% to 15% of all lung cancers.1 SCLC comprises an estimated 250,000 new cases and at least 200,000 deaths worldwide each year.2 Although diagnostic techniques have been developed, approximately 70% of the patients are diagnosed with extensive-stage SCLC (ES-SCLC) with poor overall survival (OS).3 As the gold standard for SCLC treatment, platinum combined with etoposide (EP/EC) chemotherapy has been widely used in the past 40 years. Despite the high response rate to chemotherapy, SCLC patients always inevitably relapse and progress rapidly.4 Fortunately, novel immune checkpoint inhibitors combined with chemotherapy exhibited promising efficacy in ES-SCLC as the first-line treatment.5 Topotecan is the only approved second-line drug with moderate efficacy and prognosis, accompanied by considerable adverse reactions.6 However, the third-line and above treatment options for patients with ES-SCLC remain limited and controversial.7
Angiogenesis is a complex process that plays an important role in tumor growth, invasion and metastasis. Vascular endothelial growth factor (VEGF) is the most important proangiogenic protein.8 Previous studies found that about 80% of SCLC tissues were positive for VEGF expression, and the VEGF level was an independent prognostic factor in SCLC.9 As an oral antiangiogenic tyrosine kinase inhibitor (TKI), anlotinib targets vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptors (PDGFR), fibroblast growth factor receptor (FGFR), and c-kit.10 Based on the ALTER 1202 study, anlotinib was approved by the China Food and Drug Administration (CFDA) in 2019 as the third-line and above treatment for SCLC. ALTER 1202 study showed that the median progression-free survival (PFS) of anlotinib third-line treatment for ES-SCLC was 4.1 months, which was 3.4 months longer than the placebo group. Moreover, anlotinib prolonged OS (7.3 vs 4.9 months) and improved disease control rate (DCR) (71.6% vs 13.2%).11 The 2021 American Society of Clinical Oncology (ASCO) meeting announced the preliminary results of a Phase II clinical study on the efficacy and safety of anlotinib combined with EP/EC chemotherapy regimen in the first-line treatment of ES-SCLC. Since August, 2020, 20 patients could evaluate the efficacy, of which the median PFS was 10.0 months, the median OS was 15.0 months, the objective response rate (ORR) was 90%, and the DCR was 100%. It was significantly higher than that of traditional chemotherapy.12 At present, several clinical trials of anlotinib in the treatment of SCLC are progressed in China.
In clinical trials, patients are strictly screened. However, the treatment effect is often affected by many factors in the real world, such as economic status, adverse reactions, compliance, and so on. Patients with poor conditions, such as the elderly, combined brain metastases, and the Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥2, are often excluded from clinical trials.13 Therefore, we conducted this multi-center research to investigate the real-world efficacy and safety of anlotinib in patients with ES-SCLC.
Methods
Study Design and Patients
This research is a multicenter, non-intervention, retrospective real-world study. ES-SCLC patients receiving anlotinib in the First Affiliated Hospital of Xi’an Jiaotong University, Xijing Hospital of Air Force Military Medical University, Xianyang Central Hospital, Shaanxi Nuclear Industry 215 Hospital, Hanzhong Central Hospital, and Baoji Traditional Chinese Medicine Hospital were eligible for retrospective analysis between December 1, 2018, and July 31, 2021. These tertiary hospitals are located in Shaanxi, China. The characteristics of patients were collected, including age, sex, smoking status, ECOG PS, age-adjusted Charlson comorbidity index (aCCI), TNM stage, number and location of metastases, imaging and laboratory examination, anlotinib initial dose, number of treatment lines, combined therapeutic protocol, and adverse reaction.
Inclusion and Exclusion Criteria
The inclusion criteria for patients were as follows: (1) age ≥18 years; (2) patients with ES-SCLC diagnosed by pathology have measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 standard; (3) ECOG PS ≤3; (4) without surgery; (5) using anlotinib at least 1 cycle. The exclusion criteria for patients were as follows: (1) severe lack of clinical records or loss of follow-up; (2) imaging efficacy evaluation cannot be performed; (3) patients with active bleeding or serious systemic diseases.
Therapeutic Methods
On days 1–14 of a 21-day cycle, anlotinib was taken orally once a day with an initial dose of 10–12mg. The initial dose of anlotinib and combined regimen were determined by qualified physicians. Patients with severe adverse reactions can gradually reduce the dose from 12mg to 8mg. Treatment was discontinued if the disease progressed or intolerance of the adverse reactions.
Efficacy and Safety Evaluation
According to the RECIST version 1.1 standard, two qualified physicians independently evaluated the efficacy through computed tomography (CT) or magnetic resonance imaging (MRI) every 2 cycles. The responses were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). When there was disagreement on the assessment, a third physician was requested to reevaluate. Clinical follow-up was performed regularly every 6–8 weeks. Follow-up data were collected up to October 31, 2021. PFS was defined as the time from the start of anlotinib administration until tumor progression or death from any cause before disease progression. OS was defined as the time from anlotinib initiation to death. Respectively, ORR or DCR was calculated as the addition of CRs plus PRs or CRs plus PRs plus SDs. The adverse reactions were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The primary endpoint of this study was PFS, and secondary endpoints included OS, ORR, DCR, and adverse reactions.
Statistical Analysis
Patients’ baseline characteristics were summarized as proportions for categorical variables and medians (range) for continuous variables as appropriate. Pearson Chi-square test was used to compare categorical variables and tumor responses between two groups. The median PFS, OS, and 95% confidence interval (CI) were estimated using the Kaplan–Meier method. Cox proportional hazards regression was used for the univariable and multivariable analyses and to calculate the hazard ratios (HR) with 95% CIs. All statistical analyses in this study were performed using SPSS version 18.0 for Windows 64.0, GraphPad Prism version 6.0, and R software version 3.6.1. A two-tailed P-value <0.05 was considered statistically different.
Results
Baseline Clinical Characteristics of Patients
In total, 202 patients were included in the present study. The median follow-up duration was 6.0 months. Details of the patients’ baseline clinical characteristics are shown in Table 1. There were 164 males (81.2%) and 38 females (18.8%). The median age of the patients was 61 years (range, 28 to 87 years). Of these, 93 (46.0%) patients received anlotinib in first-line treatment, and 109 (54.0%) patients received anlotinib in second-line and above treatment. Fifty-seven (28.2%) patients received radiotherapy during the treatment. Anlotinib was used alone in 78 (38.6%) patients and combined with other regimens in 124 (61.4%) patients. In combination group, 98 (79.0%) patients received anlotinib plus chemotherapy, 6 (4.8%) patients received anlotinib plus immunotherapy, and 20 (16.2%) patients received anlotinib plus chemotherapy and immunotherapy. Compared with combination group, there were more patients in monotherapy group included age ≥65 (56.4% vs 32.3%, P = 0.001), ECOG PS ≥2 (56.4% vs 33.1%, P = 0.001), aCCI >8 (50.0% vs 33.9%, P = 0.023), baseline neuron-specific enolase (NSE) >20ng/mL (79.5% vs 66.1%, P = 0.041), received anlotinib as second-line and above treatment (65.4% vs 46.8%, P = 0.010), and without radiotherapy (82.1% vs 65.3%, P = 0.010).
 |
Table 1 Baseline Clinical Characteristics of Patients |
Overall Efficacy
The median PFS of all patients was 4.8 months (95% CI 3.9–5.7), and the median OS was 7.6 months (95% CI 6.5–8.7) (Figure 1A and Figure 1B). Among them, 61 (30.2%) patients achieved PR, 115 (56.9%) patients achieved SD, and 26 (12.9%) patients achieved PD. Respectively, the ORR and DCR were 30.2% and 87.1%. The waterfall plot of tumor best response compared with measurable baseline lesions is shown in Figure 1C. We performed univariate analysis to identify potential factors associated with PFS and OS in all patients (Table S1) and included statistically significant factors (P < 0.05) and baseline imbalance factors into multivariate Cox regression analysis. Multivariate Cox regression analysis revealed that sex (male vs female: HR = 1.65, 95% CI 1.05–2.59, P = 0.030), ECOG PS (≥2 vs 0–1: HR = 2.99, 95% CI 1.94–4.61, P < 0.001), T stage (T3-4 vs T1-2: HR = 1.51, 95% CI 1.06–2.17, P = 0.024), number of treatment lines (≥2 vs 1: HR = 1.43, 95% CI 1.00–2.05, P = 0.049), plus chemotherapy/immunotherapy (yes vs no: HR = 0.49, 95% CI 0.34–0.70, P < 0.001), plus radiotherapy (yes vs no: HR = 0.62, 95% CI 0.40–0.95, P = 0.027), and post-medication hypertension (yes vs no: HR = 0.36, 95% CI 0.24–0.52, P < 0.001) were the independent influencing factors of PFS (Table 2). Smoking status (ever vs never: HR = 2.09, 95% CI 1.24–3.52, P = 0.006), ECOG PS (≥2 vs 0–1: HR = 4.32, 95% CI 2.65–7.04, P < 0.001), plus chemotherapy/immunotherapy (yes vs no: HR = 0.48, 95% CI 0.32–0.72, P < 0.001), plus radiotherapy (yes vs no: HR = 0.57, 95% CI 0.35–0.91, P = 0.019), and post-medication hypertension (yes vs no: HR = 0.26, 95% CI 0.17–0.41, P < 0.001) were the independent influencing factors of OS (Table 3). The Kaplan–Meier survival curves of PFS and OS in multivariate Cox regression analysis are presented in Figures S1 and S2.
 |
Table 2 Multivariate Cox Regression Analysis of Factors Associated with PFS in All Patients |
 |
Table 3 Multivariate Cox Regression Analysis of Factors Associated with OS in All Patients |
Efficacy in Monotherapy and Combination Groups
The median PFS was 6.0 months (95% CI 5.2–6.8) in combination group and 3.6 months (95% CI 2.9–4.3) in monotherapy group (HR = 0.49, 95% CI 0.34–0.70, P < 0.001) (Figure 2). Moreover, combination group had more favorable 6-month PFS rate (46.1% vs 20.8%, P = 0.001). The HRs for PFS were less than 1.00 across all subgroups (Figure 3). However, the upper boundaries of the 95% CIs crossed 1.00 in hepatic metastases and 10mg of anlotinib initial dose subgroups. The interaction test indicated that the treatment efficacy only varied significantly across the subgroup of sex (P = 0.047).
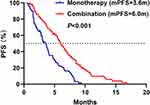 |
Figure 2 The PFS in monotherapy and combination groups. Abbreviation: PFS, progression-free survival. |
The median OS was 9.2 months (95% CI 7.5–10.9) in combination group and 4.8 months (95% CI 3.7–5.9) in monotherapy group (HR = 0.48, 95% CI 0.32–0.72, P < 0.001) (Figure 4). Remarkably, the 6-month OS rate was higher in combination group (69.5% vs 39.4%, P < 0.001). However, there was no statistical difference in the 1-year OS rate between two groups (24.1% vs 14.9%, P = 0.163). The HRs for OS were less than 1.00 across all subgroups (Figure 5), yet with the upper limits of the 95% CIs crossing 1.00 in age ≥65, ECOG PS 0–1, T1-2 stage, TNM III stage, hepatic metastases, baseline NSE ≤20ng/mL, and second-line and above treatment subgroups. The interaction test revealed that the treatment efficacy varied significantly across the subgroups of sex (P = 0.013) and the number of treatment lines (P = 0.028). Combination group had longer OS benefit, especially in the first-line treatment.
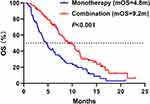 |
Figure 4 The OS in monotherapy and combination groups. Abbreviation: OS, overall survival. |
In combination group, 51 (41.1%) patients achieved PR, 63 (50.8%) patients achieved SD, and 10 (8.1%) patients achieved PD. In monotherapy group, 10 (12.8%) patients achieved PR, 52 (66.7%) patients achieved SD, and 16 (20.5%) patients achieved PD. No patients achieved CR in two groups. The ORR and DCR were significantly higher in combination group than that in monotherapy group (ORR 41.1% vs 12.8%, P < 0.001; DCR 91.9% vs 79.5%, P = 0.010) (Table 4).
 |
Table 4 Comparison of Efficacy Between Monotherapy and Combination |
In the first-line treatment, patients in combination group achieved longer median PFS (6.1 vs 3.6 months, P < 0.001) and median OS (10.5 vs 4.1 months, P < 0.001) compared with monotherapy group (Figure 6A and B). In addition, the 6-month PFS rate (50.0% vs 23.1%, P = 0.021), 6-month OS rate (74.1% vs 32.0%, P < 0.001), and 1-year OS rate (29.5% vs 8.0%, P = 0.037) were also significantly higher in the combination group. In combination group, 38 (57.6%) patients achieved PR, 22 (33.3%) patients achieved SD, and 6 (9.1%) patients achieved PD. In monotherapy group, 6 (22.2%) patients achieved PR, 14 (51.9%) patients achieved SD, and 7 (25.9%) patients achieved PD. The ORR and DCR were significantly higher in combination group than monotherapy group (ORR 57.6% vs 22.2%, P = 0.002; DCR 90.9% vs 74.1%, P = 0.042) (Table S2). In this study, 58 patients received anlotinib plus EP/EC chemotherapy as first-line treatment, the median PFS was 6.0 months (95% CI 3.5–8.5) (Figure S3A), the median OS was 10.5 months (95% CI 8.7–12.3) (Figure S3B), the ORR was 58.6%, and the DCR was 89.7%.
In the second-line and above treatment, patients in combination group had longer median PFS (5.7 vs 3.5 months, P = 0.002) (Figure 6C). The 6-month PFS rate (41.3% vs 19.6%, P = 0.023) and 6-month OS rate (63.8% vs 43.5%, P = 0.049) were also higher in combination group. Although the median OS (7.1 vs 4.8 months, P = 0.068) (Figure 6D) was longer in combination group, it did not have statistical difference. The 1-year OS rate (17.9% vs 19.0%, P = 0.899) was similar between the two groups. In combination group,13 (22.4%) patients achieved PR, 41 (70.7%) patients achieved SD, and 4 (6.9%) patients achieved PD. In monotherapy group, 4 (7.8%) patients achieved PR, 38 (74.6%) patients achieved SD, and 9 (17.6%) patients achieved PD. The ORR was higher in combination group than monotherapy group (22.4% vs 7.8%, P = 0.036). But there was no statistical difference in DCR (93.1% vs 82.4%, P = 0.084) (Table S2).
Safety
The incidence of treatment-related adverse reactions was 65.8% (133/202), and the incidence of grade 3 and above adverse reactions was 7.9% (16/202) among the participants. Dose reductions due to adverse reactions were required for 38 (18.8%) patients, and 27 (13.4%) patients discontinued the treatment. There were no treatment-related deaths in this research. The incidence of adverse reactions in combination group was higher than that in monotherapy group (75.0% vs 52.6%, P = 0.001), but the incidence of grade 3 and above adverse reactions was similar between the two groups (8.1% vs 7.7%, P = 0.924). Furthermore, the most common adverse reaction was hypertension, followed by hand-foot syndrome and fatigue, regardless of monotherapy or combination groups. The adverse reactions are listed in Table 5.
 |
Table 5 Summary of Adverse Reactions |
Discussion
SCLC has an abnormally high proliferation rate, a strong tendency for early metastasis, and a bleak prognosis.2 Although the response rate to first-line chemotherapy is high, most patients cannot escape from the destiny of disease progression or relapse. The follow-up treatment of SCLC is limited. More effective therapies and drugs in SCLC are urgently needed. Angiogenesis serves a pivotal role in tumor occurrence, invasion, and metastasis.14 However, the efficacy of antiangiogenic therapy in SCLC is limited, such as bevacizumab, sorafenib, sunitinib and so on, except for anlotinib.15–18 The ALTER 1202 study revealed that anlotinib could improve the prognosis of SCLC patients as third-line and above treatment.11 Moreover, some small sample clinical trials found that the efficacy of anlotinib combined with chemotherapy was better than chemotherapy alone.12,19,20 Nevertheless, the real-world efficacy and safety of anlotinib in ES-SCLC are still worthy of further exploration. Therefore, we conducted this multicenter real-world study to address this issue.
In this study, 38.6% of patients used anlotinib alone and 61.4% combined with other therapies. The combination group achieved more extended survival benefits and better tumor response than monotherapy group, especially in the first-line treatment. Multivariate Cox regression analysis showed that combination therapy was the independent influencing factor of PFS and OS. Antiangiogenic therapy can improve drug delivery efficiency by opening the vascular normalization window, thus exerting a synergistic effect when combined with other regimens.21 Yang et al reported that anlotinib and programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) antibodies acted synergistically to provide therapeutic benefit by promoting infiltration of natural killer cells, M1-like tumor-associated macrophages (TAM), and dendritic cells while reducing the infiltration of M2-like TAM.22 Another study showed that anlotinib reduced PD-L1 expression on vascular endothelial cells via inactivation of AKT pathway, which then leads to increase in the CD8/FoxP3 ratio in the tumor immune microenvironment.23 It appears that anlotinib changes the immunosuppressive tumor microenvironment to an immune permissive status and thus enhances the efficacy of PD-1/PD-L1 antibody. Furthermore, the combination of anlotinib with chemotherapy or target therapy has been shown to have synergistic efficacy.24,25 In addition, the non-overlapping toxicity spectrum and excellent tolerance of anlotinib allow it to be used in combination with other drugs. Our study further confirmed the superiority of anlotinib combination therapy.
ES-SCLC patients first receive chemotherapy to control the spread of metastasis. Subsequently, chest radiotherapy is recommended to control local lesions for patients who achieve CR or PR after chemotherapy.26 Some studies found that antiangiogenic therapy can increase the local oxygen partial pressure and oxygen content of tumor tissue, inhibit the angiogenesis induced by radiotherapy, and play the role of radiotherapy sensitization.27 In our study, patients combined with radiotherapy had longer median PFS (7.4 vs 3.9 months, P = 0.027) and OS (12.1 vs 5.6 months, P = 0.019). Hypertension and hand-foot syndrome are the most common adverse reactions treated with anlotinib. Interestingly, more extended PFS benefits were observed in patients with post-medication hypertension or hand-foot syndrome in Song PF’s study.28 Consistently, our study also found that post-medication hypertension was an independent influencing factor of PFS (7.1 vs 3.0 months, P < 0.001) and OS (12.0 vs 4.9 months, P < 0.001), while hand-foot syndrome was not associated with prognosis. Additionally, multivariate Cox regression analysis also found that patients with ECOG PS ≤ 1 had more favorable PFS (6.7 vs 2.4 months, P < 0.001) and OS (11.0 vs 3.6 months, P < 0.001). Furthermore, we observed that sex and T stage were associated with PFS, and smoking status was associated with OS by multivariate Cox regression analysis. But only 38 (18.8%) female patients were included in our study, which might influence the result.
Our research provided real-world data on the first-line and second-line treatment of anlotinib monotherapy in ES-SCLC firstly. In monotherapy group, the median PFS of the first-line, second-line, and third-line and above treatment was 3.6, 3.8, and 3.1 months (Figure S4A). The median OS of the first-line, second-line, and third-line and above treatment was 4.1, 5.0, and 4.2 months (Figure S4B). In first-line treatment, the median PFS was similar to second-line treatment, but the median OS was shortest compared with second-line and third-line and above treatment. It can be explained that most patients with age ≥65 and aCCI >8 were included in the first-line treatment. These patients were unwilling or unable to receive chemotherapy or immunotherapy, so that they received anlotinib alone as the first-line treatment. Han et al reported the result of a phase II clinical trial with sunitinib as second-line treatment in ES-SCLC. The median PFS was 1.4 months, and the median OS was 5.6 months.17 Most patients were unable to tolerate the therapy. The median PFS of patients using nintedanib as second-line treatment was 1.0 months, and the median OS was 9.8 months in a phase II study.29 Our study found that the PFS of patients using anlotinib was prolonged compared with sunitinib and nintedanib. Treatment-related adverse reactions were tolerable and manageable. Anlotinib may be a suitable choice for patients who are elderly, cannot tolerate chemotherapy or are unwilling to receive chemotherapy.
In ALTER 1202 study, 121 ES-SCLC patients used anlotinib or placebo as third-line and above treatment. The median PFS was 4.1 months in anlotinib arm and only 0.7 months in placebo arm, respectively (HR = 0.19, 95% CI 0.12–0.32, P < 0.001). The ORR and DCR in the anlotinib group were 4.9% and 71.6%. The median OS was 7.3 months in the anlotinib group compared to 4.9 months in the placebo group.11 In this real-world study, we revealed a shorter median PFS of 3.1 months and a shorter median OS of 4.2 months compared with the results from ALTER 1202 trial. The ORR and DCR were 10.5% and 78.9%, better than the results of ALTER 1202 study. We speculated that the following reasons might cause the above difference. First and foremost, 88.9% of patients treated with anlotinib in the ALTER 1202 study were with ECOG PS ≤ 1, while our study only included 31.6% of patients with ECOG PS ≤ 1, and the sample size was limited. Secondly, due to the non-intervention and retrospective study design in our study, the treatment effect might be affected by many factors, such as economic status, adverse reactions, compliance and so on. Our result of the primary endpoint was similar to other real-world studies.28,30 This research further confirmed that anlotinib could be a viable treatment option for ES-SCLC patients who have experienced multiple-line therapy failure.
Some small sample size phase II clinical trials have shown the superiority of anlotinib combined with EP/EC chemotherapy as first-line treatment for ES-SCLC. In 2021 ASCO meeting, the result by Kong et al showed that the median PFS was 10.0 months, the median OS was 15.0 months, the ORR was 90%, and the DCR was 100%12 of 20 ES-SCLC patients. Supported by these encouraging preliminary results, Phase III clinical trials of anlotinib combined with EP/EC chemotherapy in treatment-naive SCLC have already begun in China. In our study, 58 patients received anlotinib plus EP/EC chemotherapy as first-line treatment. The median PFS was 6.0 months, the median OS was 10.5 months, the ORR was 58.6%, and the DCR was 89.7%. Our results are similar to previous clinical trials of EP/EC chemotherapy as first-line treatment. Since there is no control group in our study, whether the efficacy of anlotinib combined with EP/EC chemotherapy is better than chemotherapy alone requires further verification by prospective studies with larger sample size.
In this research, either treating with anlotinib alone or combined with other regimens was generally well tolerated. The incidence of adverse reactions in combination group was higher than monotherapy group, but the incidence of grade 3 and above adverse reactions was similar. Similar to previous research, hypertension was the most frequent adverse reaction, followed by hand-foot syndrome and fatigue.31 As one of the serious adverse reactions of anlotinib, hemorrhage could be fatal. In our study, 30 (14.9%) patients with hemorrhage were reported, and the bleeding stopped after anlotinib was discontinued. No patients died of the treatment of anlotinib. The grade 3 and above adverse reactions were manageable with dose reduction or drug discontinuation.
This study provides real-world data of anlotinib in the treatment of ES-SCLC, especially for combination therapy and monotherapy as first-line and second-line treatment at the first time. However, as a retrospective study, our research still has some unavoidable limitations. First, as a real-world study, the Chinese undiversified population and small sample size might affect the universality of the results. Thus, future study with larger sample size is needed. Second, there were many confounding factors in this study, including different forms of previous therapy, ECOG PS, aCCI and so on, which might influence the actual efficacy and safety of anlotinib. A certain degree of heterogeneity was in clinical characteristics between monotherapy group and combination group. However, we used the multivariate Cox regression analysis to adjust for these confounding factors. Due to the problems of cross-line and unbalanced sample size, we did not compare the efficacy between different combined treatment schemes. Last but not least, the incidence of adverse reactions in this research might be lower than actual data in the real world because of the bias of the retrospective study.
Conclusion
Anlotinib appears to be well tolerated and effective in patients with ES-SCLC in the real-world. The clinical efficacy of anlotinib combined with chemotherapy or immunotherapy is better than that of monotherapy, especially in the first-line treatment. In addition, plus radiotherapy can make patients have longer PFS and OS benefits. Anlotinib alone may be a suitable choice for patients with advanced age, unable or unwilling to receive chemotherapy or immunotherapy. The incidence of adverse reactions of combination is slightly higher than that of monotherapy. The most common adverse reaction is hypertension. Patients with post-medication hypertension may have longer PFS and OS benefits.
Data Sharing Statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethical Approval and Informed Consent
This study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2019LSK-132) and conformed to the provisions of the Declaration of Helsinki. Since the study is a retrospective study, it did not damage the health and interests of patients, and protected their privacy and personal information, the study is exempted from informed consent. The consent waiver was approved by IRB.
Consent for Publication
Informed consent was obtained from the authors.
Acknowledgments
We would like to thank Xijing Hospital of Air Force Military Medical University, Xianyang Central Hospital, Shaanxi Nuclear Industry 215 Hospital, Hanzhong Central Hospital and Baoji Traditional Chinese Medicine Hospital for providing the clinical data.
Funding
This study was funded by the International Cooperation Project in Science and Technology of Shaanxi Province (No. 2019KW-074), Nation Natural Science Funding of China (No. 82002437), and Shaanxi Province Technology Innovation Team (No. 2021TD-44).
Disclosure
The authors declare that they have no competing interests.
References
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi:10.1056/NEJMra0802714
2. Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. doi:10.1038/s41572-020-00235-0
3. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. doi:10.1186/s13045-019-0736-3
4. Reck M, Horn L, Novello S, et al. Phase II study of roniciclib in combination with cisplatin/Etoposide or carboplatin/Etoposide as first-line therapy in patients with extensive-disease small cell lung cancer. J Thorac Oncol. 2019;14(4):701–711. doi:10.1016/j.jtho.2019.01.010
5. Esposito G, Palumbo G, Carillio G, et al. Immunotherapy in small cell lung cancer. Cancers. 2020;12(9). doi:10.3390/cancers12092522.
6. Garst J. Topotecan: an evolving option in the treatment of relapsed small cell lung cancer. Ther Clin Risk Manag. 2007;3(6):1087–1095.
7. De Jong WK, Ten Hacken NH, Groen HJ. Third-line chemotherapy for small cell lung cancer. Lung Cancer. 2006;52(3):339–342. doi:10.1016/j.lungcan.2006.02.005
8. Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669
9. Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4(9):1094–1103. doi:10.1097/JTO.0b013e3181a97e31
10. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
11. Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. 2021;125(3):366–371. doi:10.1038/s41416-021-01356-3
12. Kong TD, Chen L, Duan FF, et al. Efficacy and safety analysis of anlotinib combined with etoposide plus cisplatin/carboplatin as first-line therapy for extensive-stage small cell lung cancer (SCLC): the final results from a phase II single-arm trial. J Clin Oncol. 2021;39(15):8560. doi:10.1200/JCO.2021.39.15_suppl.8560
13. Nabhan C, Klink A, Prasad V. Real-world evidence-What does it really mean?. JAMA Oncol. 2019;5(6):781–783. doi:10.1001/jamaoncol.2019.0450
14. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
15. Pujol JL, Lavole A, Quoix E, et al. Randomized Phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial†. Ann Oncol. 2015;26(5):908–914. doi:10.1093/annonc/mdv065
16. Sharma N, Pennell N, Nickolich M, et al. Phase II trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs. 2014;32(2):362–368. doi:10.1007/s10637-013-0061-6
17. Han JY, Kim HY, Lim KY, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer. 2013;79(2):137–142. doi:10.1016/j.lungcan.2012.09.019
18. Montanino A, Manzo A, Carillio G, et al. Angiogenesis inhibitors in small cell lung cancer. Front Oncol. 2021;11:655316. doi:10.3389/fonc.2021.655316
19. Deng P, Yang H, Chen C, et al. The efficacy and safety profile of anlotinib with etoposide plus cisplatin/carboplatin in treatment-naive extensive-stage small cell lung cancer(SCLC) patients: results from a phase II single-arm trial. J Clin Oncol. 2020;38(15):9066. doi:10.1200/JCO.2020.38.15_suppl.9066
20. Han B, Zhang W, Zhang B, et al. Anlotinib plus etoposide and carboplatin as first-line treatment for extensive-stage small cell lung cancer: a single arm phase II trial. J Thorac Oncol. 2021;16(3):S503–S503. doi:10.1016/j.jtho.2021.01.879
21. Alshangiti A, Chandhoke G, Ellis PM. Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol. 2018;25(Suppl 1):S45–s58. doi:10.3747/co.25.3747
22. Yang Y, Li L, Jiang Z, et al. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi:10.1007/s00262-020-02641-5
23. Liu S, Qin T, Liu Z, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11(5):309. doi:10.1038/s41419-020-2511-3
24. Wang HY, Chu JF, Zhao Y, et al. A trial of the safety and efficacy of chemotherapy plus anlotinib vs chemotherapy alone as second- or third-line salvage treatment for advanced non-small cell lung cancer. Cancer Manag Res. 2020;12:3827–3834. doi:10.2147/CMAR.S249678
25. Li T, Qian Y, Zhang C, et al. Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo. Transl Lung Cancer Res. 2021;10(4):1873–1888. doi:10.21037/tlcr-21-192
26. Picardi C, Caparroti F, Di Maio M, et al. Prophylactic cranial irradiation in extensive disease small cell lung cancer: an endless debate. Crit Rev Oncol Hematol. 2019;143:95–101. doi:10.1016/j.critrevonc.2019.08.010
27. Ansiaux R, Dewever J, Grégoire V, et al. Decrease in tumor cell oxygen consumption after treatment with vandetanib (ZACTIMA; ZD6474) and its effect on response to radiotherapy. Radiat Res. 2009;172(5):584–591. doi:10.1667/RR1744.1
28. Song PF, Xu N, Li Q. Efficacy and safety of anlotinib for elderly patients with previously treated extensive-stage SCLC and the prognostic significance of common adverse reactions. Cancer Manag Res. 2020;12:11133–11143. doi:10.2147/CMAR.S275624
29. Han JY, Kim HY, Lim KY, et al. A phase II study of nintedanib in patients with relapsed small cell lung cancer. Lung Cancer. 2016;96:108–112. doi:10.1016/j.lungcan.2016.04.002
30. Gao X, Peng L, Zhang L, et al. Real-world efficacy and safety of anlotinib as third- or further-line treatment in refractory small cell lung cancer. J Cancer Res Clin Oncol. 2021. doi:10.1007/s00432-021-03848-4
31. Syed YY. Anlotinib: first global approval. Drugs. 2018;78(10):1057–1062. doi:10.1007/s40265-018-0939-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




