Back to Journals » Infection and Drug Resistance » Volume 15
Albumin Infusion May Improve the Prognosis of Critical COVID-19 Patients with Hypoalbuminemia in the Intensive Care Unit: A Retrospective Cohort Study
Authors Zhang L, Yu W, Zhao Y, Chen X , Wang P, Fan X, Xu Z
Received 2 August 2022
Accepted for publication 29 September 2022
Published 19 October 2022 Volume 2022:15 Pages 6039—6050
DOI https://doi.org/10.2147/IDR.S383818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Liren Zhang,1 Weibin Yu,2 Yuwu Zhao,1 Xiaohua Chen,3 Peng Wang,3 Xiaohong Fan,4 Zhouwei Xu1
1Department of Neurology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Radiology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Infectious Disease, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 4Department of Respiratory Medicine, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Zhouwei Xu, Department of Neurology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China, Tel +8615921704083, Email [email protected]
Background: The coronavirus disease 2019 (COVID-19) pandemic has caused enormous mortality worldwide. Low albumin level is a risk factor for increasing mortality among patients in the intensive care unit (ICU). This study investigated the effect of albumin infusion on critical COVID-19 patients with hypoalbuminemia.
Methods: A total of 114 COVID-19 ICU patients with hypoalbuminemia were recruited from Wuhan Leishenshan Hospital and Zhongnan Hospital of Wuhan University. Clinical features and laboratory variables were collected through electronic medical records. The cohorts were divided into two groups: albumin infusion and non-albumin infusion. Propensity-matched analysis was used to compare patients who received albumin to controls. Statistical analyses were used to investigate the survival time and inflammation-related blood biomarkers between groups.
Results: Lactate dehydrogenase, interleukin (IL)-6, IL-2 receptor, and IL-8 levels were significantly downregulated in the albumin infusion group. Significant upregulations of lymphocyte counts and IL-10 were found in the albumin infusion group. There was a negative association between albumin level and D-dimer or procalcitonin levels after treatment. The albumin infusion group had a significantly longer survival time and shorter hospitalization time than control patients. Notably, a 1g increase in albumin level reduced the risk of death by approximately 7.3% after adjusting for age and sex. Patients with increased albumin levels after treatment had better prognoses than those without.
Conclusion: Albumin administration can regulate COVID-19-related biomarkers and reduce the risk of death in critical patients with hypoalbuminemia. Clinicians should pay more attention to these risk factors. Targeted clinical interventions should be implemented to minimize the negative impacts of hypoalbuminemia and improve disease outcomes.
Keywords: albumin, COVID-19, critical, prognosis, hypoalbuminemia
Introduction
Since late 2019, coronavirus disease 2019 (COVID-19) has evolved into a global pandemic and caused a high death toll worldwide.1 Previous reports concluded that serum albumin level was associated with the severity of several critical medical conditions including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS),2 which are characterized by dysregulated inflammatory processes.3 Similarly, one group reported that COVID-19 patients with low serum albumin had longer hospitalizations and a worse prognosis.4 Hypoalbuminemia plays a key role in damaging the function of vital organs, leading to shock or death in intensive care unit (ICU) patients. Albumin is a single 67-kDa peptide chain protein that constitutes a large part of the colloid oncotic pressure of the vasculature and helps maintain interstitial fluid homeostasis.5 Human serum albumin, which is currently derived from human serum, is a critical therapeutic used in the treatment of trauma, injury, hypoalbuminemia, and hypoproteinemia.6 Albumin infusion could promote plasma volume expansion and exert significant anti-inflammatory and antioxidative effects.7 One study showed that albumin infusion could be used to reduce lung injury in human ARDS.8 This treatment was also found to improve the prognosis of other chronic diseases including liver cirrhosis.9 COVID-19 is characterized by an inflammation-related cytokine storm that can further increase vessel permeability and facilitate albumin escape from the interstitial space.10 More recent studies have revealed that lymphopenia, high D-dimer levels, and hypoalbuminemia are risk factors of severe or critical COVID-19 and could be used to predict patient prognosis, independent of age and comorbidities.11,12 To the best of our knowledge, no study has assessed the effects of albumin administration on COVID-19-related biomarkers or the prognosis of ICU patients with severe illness. The relation between inflammation markers, patient survival and albumin infusion is unclear and needs to be explored. The aim of this retrospective cohort study was to investigate whether albumin infusion could improve the biomarkers and prognoses of COVID-19 patients in ICUs.
Methods
Study Design and Participants
A retrospective study was conducted from February 15, 2020, to July 30, 2021. All COVID-19 patients were definitively diagnosed in accordance with the seventh version of diagnosis and treatment protocol for COVID-19 issued by the National Health Committee of the People’s Republic of China (http://www.nhc.gov.cn/). All of the cases were critical subtypes of COVID-19 and hospitalized in the ICUs of Lei Shenshan Hospital and Zhongnan Hospital of Wuhan University. Leishenshan Hospital and Zhongnan Hospital of Wuhan University were two medical centers designed and built by the Chinese government in Wuhan to treat the overwhelming number of critical COVID-19 patients early in the pandemic. Nasopharyngeal swabs for laboratory diagnosis of COVID-19 were performed in duplicate with the reverse transcription-polymerase chain reaction (RT-PCR).
All patients met the following inclusion criteria:
- Age ≥18 years old,
- Serum albumin level <40 g/L (normal range 45 g/L-55g/L),
- The initial blood tests were completed within 24 hours after admission, with additional tests performed after albumin infusion.
Patients were excluded if they met any one of the following exclusion criteria:
- Age <18 years old,
- History of other diseases that could affect albumin levels (eg, liver cirrhosis, renal dialysis, etc.),
- Received albumin infusion before ICU admission,
- Incomplete medical records.
Data Collection
Demographic and baseline clinical features were collected, including age, sex, education years, body mass index, hospitalization duration, history of chronic diseases, highest body temperature during hospitalization, the time of negative nucleic acid testing (period between the first positive PCR to the first negative PCR), and the time from symptom onset to intubation. The time from symptom onset to ICU admission and the initial blood test results within 24 hours of admission were collected from electronic medical records. Participants were divided into non-albumin infusion and albumin infusion groups according to whether the patient received albumin infusion treatment for at least 3 consecutive days after ICU admission. A multivariate logistic regression model was applied to estimate the propensity score based on the covariates including sex, age, comorbidity, lymphocyte count, and the time from symptom onset to ICU admission. The propensity score was defined as the predicted probability of a patient receiving albumin administration, and a match tolerance of 0.2 was set. The second blood tests were performed at the end of the 3-day consecutive albumin administration treatment.
Ethics Approval
The data used in the study were obtained from routine clinical diagnosis and treatment, and all patients or their families signed an informed consent form authorizing the use of their clinical data on admission. Therefore, this retrospective study was approved by the ethical committee of Shanghai Sixth People‘s Hospital Affiliated to Shanghai Jiao Tong University School of medicine(2020-KY-117), and the need for informed consent was waived. All patient data were treated with confidentiality and in compliance with the Declaration of Helsinki.
Statistical Analysis
Categorical variables are expressed as frequencies and percentages. Normally distributed continuous variables are summarized as means and standard deviations (SDs). Single-sample Kolmogorov–Smirnov tests were used to determine whether data were normally distributed. Non-normally distributed continuous variables are presented as median and first, third quartiles. Student’s t-tests were used to compare differences between two groups if the data were normally distributed, otherwise we performed Mann–Whitney U-tests. Differences between categorical variable differences were examined with χ2 tests. Kaplan-Meier curves and Log rank tests were employed to compare survival rates between groups. A Cox proportional regression model was used to calculate the hazard ratios and 95% confidence intervals for each factor affecting prognosis. Differences were considered statistically significant at P<0.05. All data analyses were performed using SPSS 20.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Clinical Feature Comparison Between Groups
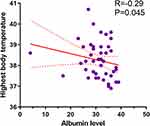
A total 257 critical COVID-19 patients were screened in the study. 158 (61.5%) patients demonstrated hypoalbuminemia. Figure 1 illustrates the process of patient recruitment and exclusion. A propensity score-matched (PSM) cohort of 114 patients were included in the final analyses; they were divided into non-albumin infusion (n=65) and albumin infusion (n=49) groups. Their clinical features are summarized in Table 1. There were no significant differences in albumin levels between the two groups on admission (P=0.148). In the non-albumin infusion group, the albumin level was negatively correlated with highest body temperature during hospitalization (Figure 2, P=0.045). However, the albumin infusion group had a shorter hospitalization duration and lower body temperature during the study period than the group without albumin infusion (P=0.014 and P=0.004, respectively). Compared with the albumin infusion group, more patients in the non-albumin infusion group were intubated (P=0.033), but there was no significant difference in the timing of intubation between the two groups (P=0.098). There were no statistical differences in other clinical categories including comorbidities and mortality.
 |
Table 1 Comparison of Clinical Features Between Groups |
 |
Figure 1 Patient recruitment flow chart. |
Comparison of Laboratory Variables Between Groups After Albumin Infusion
Laboratory findings in both groups are summarized in Table 2. The number of lymphocytes, levels of C-reactive protein and IL-10 were significantly upregulated in the albumin infusion group (P=0.008, P=0.006, P=0.001). Compared with the non-albumin infusion group, the lactate dehydrogenase level was significantly reduced in the albumin infusion group (P=0.029). The expression levels of inflammation-related proteins including interleukin (IL)-6, IL-2 receptor and IL-8 were significantly higher in the non-albumin infusion group (P=0.006, P=0.017 and P=0.005, respectively).
 |
Table 2 Comparison of Laboratory Variables Between Groups |
Correlations Between Post-Infusion Albumin Level and Other Laboratory Variables
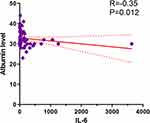
We next explored the correlations between albumin levels and laboratory variables after 24 hours with or without albumin infusion using Spearman correlation tests (Table 3). There was a significantly negative correlation between levels of albumin and IL-6 in either the non-albumin infusion group (P=0.020) or albumin infusion group (P=0.012, Figure 3). Albumin level was also negatively associated with COVID-19-related biomarkers including white cell count, D dimer, aspartate aminotransferase (AST), amylase, LDH, procalcitonin, myoglobin, and IL-8 in patients who did not receive intravenous albumin (P=0.026, P=0.001, P=0.024, P=0.019, P=0.020, P=0.001, P=0.030; Table 3). Interestingly, these correlations were not observed in the albumin infusion group.
 |
Table 3 Associations Between Albumin Concentrations After Infusion and Laboratory Variables |
Albumin Infusion Prolonged Survival Time
We then investigated the effect of albumin infusion on survival. As shown in Figure 4, COVID-19 patients with albumin infusion had significantly longer survival than those without albumin infusion (P=0.045). We then performed Cox regression analysis of the combined effects of albumin and other clinical variables on survival after infusion; the models included albumin level after treatment, age, and sex are shown in Table 4. A1g upregulation in the level of albumin can reduce the hazard ratio of death by 7.3% after adjusting for age and sex (P=0.005).
 |
Table 4 Cox Regression Model Based on Albumin Level After Infusion |
 |
Figure 4 Kaplan-Meier survival curves of the non-albumin infusion and albumin infusion groups. |
Relationship Between Survival Time and Number of Albumin Infusions
We then explored the relationship between the number of albumin infusions and survival time. We divided the albumin infusion group into two subgroups based on the median number of treatments (≤4 and >4 times groups). There was no difference between the two groups in terms of survival time (P=0.415, Figure 5).
 |
Figure 5 Kaplan-Meier survival curves of the ≤4 and >4 times infusion groups. |
Relationship Between Survival Time and Albumin Levels After Infusion
We further investigated whether survival time was correlated with post-treatment albumin levels. Patients in the albumin infusion treatment were subdivided into increased and non-increased groups based on whether levels were higher after treatment. There was significant difference between the two groups in terms of survival time (P=0.038, Figure 6).
 |
Figure 6 Kaplan-Meier survival curves of increased and non-increased groups. |
Discussion
This work focused on the effect of albumin administration on critical COVID-19 patient outcomes in the ICU. To our best knowledge, this is the first study to report that intravenous albumin infusion could reduce the risk of death in COVID-19 patients with low albumin levels. Hypoalbuminemia is very common and can be observed in the early stage of this disease. Previous investigations reported that the incidence rate of hypoalbuminemia or malnutrition in critical COVID-19 patients was around 50%.13,14 The prevalence of hypoalbuminemia in our cohort was around 61.5% in the early screening stage. This increased rate is partly due to the fact that the Leishenshan Hospital was the main referral medical center for critical COVID-19 patients during the initial outbreak, so disease severity and progression were more advanced in our cohort. Serum albumin is mainly synthesized by the liver, and it plays a pivotal role in maintaining pH and normal microvascular permeability.15 Recent studies illustrated that patients with COVID-19 and hypoalbuminemia have a much poorer prognosis than the patients with normal serum albumin levels, which is also consistent with other infectious diseases.12,16 In the group without albumin treatment, the albumin level was negatively associated with maximum temperature during hospitalization. Our results provide additional support for the hypothesis that low albumin level is a risk factor for poor COVID-19 outcomes.
Hypoalbuminemia also reflects the body’s nutrition status. One study showed that serum albumin level and total lymphocyte count could be used as surrogate predictors of malnutrition in surgical inpatients.17 Kurtz et al found that long-term malnutrition could enhance COVID-19 disease severity.18 Human serum albumin contains abundant thiols that inhibit oxidative stress-related clotting and platelet activation.19,20 In our cohort, albumin level negatively correlated with the D-dimer concentration in the non-albumin infusion group, but there was no statistical relationship between these two variables in the albumin infusion group. We hypothesized that albumin infusion could reduce clotting by regulating D dimer activity. Two studies have reported that D-dimer level is associated with disease severity or mortality and is a reliable blood biomarker for COVID-19 patient prognosis.11,21 Procalcitonin is a peptide precursor of calcitonin, which is upregulated in other infectious diseases and has been considered a reliable diagnostic marker of sepsis.22 Hu et al reported that procalcitonin could be used as an indicator of prognosis and contribute to determining the severity of COVID-19.23 A statistically negative association was found between the level of albumin and the level of procalcitonin in the non-albumin group. However, there was no evident relationship in the albumin infusion group, and the interactive mechanisms between albumin and D dimer or procalcitonin should be further investigated.
In the non-albumin infusion group, AST and amylase were significantly negatively and positively associated with the albumin level, respectively. Evidences indicate that the COVID-19 virus can infect different organ systems through binding the angiotensin-converting enzyme 2 receptor, which is expressed in many tissues including the liver, heart, and muscle.24 Virus-induced cell death may contribute to liver function test abnormalities through its effects on portal circulation.25 Interestingly, AST elevation was found to be more frequent than alanine aminotransferase abnormalities in Chinese COVID-19 patients.26 AST is an enzyme that plays a key role in metabolizing amino acids. Elevated AST levels may indicate liver, heart, or muscle damage. de-Madariahat et al reported that increased levels of amylase or lipase does not necessarily indicate pancreatic injury in COVID-19.27 However, the albumin reduction was not associated with the level of liver dysfunction in COVID-19 patients, but it was closely related with the severity of cytokine storm syndrome (CSS) severity.28 CSS is caused by the excessive or uncontrolled release of pro-inflammatory cytokines and is associated with multiple organ dysfunction. It is still unknown if alterations in AST and amylase are a primary outcome of damage caused by COVID-19 virus infection or a secondary result due to CSS. Regardless, it is notable that these organ dysfunction were attenuated following albumin infusion.
One study indicated that albumin level is a better prognosis predictor for patients with respiratory distress syndrome than other inflammatory factors including cytokines or CRP.29 Another study showed that intravenous albumin infusion with diuretics could enhance the oxygenation level by improving cardiac output and ventilation–perfusion matching in the patients with ARDS.30 Lucijanić et al reported that the CRP/albumin ratio in COVID19 patients has been shown to be associated not only with higher 30 days mortality but also with post discharge mortality, and to be predictive of tendency for respiratory deterioration, higher VTE and bacteriemia occurrence.31 Interestingly, we found that CRP levels were significantly upregulated in the albumin infusion group. CRP is an acute-phase serum protein that can increase microcirculation permeability and lead to albumin extravasation. One study demonstrated that human CRP injection could protect mice against pneumococcal pneumonia.32 We hypothesized that albumin infusion could upregulate CRP expression and thus play an anti-inflammatory role in the initial stage of COVID-19 infection. The interplay between CRP and albumin requires further investigation.
COVID-19 infection could decrease serum albumin through multiple mechanisms. In our cohort, there was a significant negative correlation between albumin level after treatment and the IL-6 concentration, which suggests that the inflammation process is paralleled with a change in albumin concentration. IL-6 and tumor necrosis factor (TNF)-α produced during inflammatory processes could lead to hypoalbuminemia both directly (due to reduced albumin synthesis) and indirectly (due to elevated microcirculatory permeability). IL-6 is the hallmark biomarker of COVID-19, and albumin infusion could modulate the inflammation process and reduce oxidative stress. Albumin could also play a role in protecting host cells from the oxidative burst that occurs in response to the viral infection. IL-8 and the IL-2 receptor were significantly downregulated in the albumin infusion group. One study reported that IL-8 helps neutrophils reach sites of infection and was positively correlated with polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs), which could lead to the fatal outcome of the disease.33 Another group showed that COVID-19 patients with high serum IL-2α receptor levels took longer to recover than those with low levels, presumably due to the reduced T-cell activation.34 Albumin infusion could downregulate IL-8 and further reduce PMN-MDSC numbers. The IL-10 receptor is found highly expressed in monocytes and macrophages. IL-10 activates the JAK1-TYK2-STAT3 pathway and further upregulates STAT3-mediated transcription of genes to dampen the inflammatory response.35 Albumin treatment may upregulate IL-10 and protect against host cell damage by inducing SHIP1-STAT3 complex formation.36 We observed that high LDH levels were reversed in the albumin infusion group. LDH is expressed by all living cells and related to cellular metabolism and death.37 The present findings suggest that albumin administration could downregulate LDH levels and further reduce the systemic inflammatory response. Interestingly, we did not find a relationship between times of albumin infusion and survival time. One study found no significant difference in major surgery patients with slow versus rapid albumin administration rate.38 In our study, critical COVID-19 survival time was correlated with post-treatment albumin levels. The relation between the rate of albumin infusion and the targeted albumin levels is unclear and needs to be further investigated.
Our results should be considered in the context of several limitations. First, the retrospective design has its inherent caveats. We chose the PSM method to reduce selection bias associated with albumin treatment. The relatively small sample size may have limited our ability to identify risk factors due to low statistical power and underestimation of covariate influence the on the results. Randomized, multicenter studies would provide more robust evidence to confirm the relationship between albumin level and mortality. Secondly, we were unable to standardize all treatments for the two groups, so there was some bias of measurements in the clinical setting. Third, we only monitored patients after albumin infusion, and future studies should assess the longitudinal change of albumin. While we demonstrated that increased albumin level after infusion could reduce the hazard odds of death, the optimal albumin level to achieve with treatment is still unknown.
Conclusion
In summary, our results suggest that albumin infusion could shorten the hospitalization days, downregulate the expression of inflammation-related biomarkers and improve the prognosis of COVID-19 patients. Clinicians should pay more attention to addressing hypoalbuminemia in hospitalized patients. Targeted interventions should be implemented to minimize the negative impacts of hypoalbuminemia, which could reduce mortality and improve disease outcomes.
Data Sharing Statement
The data used in this study are available from the corresponding author on reasonable request.
Ethics Approval Information
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethical committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (2020-KY-117).
Acknowledgments
The authors thank the patients and ICU unit team members in Wuhan Lei Shengshan Hospital and Zhongnan Hospital of Wuhan University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by the Shanghai Sailing Program.
Disclosure
The authors declare that they have no competing interests.
References
1. Wang H, Paulson KR, Pease SA. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. doi:10.1016/S0140-6736(21)02796-3
2. Chen C, Zhang Y, Zhao X, Tao M, Yan W, Fu Y. Hypoalbuminemia - an indicator of the severity and prognosis of COVID-19 patients: a multicentre retrospective analysis. Infect Drug Resist. 2021;14:3699–3710. doi:10.2147/IDR.S327090
3. Turcato G, Zaboli A, Kostic I, et al. Severity of SARS-CoV-2 infection and albumin levels recorded at the first emergency department evaluation: a multicentre retrospective observational study. Emerg Med J. 2021;39(1):63–69. doi:10.1136/emermed-2020-210081
4. de la Rica R, Borges M, Aranda M, et al. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain: a retrospective cohort study. Microorganisms. 2020;8(8):1106. doi:10.3390/microorganisms8081106
5. Tabata F, Wada Y, Kawakami S, Miyaji K. Serum albumin redox states: more than oxidative stress biomarker. Antioxidants. 2021;10(4):503. doi:10.3390/antiox10040503
6. Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care. 2014;18(4):231. doi:10.1186/cc13991
7. Bonifazi M, Meessen J, Pérez A, et al. Albumin oxidation status in sepsis patients treated with albumin or crystalloids. Front Physiol. 2021;12:682877. doi:10.3389/fphys.2021.682877
8. Mendes RS, Oliveira MV, Padilha GA, et al. Effects of crystalloid, hyper-oncotic albumin, and iso-oncotic albumin on lung and kidney damage in experimental acute lung injury. Respir Res. 2019;20(1):155. doi:10.1186/s12931-019-1115-x
9. Romanelli RG, La Villa G, Barletta G, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12(9):1403–1407. doi:10.3748/wjg.v12.i9.1403
10. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther. 2021;6(1):255. doi:10.1038/s41392-021-00679-0
11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
12. Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92(10):2152–2158. doi:10.1002/jmv.26003
13. Bassoli C, Oreni L, Ballone E, et al. Role of serum albumin and proteinuria in patients with SARS-CoV-2 pneumonia. Int J Clin Pract. 2021;75(4):e13946. doi:10.1111/ijcp.13946
14. Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. doi:10.1038/s41430-020-0642-3
15. Omiya K, Sato H, Sato T, et al. Albumin and fibrinogen kinetics in sepsis: a prospective observational study. Crit Care. 2021;25(1):436. doi:10.1186/s13054-021-03860-7
16. Minatoguchi S, Nomura A, Imaizumi T, et al. Low serum albumin as a risk factor for infection-related in-hospital death among hemodialysis patients hospitalized on suspicion of infectious disease: a Japanese multicenter retrospective cohort study. Ren Replace Ther. 2018;4(1):30. doi:10.1186/s41100-018-0173-8
17. Morey VM, Song YD, Whang JS, Kang YG, Kim TK. Can serum albumin level and total lymphocyte count be surrogates for malnutrition to predict wound complications after total knee arthroplasty? J Arthroplasty. 2016;31(6):1317–1321. doi:10.1016/j.arth.2015.12.004
18. Kurtz A, Grant K, Marano R, et al. Long-term effects of malnutrition on severity of COVID-19. Sci Rep. 2021;11(1):14974. doi:10.1038/s41598-021-94138-z
19. Violi F, Cangemi R, Romiti GF, et al. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal. 2021;35(2):139–142. doi:10.1089/ars.2020.8142
20. Kunutsor SK, Seidu S, Katechia DT, Laukkanen JA. Inverse association between serum albumin and future risk of venous thromboembolism: interrelationship with high sensitivity C-reactive protein. Ann Med. 2018;50(3):240–248. doi:10.1080/07853890.2018.1441537
21. Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13(11):1265–1275. doi:10.1080/17474086.2020.1831383
22. Schmidt de Oliveira-Netto AC, Morello LG, Dalla-Costa LM, et al. Procalcitonin, C-reactive protein, albumin, and blood cultures as early markers of sepsis diagnosis or predictors of outcome: a prospective analysis. Clin Pathol. 2019;12:2632010X1984767. doi:10.1177/2632010X19847673
23. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2):106051. doi:10.1016/j.ijantimicag.2020.106051
24. Zamorano Cuervo N, Grandvaux N. ACE2: evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife. 2020;9:e61390. doi:10.7554/eLife.61390
25. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi:10.1126/science.abc1669
26. Bertolini A, van de Peppel IP, Bodewes F, et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864–1872. doi:10.1002/hep.31480
27. de-Madaria E, Siau K, Cárdenas-Jaén K. Increased amylase and lipase in patients with COVID-19 pneumonia: don’t blame the pancreas just yet! Gastroenterology. 2021;160(5):1871. doi:10.1053/j.gastro.2020.04.044
28. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi:10.1016/j.ebiom.2020.102763
29. Hoeboer SH, Straaten HMO-V, Groeneveld AJ, et al. Albumin rather than C-reactive protein may be valuable in predicting and monitoring the severity and course of acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new onset fever. BMC Pulm Med. 2015;15(1):22. doi:10.1186/s12890-015-0015-1
30. Uhlig C, Silva PL, Deckert S, Schmitt J, de Abreu MG. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014;18(1):R10. doi:10.1186/cc13187
31. Lucijanić M, Stojić J, Atić A, et al. Clinical and prognostic significance of C-reactive protein to albumin ratio in hospitalized coronavirus disease 2019 (COVID-19) patients: data on 2309 patients from a tertiary center and validation in an independent cohort. Wien Klin Wochenschr. 2022;134(9–10):377–384. doi:10.1007/s00508-021-01999-5
32. Singh SK, Ngwa DN, Agrawal A. Complement activation by C-reactive protein is critical for protection of mice against pneumococcal infection. Front Immunol. 2020;11:1812. doi:10.3389/fimmu.2020.01812
33. Sacchi A, Grassi G, Bordoni V, et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11(10):921. doi:10.1038/s41419-020-03125-1
34. Ma A, Zhang L, Ye X, et al. High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front Immunol. 2021;12(12). doi:10.3389/fimmu.2021.626235
35. Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15(1):23. doi:10.1186/s12964-017-0177-y
36. Islam H, Chamberlain TC, Mui AL, Little JP. Elevated interleukin-10 levels in COVID-19: potentiation of pro-inflammatory responses or impaired anti-inflammatory action? Front Immunol. 2021;12:2485. doi:10.3389/fimmu.2021.677008
37. Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107(5):2037–2042. doi:10.1073/pnas.0914433107
38. Statkevicius S, Bonnevier J, Fisher J, et al. Albumin infusion rate and plasma volume expansion: a randomized clinical trial in postoperative patients after major surgery. Crit Care. 2019;23(1):191. doi:10.1186/s13054-019-2477-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.