Back to Journals » Journal of Inflammation Research » Volume 16
Utilizing the Lactate Dehydrogenase-to-Albumin Ratio for Survival Prediction in Patients with Bladder Cancer After Radical Cystectomy
Authors Xu H, Lin T , Ai J , Zhang J, Zhang S, Li Y, Zheng X, Zhang P, Wei Q , Tan P, Yang L
Received 6 August 2022
Accepted for publication 18 January 2023
Published 18 April 2023 Volume 2023:16 Pages 1733—1744
DOI https://doi.org/10.2147/JIR.S384338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Ning Quan
Hang Xu,1,2,* Tianhai Lin,1,2,* Jianzhong Ai,1,2,* Jiapeng Zhang,1,2 Shiyu Zhang,1,2 Yifan Li,1,2 Xiaonan Zheng,1,2 Peng Zhang,1,2 Qiang Wei,1,2 Ping Tan,1,2 Lu Yang1,2
1Department of Urology, West China Hospital, Sichuan University, Sichuan, People’s Republic of China; 2Institute of Urology, West China Hospital, Sichuan University, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Tan; Lu Yang, Department of Urology, West China Hospital, Sichuan University, Sichuan, People’s Republic of China, Tel +86 18980606829 ; +86 15208212056, Email [email protected]; [email protected]
Purpose: Previous studies have suggested that the preoperative lactate dehydrogenase-to-albumin ratio (LAR) is correlated with survival in several cancers except bladder cancer (BCa). This study aimed to determine the prognostic value of the LAR in patients with urothelial carcinoma of the bladder (UCB) after radical cystectomy (RC).
Patients and Methods: A total of 595 UCB patients with RC in West China Hospital from December 2010 to May 2020 were enrolled. A receiver operating characteristic (ROC) curve was used to determine the optimal cutoff value of the LAR. Kaplan–Meier curves and Cox regression analyses were applied to evaluate the association of the LAR with overall survival (OS) and recurrence-free survival. Independent factors in multivariate analyses were selected to construct nomograms. Calibration curves, ROC curves, concordance index (C-index) and decision curve analyses were used to evaluate the performance of the nomograms.
Results: The optimal cutoff value of the LAR was determined to be 3.8. Preoperative low LAR was associated with decreased OS (P < 0.001) and RFS (P < 0.001), especially in patients with ≥ pT2 disease. LAR was an independent factor for OS (hazard ratio [HR]: 1.719; P < 0.001) and RFS (HR: 1.429; P = 0.012). The addition of the LAR into nomograms could result in better prediction performance. The areas under the curves of the nomograms were 0.821 and 0.801 for the prediction of 3-year OS and RFS, respectively. The C-indexes of the nomograms were 0.760 and 0.741 for the prediction of OS and RFS, respectively.
Conclusion: The preoperative LAR is a novel and reliable independent prognostic biomarker for survival in UCB after RC.
Keywords: lactate dehydrogenase, albumin, lactate dehydrogenase to albumin ratio, bladder cancer, prognosis
Introduction
Bladder cancer (BCa) ranks as the tenth most commonly diagnosed malignant tumor in all populations globally and the fourth most prevalent cancer in America.1,2 The estimated number of new cases was 81,180, and the estimated number of new deaths will reach 17,100 in 2022.3 Radical cystectomy (RC) with urinary diversion represents the gold-standard treatment modality for localized muscle-invasive bladder cancer (MIBC) and selected non-muscle-invasive bladder cancer (NMIBC) patients,4,5 and it is also viable in the elderly patients aged above 85 years.6 Despite the development of new technologies and therapeutic drugs, the 5-year relative survival rate is 77% in all stages and decreases to 14% in those diagnosed with stage IV disease.1,7 Therefore, utilizing preoperative factors to predict survival endpoints is helpful for clinical decisions and postoperative interventions.
Several preoperative parameters have been studied in BCa, such as the neutrophil-to-lymphocyte ratio,8 lymphocyte-to-monocyte ratio,9 prognostic nutritional index,10 and controlling nutritional status score.11 Lactate dehydrogenase (LDH), an enzyme involved in the glycolytic pathway, is reported to participate in carcinogenesis, the immune microenvironment and cancer progression.12 A pretreatment high LDH level is correlated with poor survival in cancer.13 In addition, the serum albumin level, an indicator of nutritional status, is correlated with prognosis in BCa.14 However, a combination of LDH and albumin, named the lactate dehydrogenase-to-albumin ratio (LAR), which reflects both inflammation and nutrition status, has not been investigated in BCa.
Therefore, this study aimed to probe the prognostic significance of the preoperative LAR for urothelial carcinoma of the bladder (UCB) prognosis. Moreover, we also tried to evaluate whether the addition of the LAR would improve the performance of our nomograms for survival prediction.
Materials and Methods
Population
Patients with BCa who underwent RC at West China Hospital, Sichuan University, were retrospectively reviewed between December 2010 and May 2020. A total of 1175 consecutive patients who received RC at our institution were initially retrieved. We excluded the following patients: 1) metastatic disease (17 patients); 2) neoadjuvant therapy (40 patients); 3) main histology was not UCB (36 patients); 4) missing data on pathological information (163 patients), perioperative LDH/albumin levels (97 patients) or other required clinical information (129 patients); and 5) lost to follow-up (98 patients). Finally, a total of 595 patients were included. Information on LDH and albumin was obtained within 1 week before RC. This study obtained approval from the Ethics Committee of West China Hospital, Chengdu, Sichuan, China. In addition, we adhered to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The method of RC, which included open, laparoscopic and robot-assisted surgery accompanied by urinary diversion, was chosen by the surgeon’s discretion. In addition, whether lymph node dissection was performed was decided by the surgeons.
Patient information was acquired from the medical charts. Pathological tumor grade was determined by the 2004/2016 World Health Organization (WHO) classification.2 Pathological tumor stage was determined according to the 2017 American Joint Committee on Cancer (AJCC) cancer staging manual.15 Variant histological subtypes were defined according to the 2016 WHO Classification.16,17 Pathologic information was independently evaluated by two experienced pathologists.
LAR
LAR was computed as LDH (U/L)/serum albumin (g/L). Receiver operating characteristic (ROC) curves were applied to assess the optimal cutoff values of LDH, albumin and LAR. We calculated the Youden index, which was calculated as “sensitivity + specificity – 1”, and we selected the optimal cutoff value based on the maximum Youden index.
Follow-Up and Outcomes
After RC, patients were regularly followed every three months in the first year, twice a year in the next two or three years and once a year thereafter. Overall survival (OS) and disease recurrence-free survival (RFS) were the primary outcomes in this study. Disease recurrence was defined as local recurrence or distant metastasis.
Statistical Analysis
Patients were divided into two groups based on the optimal cutoff value of the LAR. Continuous variables are presented as medians with interquartile ranges (IQRs). The chi-squared test, Student’s t test or Wilcoxon’s rank-sum test were applied as appropriate. Kaplan–Meier curves with the Log rank test were applied to assess the impact of the LAR on OS and RFS. In addition, subgroup analysis based on tumor stage was performed. Variables with p values less than 0.1 in univariate Cox analysis were included in multivariate Cox analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed. Predictors with a P value < 0.05 in multivariate analyses were used to construct nomograms for 3-year and 5-year OS/RFS prediction. The performance of the nomogram was assessed using calibration curves, Harrell’s concordance index and time-dependent ROC curves. Decision curve analyses were used to evaluate the net benefit of the nomograms. A P value less than 0.05 on both sides was deemed statistically significant. Statistical analyses were conducted using SPSS 25.0 and R (Version 3.6.3).
Results
Patient Clinical Features
A total of 595 patients met our inclusion criteria. The clinical features of the patients are presented in Table 1. There were 507 males and 88 females, and the median age was 65 years (IQR, 58–72). A significant proportion of patients had high-grade disease (87.2%), and 346 (58.2%) patients were diagnosed with ≥ pT2 disease. Positive surgical margins were found in only 37 (6.2%) patients. Concomitant variant histology was found in 117 (19.7%) patients, of which squamous (7.7%) and glandular differentiation (7.4%) were the main variant histological subtypes (Table S1). The median follow-up was 40 months. The median LDH was 150 U/L, and the median albumin level was 41 g/L. The ROC curves showed that the prediction of OS was higher based on the LAR than based on LDH or albumin alone (Figure S1). The Youden index was 0.270, 0.174 and 0.297 for LDH, albumin and LAR, respectively (Table S2). The optimal cutoff value was determined to be 3.8. Consequently, 345 (58.0%) patients were divided into the low LAR group, and 250 (42.0%) patients were divided into the high LAR group. No significant differences were found between groups regarding body mass index (BMI), sex, hypertension, diabetes, tumor grade, surgical margins, variant histology or adjuvant therapy. Nevertheless, we observed marked discrepancies with regard to age, smoking status, surgical approach, tumor stage, LDH and albumin levels (Table 1).
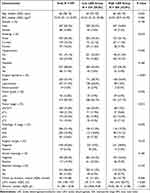 |
Table 1 Study Characteristics |
LAR and OS/RFS
Kaplan–Meier analyses demonstrated that a high preoperative LAR was correlated with decreased OS (log-rank P < 0.001, Figure 1A) and RFS (log-rank P = 0.002, Figure 1B). In addition, we performed subgroup analysis based on tumor stage. We observed that the prognostic significance of the LAR for OS (Figure 1C) and RFS (Figure 1D) did not exist in patients with < pT2 disease. In contrast, it remained a prognostic factor for OS (Figure 1E) and RFS (Figure 1F) in patients with ≥ pT2 disease. Univariate Cox regression analysis demonstrated that the LAR was linked to poor OS (HR: 2.141; 95% CI: 1.641–2.794; P < 0.001; Table 2) and poor RFS (HR: 1.743; 95% CI: 1.330–2.283; P < 0.001; Table 3). When confounding predictors were adjusted in multivariate Cox regression analysis, we found that a high preoperative LAR independently predicted decreased OS (HR: 1.719; 95% CI: 1.306–2.262; P <0.001; Table 2) and RFS (HR: 1.429; 95% CI: 1.082–1.886; P = 0.012; Table 3).
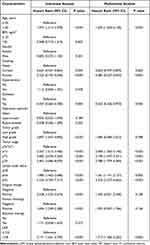 |
Table 2 Univariate and Multivariate Cox Regression Analysis for Overall Survival |
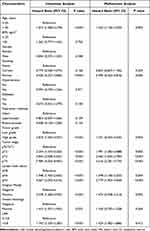 |
Table 3 Univariate and Multivariate Cox Regression Analysis for Recurrence-Free Survival |
Nomogram Construction and Performance
Based on the results from the multivariate Cox analyses, six variables (including age, smoking, diabetes, tumor stage, lymph node status and LAR) were selected to build nomograms for 3-year and 5-year OS prediction (Figure 2A), and five variables (including age, smoking, tumor stage, lymph node status and LAR) were selected to build nomograms for 3-year and 5-year RFS prediction (Figure 2B). The calibration curves revealed that the predicted probabilities of the nomograms matched the actual observations in terms of 3-year or 5-year OS (Figure 2C) and RFS (Figure 2D). Next, we calculated the C-index to assess the predictive accuracies of the nomograms (Table 4). The addition of the LAR improved the predictive accuracies to 0.760 for OS and 0.741 for RFS (Table 4).
 |
Table 4 C- Index of the Nomogram for the Prediction of Survival Outcomes |
We further used time-dependent ROC curves to evaluate the prediction accuracies of the nomograms. The results showed that the nomograms had sound performance in predicting OS and RFS. The AUCs were 0.821 and 0.801 for 3-year OS (Figure 3A) and RFS (Figure 3B), respectively. Moreover, the clinical utility of the nomograms was evaluated through decision curve analyses. For 3-year and 5-year OS prediction, in the comparison of LAR or tumor stage, our nomograms significantly improved the net benefits at a threshold of between 10% and 80% (Figure 3C and D). For 3-year and 5-year RFS prediction, our nomogram also significantly improved the net benefits at a threshold of between 10% and 80% compared to the LAR or tumor stage (Figure 3E and F).
Discussion
In this study, we proposed a novel biomarker, the LAR, for predicting survival endpoints in UCB. We included a relatively large number (approximately six hundred) of patients from a single institution in China. Our results indicated that a high preoperative LAR was an independent factor for both OS and RFS. Furthermore, we constructed nomograms based on the LAR and demonstrated the predictive accuracies and clinical utilities of our nomograms. To our knowledge, this is the first study investigating the role of the LAR in UCB prognosis prediction.
Deregulating cellular metabolism is a major component of the hallmarks of cancer.18 Aberrant cancer metabolism, such as aerobic glycolysis, plays important roles in cancer biology, including carcinogenesis, inflammation, drug resistance and cancer progression.19 LDH, an enzyme involved in the glycolytic pathway, participates in the conversion of pyruvate to lactic acid. A pretreatment high LDH level is reported to be related to poor survival in cancer.13 In addition, serum albumin is the most abundant protein in human plasma is considered a reflection of individual nutrition status. Malnutrition could predict poor prognosis in patients with cancer.20 In addition, low serum albumin is also reported to be associated with worse bladder cancer prognosis.14 Therefore, a combination of LDH and albumin reflects both the inflammation and nutrition status of an individual and is more powerful than LDH or albumin alone in survival prediction.
Numerous prognostic factors, including hematologic, physiological, molecular and pathological markers, have been explored to be associated with survival outcomes in UCB patients after RC.21–28 Compared with these molecular and pathological predictors, the LAR is relatively cost-effective and easily accessed, and preoperative interventions might be administered for those at high risk of disease recurrence or death (with high LAR). With regard to novel hematologic indexes based on albumin levels, the albumin-to-blood glucose ratio,27 albumin-to-fibrinogen ratio,28 and albumin-globulin ratio29 have been reported to be prognostic factors for oncological outcomes in BCa. In addition, other hematological biomarkers, such as hemoglobin and C-reactive protein, have also been proven to be independent predictors in BCa after RC.30 Compared with these studies, we not only proposed the LAR, another novel index based on LDH and albumin levels, as an independent prognostic biomarker for worse OS and RFS in patients with UCB after RC but also demonstrated the clinical usefulness of integrating this novel index into prognostic models by constructing nomograms.
In our study, we showed that the Youden index was much higher in the LAR group (0.297) than in the LDH (0.270) or albumin (0.174) groups (Table S2). The prognostic role of the LAR has been reported in several cancers. In hepatocellular carcinoma, Gan et al included 1041 patients and found that preoperative LAR was an independent factor for both OS and RFS.31 In pancreatic cancer, Gan et al enrolled 139 patients treated with intervention chemotherapy and found that a high LAR was associated with poor OS.32 In esophageal squamous cell carcinoma, Feng et al enrolled 346 patients and found that the LAR was an independent factor for cancer-specific survival.33 Thereafter, the prognostic significance of the LAR was validated in nasopharyngeal carcinoma,34,35 gastric cancer,36 and colorectal cancer.37,38 In line with previous findings, our study provided additional evidence that the LAR could serve as an independent prognostic parameter in UCB, and the addition of the LAR to the prognostic nomograms would increase the predictive accuracies and clinical utilities.
It should be noted that the cutoff value of the LAR varied across studies. Different cutoff values of LAR were determined, including 5.5,31,33 6.54,32 4.04,34 4.5,37 4.9138 and 3.67.35 In our study, this value was 3.8. Discrepancies in tumor types, sample sizes, geographic regions, outcome measurements, laboratory equipment and definition methods might explain this diversity.
Limitations should be noted as well. First, this study was a single-center retrospective study without external validation, even though it had a relatively large number of cases, which might lead to potential bias regarding the optimal threshold for LAR evaluation. The retrospective nature of our study might contribute to selection bias as well. Second, even though we proposed and evaluated the nomograms through multiple methods to predict 3-year and 5-year survival outcomes in UCB, the application of these nomograms in the clinic warrants external validation. Third, cancer-specific survival was not assessed in this study because it was difficult to define whether it was cancer-related death (telephone was the major source of our follow-up methods). Fourth, we excluded patients who received neoadjuvant chemotherapy considering its potential influence on LDH and albumin levels. The impact of neoadjuvant chemotherapy on UCB prognosis will be explored in our further prospective studies. Finally, we only assessed the LAR prior to surgery instead of monitoring the dynamic change in this index throughout the cancer treatment. This might be accomplished in our future studies.
Conclusion
In summary, our study confirmed the preoperative LAR as an independent prognostic biomarker in UCB. The addition of this cost-effective and reliable biomarker into the prognostic nomograms of UCB could bring more net benefits for OS and RFS than nomograms alone. Further studies are warranted to validate our results.
Abbreviations
BCa, bladder cancer; UCB, urothelial carcinoma of the bladder; RC, radical cystectomy; MIBC, muscle-invasive bladder cancer; NMIBC, non-muscle-invasive bladder cancer; LDH, lactate dehydrogenase; LAR, lactate dehydrogenase-to-albumin ratio; BMI, body mass index; WHO, World Health Organization; AJCC, American Joint Committee on Cancer; ROC, receiver operating characteristic; OS, overall survival; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; IQR, interquartile range.
Data Sharing Statement
All data generated involved in the research are available from the corresponding author Lu Yang on reasonable request.
Ethics Approval and Consent to Participate
This study obtained approval from the Ethics Committee of West China Hospital, Chengdu, China. The retrospective design enabled this study to be conducted under waiver of informed consent by the local institutional review board. The data has been analyzed anonymously and all personal information of the participants was confidential. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
Hang Xu, Tianhai Lin and Jianzhong Ai are co-first authors. We appreciate Dr. Xin Han (West China Biomedical Big Data Center, West China Hospital, Sichuan University, Sichuan, China) for helping to reanalyze and interpret the data.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070784, 81702536) to J. A., a grant from Science & Technology Department of Sichuan Province, China (2022JDRC0040) to J. A., grants from The National Natural Science Foundation of China (Grant Nos. 81974099, 82170785) to L.Y., a grant from Science and Technology Department of Sichuan Province (Grant Nos. 2021YFH0172) to L.Y., a grant from Young Investigator Award of Sichuan University 2017 (Grant No. 2017SCU04A17) to L.Y., a grant from Sichuan University--Panzhihua science and technology cooperation special fund (2020CDPZH-4) to L.Y., grants from The National Natural Science Foundation of China (Grant Nos. 81974099, 82170785) to Q.W. China Postdoctoral Science Foundation (2021M692306; 2022T150455) and PostDoctor Research Project of West China Hospital of Sichuan University (2021HXBH025) to X.Z.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409.
2. Sylvester RJ, Rodríguez O, Hernández V, et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: an Update from the EAU NMIBC Guidelines Panel. Eur Urol. 2021;79(4):480–488.
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
4. Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94.
5. Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: summary of the 2020 Guidelines. Eur Urol. 2021;79(1):82–104.
6. Tamalunas A, Volz Y, Schlenker BA, et al. Is It Safe to Offer Radical Cystectomy to Patients above 85 Years of Age? A Long-Term Follow-Up in a Single-Center Institution. Urol Int. 2020;104(11–12):975–981.
7. Alexopoulos G, Zhang J, Karampelas I, et al. Long-Term Time Series Forecasting and Updates on Survival Analysis of Glioblastoma Multiforme: a 1975–2018 Population-Based Study. Neuroepidemiology. 2022;56(2):75–89.
8. Herzberg H, Lifshitz K, Golan S, et al. Association between early change in neutrophil-to-lymphocyte ratio after radical cystectomy and treatment outcomes. BJU Int. 2022;72:409.
9. Rajwa P, Życzkowski M, Paradysz A, Bujak K, Bryniarski P. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci. 2018;22(10):3027–3037.
10. Peng D, Gong YQ, Hao H, et al. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival with Bladder Cancer after Radical Cystectomy: a retrospective study. BMC Cancer. 2017;17(1):391.
11. Peng L, Du C, Meng C, et al. Controlling Nutritional Status Score Before Receiving Treatment as a Prognostic Indicator for Patients With Urothelial Cancer: an Exploration Evaluation Methods. Front Oncol. 2021;11:702908.
12. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692.
13. Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep. 2015;5:9800.
14. Li J, Cheng Y, Liu G, Ji Z. The association of pretreatment serum albumin with outcomes in bladder cancer: a meta-analysis. Onco Targets Ther. 2018;11:3449–3459.
15. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
16. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: prostate and Bladder Tumours. Eur Urol. 2016;70(1):106–119.
17. Compérat EM, Burger M, Gontero P, et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur Urol Focus. 2019;5(3):457–466.
18. Hanahan D. Hallmarks of Cancer: new Dimensions. Cancer Discov. 2022;12(1):31–46.
19. Park JH, Pyun WY, Park HW. Cancer Metabolism: phenotype, Signaling and Therapeutic Targets. Cells. 2020;9:10.
20. Bullock AF, Greenley SL, McKenzie GAG, Paton LW, Johnson MJ. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur J Clin Nutr. 2020;74(11):1519–1535.
21. Mertens LS, Claps F, Mayr R, et al. Prognostic markers in invasive bladder cancer: FGFR3 mutation status versus P53 and KI-67 expression: a multi-center, multi-laboratory analysis in 1058 radical cystectomy patients. Urol Oncol. 2022;40(3):110.e111–110.e119.
22. Mori K, Miura N, Mostafaei H, et al. Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25(8):1459–1474.
23. Mayr R, Fritsche HM, Zeman F, et al. Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol. 2018;36(8):1201–1207.
24. Claps F, van de Kamp MW, Mayr R, et al. Risk factors associated with positive surgical margins’ location at radical cystectomy and their impact on bladder cancer survival. World J Urol. 2021;39(12):4363–4371.
25. Claps F, Mir MC, Zargar H. Molecular markers of systemic therapy response in urothelial carcinoma. Asian J Urol. 2021;8(4):376–390.
26. Mir MC, Campi R, Loriot Y, et al. Adjuvant Systemic Therapy for High-risk Muscle-invasive Bladder Cancer After Radical Cystectomy: current Options and Future Opportunities. Eur Urol Oncol. 2021;2:56.
27. Cheng Q, Gu L, Zhao X, et al. A new index (A/G) associated with early complications of radical cystectomy and intestinal urinary diversion. Urol Oncol. 2021;39(5):301.e311–301.e316.
28. Claps F, Rai S, Mir MC, et al. Prognostic value of preoperative albumin-to-fibrinogen ratio (AFR) in patients with bladder cancer treated with radical cystectomy. Urol Oncol. 2021;39(12):835.e839–835.e817.
29. Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):484.e481–484.e488.
30. Tamalunas A, Buchner A, Kretschmer A, et al. Impact of Routine Laboratory Parameters in Patients Undergoing Radical Cystectomy for Urothelial Carcinoma of the Bladder: a Long-Term Follow-Up. Urol Int. 2020;104(7–8):551–558.
31. Gan W, Zhang MX, Wang JX, et al. Prognostic impact of lactic dehydrogenase to albumin ratio in hepatocellular carcinoma patients with Child-Pugh I who underwent curative resection: a prognostic nomogram study. Cancer Manag Res. 2018;10:5383–5394.
32. Gao S, Wu M, Chen Y, et al. Lactic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol. 2018;14(14):1377–1386.
33. Feng JF, Wang L, Yang X, Jiang YH. Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7243–7251.
34. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram Based on Lactate Dehydrogenase-to-Albumin Ratio (LAR) and Platelet-to-Lymphocyte Ratio (PLR) for Predicting Survival in Nasopharyngeal Carcinoma. J Inflamm Res. 2021;14:4019–4033.
35. Zhao R, Liang Z, Chen K, Zhu X. Nomogram Based on Inflammatory Biomarkers and Nutritional Indicators for Predicting Overall Survival in Locoregionally Advanced Nasopharyngeal Carcinoma. J Inflamm Res. 2022;15:2971–2981.
36. Nakazawa N, Sohda M, Yamaguchi A, et al. An Elevated Serum Lactate Dehydrogenase-to-albumin Ratio Is a Useful Poor Prognostic Predictor of Nivolumab in Patients With Gastric Cancer. Anticancer Res. 2021;41(8):3925–3931.
37. Hu Y, Zhou Y, Cao Y, et al. Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer. Int J Med Sci. 2022;19(6):1003–1012.
38. Xie Z, Zhou H, Wang L, Wu Y. The Significance of the preoperative lactate dehydrogenase/albumin Ratio in the Prognosis of Colon Cancer: a retrospective study. PeerJ. 2022;10:e13091.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



