Back to Journals » Cancer Management and Research » Volume 14
A Nomogram to Identify the Optimal Candidates for Induction Chemotherapy in Advanced N-Stage Nasopharyngeal Carcinoma
Authors Jiang YT , Chen KH, Liang ZG, Yang J, Qu S, Li L, Zhu XD
Received 17 June 2022
Accepted for publication 21 August 2022
Published 31 August 2022 Volume 2022:14 Pages 2583—2596
DOI https://doi.org/10.2147/CMAR.S377731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Yu-Ting Jiang,1 Kai-Hua Chen,1 Zhong-Guo Liang,1 Jie Yang,1 Song Qu,1,2 Ling Li,1,2 Xiao-Dong Zhu1– 3
1Department of Radiation Oncology, Guangxi Medical University Cancer Hospital, Guangxi, People’s Republic of China; 2Key Laboratory of Early Prevention and Treatment for Regional High‐Incidence‐ Tumor, Guangxi Medical University, Ministry of Education, Guangxi, People’s Republic of China; 3Department of Oncology, Affiliated Wuming Hospital of Guangxi Medical University, Guangxi, People’s Republic of China
Correspondence: Xiao-Dong Zhu, Tel/Fax +86-771-5331466, Email [email protected]
Purpose: We aimed to select optimal candidates benefiting from the addition of induction chemotherapy (IC) to concurrent chemoradiotherapy (CCRT) in advanced N-stage nasopharyngeal carcinoma (NPC).
Patients and Methods: A total of 624 NPC patients with N2-3 stage received CCRT with or without IC were retrospectively reviewed. We constructed a nomogram for predicting overall survival (OS) based on the result of the multivariate analysis in the training cohort (n = 468) and then tested it on the validation cohort (n = 156). Harrell’s concordance indices (C-index) and time-independent receiver operating characteristic (tdROC) analysis were applied to evaluate the discriminatory ability of the nomogram and compare it with TNM staging. IC plus CCRT was compared with CCRT in the whole cohort and two risk groups based on the nomogram with balanced baseline characteristics. In addition, acute toxicities were compared between different treatment groups.
Results: The nomogram showed good prognostic accuracy with a C-index of 0.716 (95% CI 0.669– 0.763) in the validation cohort. The 5-year OS of low and high-risk groups stratified by the nomogram were significantly different. IC+CCRT was significantly associated with superior OS as compared with CCRT (75.4 vs 52.6%, p = 0.009) in the high-risk group. However, no significant difference between IC plus CCRT and CCRT was observed (p = 0.843) in the low-risk group. IC plus CCRT was associated with more grade 1– 4 acute toxicities.
Conclusion: Our study can help clinicians select NPC patients with advanced N stage who benefit from IC.
Keywords: nasopharyngeal carcinoma, advanced N-stage, induction chemotherapy, survival, nomogram
Introduction
Nasopharyngeal carcinoma (NPC) is a special kind of head and neck cancer that has the highest incidence in South Asia.1 Despite advances in radiotherapy technique and chemotherapy strategies have greatly improved the survival prognosis of NPC, treatment outcomes of locoregionally advanced NPC (LANPC) remain unsatisfactory, with approximately 30% of patients suffering treatment failure and 5-year overall survival (OS) of 67–77%.2–4 Unfortunately, over 70% of cases present with LANPC at initial diagnosis.5,6 Management of these patients remains a challenge for clinicians.
During the past decade, the application of induction chemotherapy (IC) before radical radiotherapy has been widely proven a feasible neoadjuvant treatment as it could improve survival in LANPC.7–10 However, additional IC is also accompanied by more toxicity10 and increases the economic burden. Also, it should be noted that not all patients with LANPC could benefit from the use of IC.11–13 Thus, it is essential to identify the optimal candidates for IC to improve the management of LANPC. According to the American Joint Commission on Cancer (AJCC) classification, patients with NPC are classified as N0-N3.14 Recent evidence15 has suggested that over 75% of treatment failure is concentrated in the advanced N-stage (N2–N3). Namely, advanced N-stage NPC might be used as an indication of IC selection. Furthermore, previous studies have found that the use of IC might be more effective in improving OS for NPC patients with advanced N disease.16,17
The tumor, node, metastasis (TNM) staging system is recognized to be the internationally staging standard for evaluating the prognosis and guiding treatment of cancer patients. However, this system may not be clinically satisfactory since patients with similar TNM stages have been shown to have varying efficiency to the same treatment. Hence, it remains unclear whether all NPC patients with the N2-3 stage would benefit from the addition of IC to CCRT. In addition to common serum biomarkers such as Epstein-Barr virus DNA (EBV DNA), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), etc., and some magnetic resonance imaging (MRI) features of the primary tumor (PT) and regional lymph nodes (LNs) also attracted the attention of scholars in recent years, and have been proved by some studies to have prognostic significance for NPC patients.18–20
Nomograms can be used to incorporate various risk factors to create a simple graphical model that predicts the prognoses of patients. Recently, nomograms function as new reliable tools in predicting prognosis in guiding treatments in carcinomas.11,21–23 Therefore, we conducted this study to establish a comprehensive model to guide risk stratification and further identify optimal candidates for IC in advanced N-stage NPC.
Materials and Methods
Study Population
This study included 624 LANPC patients treated at Guangxi Medical University Cancer Hospital from January 2014 to December 2018 who met the following inclusion criteria: (1) stage III–IVA NPC with N2–3 disease based on the AJCC staging system (8th edition); (2) treated by CCRT with or without IC; (3) available pretreatment MRI information; (4) no history of malignancies or synchronous cancer; (5) complete clinical and treatment information. This study was approved by the Institutional Review Board of Guangxi Medical University Cancer Hospital and conformed to the Declaration of Helsinki. Informed consent was not obtained given the retrospective nature of the study.
Imaging Protocol
Before treatment, all patients received MRI scanning from the suprasellar cistern to the inferior margin of the sternoclavicular joint with a 1.5- system. All images were assessed by two clinicians who specialized in head and neck cancers separately. We collected some common information of PT and metastatic LNs acquired via MRI that could not be reflected in the 8th AJCC TNM staging system, including necrosis, extranodal extension (ENE), nodal grouping (NG), and retropharyngeal LN (RLN) laterality. Previous studies have confirmed the prognostic potential of these factors.18–20 Detailed MRI sequences and definitions of MRI based-information of PT and metastatic LNs were presented in the Supplementary Material.
Treatment
All patients received platinum-based chemotherapy with an interval of 21 days. The common IC regimens include docetaxel-cisplatin-5-fluorouracil (TPF), docetaxel-cisplatin (TP), cisplatin-5-fluorouracil (PF), and gemcitabine-cisplatin (GP). Concurrent chemotherapy consisted of cisplatin (80 or 100 mg/m2) for 2–3 cycles during radiotherapy. The radiotherapy technique was intensity-modulated radiotherapy (IMRT). Detailed information on the method of treatment was available in Supplementary Materials. The treatment-elated acute toxicities were graded based on the Common Terminology Standard for Adverse Events (CTCAE version 4.0).
Follow-Up and Study Endpoints
Follow-up was conducted every 3 months in the first 2 years after radiotherapy, every 6 months in the third year, and once a year thereafter. The primary endpoint was OS (time from diagnosis to death from any cause). The second endpoints were distant metastasis-free survival (DMFS, time from diagnosis to first distant metastasis), locoregional relapse-free survival (LRRFS, time from diagnosis to first local or regional recurrent or both), and disease-free survival (DFS, time from diagnosis to disease progression or death from any cause).
Statistical Analysis
All continuous variables were converted to categorical variables based on the cut-off values by ROC curves. We compared the baseline characteristics and toxicities in different treatment groups with the X2 test or Fisher’s exact test. To balance baseline characteristics between the groups, the propensity score matching (PSM) method without replacement was conducted using the nearest-neighbor method with a stringent caliper of 0.05. The survival rates were calculated by the Kaplan–Meier method and compared using the Log rank tests. The random number generators function of SPSS soft was used to classify patients into a training cohort and validation cohort. Variables with p < 0.1 in the univariable Cox regression analysis were included in the multivariable analyses. Multivariable analyses in the training cohort were used to select the independent prognosis factors. A nomogram based on the Cox regression model was developed to predict the 1-, 3- and 5-year OS in the training cohort. The C-index was calculated to estimate the predictive accuracy and discriminative ability of the nomogram. Calibration curves were generated to assess the performance of the nomogram along with bootstrap validation. To evaluate the reproducibility of our model’s prognostic performance, we repeated the randomized assignment of training/test sets 10 times. We compared the area under the time-dependent ROC curve (tdAUC) of the nomogram and the 8th TNM staging in the prediction of 3-year and 5-year OS.
We calculated the risk scores for all patients with the aim of categorizing patients into different groups (low and high risk) according to the nomogram. We tried to find patients that would benefit most from additional IC by performing individual comparisons of IC plus CCRT versus CCRT alone for each risk group. The PSM method was used to reduce the effect of potential confounders.
All these statistical analyses were performed with IBM SPSS Statistics 23.0 (IBM Co., Armonk, NY) or R 3.6.3 (R project, http://www.R-project.org/). A difference with a two-sided p-value < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
From 2014 to 2018, 624 LANPC patients with N2-3 stage were enrolled in the present study. The clinical characteristics grouped by treatment strategy are shown in Table 1. In the original cohort, 408 patients were applied IC+ CCRT and 216 cases applied CCRT alone. Compared with the CCRT group, patients with age ≤ 50 (57.4 vs 70.1%, p = 0.002) or with advanced disease (T4, N3, and stage IVA disease; all p < 0.001) were significantly more likely to receive IC+CCRT. Yet, the PSM method balanced all the baseline characteristics between the two groups. A total of 178 pairs were identified for the matched analysis.
 |
Table 1 Baseline Characteristics of Patients Before and After Matching |
The median follow-up duration was 49.0 months (range, 2.3–101 months) for the whole cohort. Totally, 133 patients (21.3%) died, 112 (17.9%) suffered distant metastasis, and 33 (5.3%) suffered locoregional relapse. The 5-year OS, LRRFS, DMFS, and DFS were 76.9%, 93.2%, 79.1%, and 68.6%, respectively.
Survival Outcomes
Briefly, before matching, all survival outcomes were similar between the two treatment groups (all p > 0.1, Supplementary Figure S1A–D). In terms of the matched cohort, the addition of IC had a favorable effect on 5-year OS rate (80.0% vs 73.5%, p = 0.042; Figure 1A) compared with CCRT alone, but not for 5-year LRRFS (91.5% vs 94.9%, p = 0.335; Figure 1B), DMFS (84.5% vs 80.8%, p = 0.572; Figure 1C), and DFS (74.5% vs 69.4%, p = 0.319; Figure 1D). The multivariable analysis in the matched data set also indicated that IC+CCRT was an independent prognostic factor of OS (IC+CCRT vs CCRT: HR 0.599, 95% CI 0.374–0.960, p = 0.033, Supplementary Table 1).
Establishment and Validation of a Nomogram Model for Overall Survival
We used computer-generated random numbers to classify patients into a training cohort (n = 468) and a validation cohort (n = 156, Supplementary Table 2). We included all clinicopathologic factors in the univariable analysis (Table 2) for OS. At last, when the multivariate Cox proportional hazard model was performed, five factors (age, N stage, EBV DNA, LMR, RLN_ necrosis, and CLN_ necrosis) significantly influenced OS (all p < 0.05; Table 2).
 |
Table 2 Identification of Risk Factors of OS by Univariate and Multivariate Cox Models |
The nomogram was developed according to the results of multivariate analysis for OS in the training cohort (Figure 2A). Although the T stage had a non-significant p-value of 0.082 for OS, we still regarded it as an important variable for clinical decision and put it into the establishment of the final nomogram. By adding the scores for each factor and determining the total score on the score table, each patient’s probability of death from any cause at 1, 3, and 5 years could be predicted. The C-indexes for the nomogram were 0.700 (95% CI 0.676–0.724) and 0.716 (95% CI 0.669–0.763) in the training and validation cohorts. The calibration curves demonstrated that the nomogram showed acceptable agreement between the nomogram-predicted and observed values for 1-, 3- and 5-year OS in the two cohorts (Figure 2B and C). We randomly split the training cohort into paired training (75%) and validation (25%) sets 10 times to evaluate our predictive model repeatedly, generating a median AUC of 0.713 (IQR, 0.652–0.746). Furthermore, we compared the predictive ability of the nomogram and 8th TNM staging through tdROC analysis and found that the nomogram had higher AUC values than the TNM staging (Figure 3).
Risk Stratification
All patients’ risk scores were calculated based on the nomogram. The patients were divided into high- and low-risk groups by the optimal cutoff value of 23 determined by the ROC curve. Consequently, 137 (29.3%) in the training cohort and 60 (38.5%) in the validation cohort with scores > 23 were classified as a high-risk group, and 331 (70.7%) and 96 (61.5%) in the training and validation sets with scores ≤ 23 as a low-risk group. For low-risk vs high-risk group, the 5-year OS rate was 80.7% vs 64.5% (p < 0.001) in training cohort. Similarly, patients with high risk also achieved worse DMFS (81.3% vs 70.3%; p = 0.007) and DFS (71.9% vs 55.2%; p = 0.001). In the validation cohort, the 5-year OS (90.0% vs 69.2%; p = 0.004) and PFS (82.3% vs 63.6%; p = 0.017) of patients with high and low risk were also significantly different. The survival curves were shown in Supplementary Figure S2A–H.
The Efficacy of Induction Chemotherapy in Risk-Based Subgroups
For the whole cohort, the use of IC achieved significant improvements in OS. Considering that patients in different risk subgroups had different survival prognoses, we explored the value of IC for low- and high-risk patients and interestingly found that it differed between the two subgroups. The baseline characteristics of the patients receiving IC+CCRT and patients receiving CCRT alone were unbalanced in the two risk subgroups, including more advanced tumor stage in the IC+CCRT groups and older patients in the CCRT group. Through 1-to-1 PSM, 47 and 128 pairs of patients with balanced basic characteristics were selected to compare the efficiency of IC + CCRT and CCRT alone in the high- and low-risk subgroups. Detailed characteristics of the unmatched/well-matched risk subgroups are listed in Supplementary Tables S3 and S4. After matching, in the high-risk group, IC+CCRT group achieved better 5-year OS and DFS than did CCRT group (OS: 75.4 vs 52.6%, P = 0.009; DFS: 69.1 vs 44.8%, p = 0.022), but had similar 5-year LRFS and DMFS (Figure 4). However, in the low-risk subgroup, non-significant differences were observed in OS (p = 0.843), LRRFS (p = 0.524), DMFS (p = 0.687), and DFS (p = 0.386) between the two treatment groups (Supplementary Figure S3A–D).
Acute Toxicity
We evaluated acute toxicity between IC+CCRT and CCRT groups during the whole treatment period in the PSM cohort, details of the information were showed in Table 3. The patients in the IC+CCRT group had higher incidences of grade 1–4 hematologic toxicities, hepatotoxicity, and gastrointestinal reaction, as compared to those in the CCRT group. Moreover, more patients in the IC+CCRT group suffered from Grade 3–4 leucopenia (38.2 vs 23.0%, p = 0.002), neutropenia (33.1 vs 13.5%, p < 0.001), and gastrointestinal reaction (7.3 vs 2.2%, p = 0.044). Between-group differences in other Grade 3–4 acute toxicities, such as anemia, thrombocytopenia or hepatotoxicity were not significant.
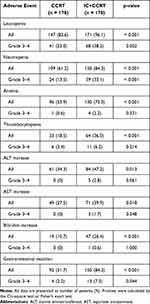 |
Table 3 Acute Adverse Events of Patients in the CCRT and IC+CCRT Groups |
Discussion
In the current study, a nomogram incorporating the TNM staging system, serum biomarkers, and MRI based-tumor features was established to predict OS in advanced N-stage NPC. The nomogram performed better than the 8th TNM stage and successfully classified patients into low and high-risk groups with significantly different 5-year OS rates. Moreover, we showed that the addition of IC before CCRT was likely to benefit patients in the high-risk group, but not patients with low risk. Our findings demonstrated that the nomogram was useful in risk stratification and identifying the optimal candidates for IC.
Recently, several clinical trials have provided evidence that the application of IC before CCRT could decrease the risk of distant metastasis and further improve survival.7–10 Although IC+CCRT was regarded as the most recommended treatment for LANPC patients according to National Comprehensive Cancer Network, clinicians did not reach a consensus on the optimal beneficiaries of IC in LANPC. Notably, the study population of the three clinical trials covered all LANPC but not T3N0–17 or T3–4N010,24 patients, since these subgroups were initially considered to have a low risk of treatment failure and did not need additional IC. Also, previous studies have reported that IC might not improve survival for patients with T3-4N0-1 NPC but was associated with higher incidences of acute toxicities.25–28 These findings suggest that IC might improve survival only in patients with a larger tumor burden. Compare to N0-1 patients, patients with large and/or extensive lymphnode disease (N2-3) are high-risk subgroups. In clinical practice, CCRT alone may not be sufficiently intensive and IC is expected to bring benefits to these patients. However, we should note that cancer is a heterogeneous disease. Thus, it is not rigorous enough to determine patients who might benefit from IC only according to the TNM stage. Being a double-edged sword, IC has positive value in eliminating micrometastases, while it also increased both wait time for radical radiotherapy and the incidence of toxicities. Our data showed that the addition of IC was associated with more acute toxicities, which is similar to previous studies.10,13 In that case, there is an urgent need for a more comprehensive prognostic model to precisely predict patient clinical survival and guide individualized treatment based on risk stratification.
Patients with a higher risk of treatment failure are generally considered to benefit more from additional IC. Several studies followed up on this experience and confirmed the benefit of IC in NPC patients with high risk through the prognostic models they built.11–13 Most previous studies utilized tumor stage and serum biomarkers to develop a prognostic model and guide individualized treatment. In our study, five characteristics (age, T stage, N stage, LMR, and EBV DNA level) were identified as independent factors and included in the nomogram. As a regular examination before treatment, MRI is now widely used to accurately assess the prognosis of NPC, determine the TNM stage, and conduct follow-up. Attempts have been made in investigating the prognostic value of some MRI based-tumor features for patients with NPC, including nodal laterality, extranodal neoplastic spread (ENS), and necrosis,18–20 and reported that patients with these tumor features had a higher risk of treatment failure and need intensive treatment. Liu et al indicated that compared with CCRT, IC + CCRT improved survival outcomes for patients with tumor necrosis, but not for patients without tumor necrosis.29 Hence, our prognostic model utilized the information obtained from pretreatment MRI without increasing the physical or financial burden. With the integration of tumor stage, tumor features via MRI, and other clinical characteristics, the nomogram yielded better performance in predicting survival than the TNM stage. Our nomogram might provide a simple and precise method for predicting OS in LANPC with advanced N-stage.
In this study, it is notable that in all LANPC patients with N2-3 stage, IC + CCRT was associated with improved OS compared with CCRT alone. This result is consistent with other studies and clinical experience,16,17 indicating that the N stage can be used as an indication of IC. However, we found that the effects of IC varied among patients with different risks. The individualized risk stratification based on nomogram was used to identify a subgroup that would be advantageous for the addition of IC before CCRT to improve survival. Our data confirmed that IC for high-risk patients achieved significant improvements in OS and PFS but this was not observed in those categorized as low-risk patients, although the characteristics were balanced between the two treatment arms. The most likely explanation for IC only benefiting high-risk patients in the present study is that these patients represent a greater tumor burden and higher risk of treatment failure. Thus, intensive treatment may be reasonable for patients with a high risk if they can tolerate such therapy based on the comprehensive assessment before treatment. The reason for the lower efficacy of IC for low-risk patients may be that they used CCRT alone to achieve a relatively satisfactory survival prognosis, while IC could result in prolonged wait time for radical radiotherapy. The adverse effects of delayed radiotherapy on patients with NPC have been proved in previous studies.30–32 Additionally, similar to other studies, we found that the use of IC increased the incidence of severe adverse events. The additional acute toxicities such as hematologic toxicities or hepatoxicity caused by IC may affect the treatment efficiency. Considering the toxicities, cost, and increasing wait time for radiotherapy, the low-risk patients may prefer to avoid IC, particularly due to the relatively small expected efficiency.
Our study shows great potential for application in clinical practice. The nomogram would particularly help to have one-to-one discussions with patients and in making treatment decisions. Consider the following examples that illustrate the value of this model in terms of individualized treatment: A 40-year-old patient (0 points) with T4 (3.25 points), N2 stage (0 points) NPC, and -an EBV-DNA level of 5000 copies/mL (0 points), LMR less than 3.80 (10 points), and with tumor characteristics of RLN necrosis (7.75 points) and CLN necrosis (4.5 points), would obtain a total score of 25.5 and a corresponding 5-year OS of 67%. Therefore, this patient will be classified as a high-risk case and the addition of IC to CCRT was more beneficial for s/he. Nevertheless, the administration of IC should involve a thorough evaluation of not only treatment efficiency, but also some details that cannot be included in this nomogram, such as patients’ tolerance, quality of life, financial status, and so on. For example, in this study, compared with the CCRT group, the proportion of young patients in the IC + CCRT group is higher. The possible reason is that the addition of IC is usually related to more side effects and may not be tolerated by older patients. Hence, the proposed model can serve as a useful reference, but should not be regarded as the sole basis for decisions making of NPC patients.
The limitations of this study lie in the following aspects. First of all, this study was performed from a single center. For this reason, the generalizability of the findings to other patient populations is not determined, and external validation may be warranted in the future. Another limitation was the heterogeneity of the IC regimen due to the retrospective study nature. Finally, as an emerging field in oncology,11,33 radiomics was not used in the present study because it was not a routine technology in our center. In future research, radiomics should be taken into analysis.
Conclusion
In conclusion, we built a nomogram to predict OS rates for advanced N-stage NPC in the endemic area. The nomogram successfully stratified patients into high- and low-risk groups, and thereby may be a potential tool for identifying the optimal candidates for IC: the addition of IC before CCRT brought survival benefits for high-risk patients, but it failed to retain benefits for patients with low risk. Future prospective studies with external validation are needed to test our results.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (LW2022105), in compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key Research and Development Program Project of Guangxi Zhuang Autonomous Region (Grant No. GuikeAB18221007), Youth Science Foundation Project of Guangxi Medical University (Grant No. GXMUYSF202122), the Independent Project of Key Laboratory of Early Prevention & Treatment for Regional High-Incidence-Tumor (Grant No. GKE-ZZ202014), and Self funded research project of Health Commission of Guangxi Zhuang Autonomous Region (Z20210913).
Disclosure
The authors declare that there is no conflict of interests.
References
1. Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):90. doi:10.1186/s40880-017-0257-9
2. Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group Phase II trial 0225. J Clin Oncol. 2009;27(22):3684–3690. doi:10.1200/JCO.2008.19.9109
3. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi:10.1016/j.radonc.2013.10.020
4. Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65(1):161–168. doi:10.1016/j.ijrobp.2005.12.003
5. Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–1334. doi:10.1016/j.ijrobp.2008.07.062
6. Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(4):546–558. doi:10.1002/cncr.29795
7. Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. doi:10.1016/j.ejca.2016.12.039
8. Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol. 2018;29(3):731–736. doi:10.1093/annonc/mdx770
9. Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–249. doi:10.1200/JCO.2008.18.1545
10. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-7
11. Peng H, Dong D, Fang MJ, et al. Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2019;25(14):4271–4279. doi:10.1158/1078-0432.CCR-18-3065
12. Sun XS, Xiao BB, Lu ZJ, et al. Stratification of candidates for induction chemotherapy in stage III-IV nasopharyngeal carcinoma: a large cohort study based on a comprehensive prognostic model. Front Oncol. 2020;10:255. doi:10.3389/fonc.2020.00255
13. Xie HJ, Yu YF, Sun XS, et al. Identifying optimal candidates for induction chemotherapy among stage II-IVa nasopharyngeal carcinoma based on pretreatment Epstein-Barr virus DNA and nodal maximal standard uptake values of [(18) F]-fluorodeoxyglucose positron emission tomography. Cancer Med. 2020;9(23):8852–8863. doi:10.1002/cam4.3500
14. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
15. Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016;35(1):103. doi:10.1186/s40880-016-0167-2
16. Peng H, Chen L, Li WF, et al. Optimize the cycle of neoadjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: a propensity score matching analysis. Oral Oncol. 2016;62:78–84. doi:10.1016/j.oraloncology.2016.10.014
17. Wei J, Feng H, Xiao W, et al. Cycle number of neoadjuvant chemotherapy might influence survival of patients with T1-4N2-3M0 nasopharyngeal carcinoma. Chin J Cancer Res. 2018;30(1):51–60. doi:10.21147/j.issn.1000-9604.2018.01.06
18. Ai QY, King AD, Poon DMC, et al. Extranodal extension is a criterion for poor outcome in patients with metastatic nodes from cancer of the nasopharynx. Oral Oncol. 2019;88:124–130. doi:10.1016/j.oraloncology.2018.11.007
19. Huang L, Zhang Y, Liu Y, et al. Prognostic value of retropharyngeal lymph node metastasis laterality in nasopharyngeal carcinoma and a proposed modification to the UICC/AJCC N staging system. Radiother Oncol. 2019;140:90–97. doi:10.1016/j.radonc.2019.04.024
20. Wan Y, Tian L, Zhang G, et al. The value of detailed MR imaging report of primary tumor and lymph nodes on prognostic nomograms for nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiother Oncol. 2019;131:35–44. doi:10.1016/j.radonc.2018.11.001
21. Dong D, Zhang F, Zhong LZ, et al. Development and validation of a novel MR imaging predictor of response to induction chemotherapy in locoregionally advanced nasopharyngeal cancer: a randomized controlled trial substudy (NCT01245959). BMC Med. 2019;17(1):190. doi:10.1186/s12916-019-1422-6
22. Luo WJ, Zou WQ, Liang SB, et al. Combining tumor response and personalized risk assessment: potential for adaptation of concurrent chemotherapy in locoregionally advanced nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Radiother Oncol. 2021;155:56–64. doi:10.1016/j.radonc.2020.10.005
23. Xu C, Chen YP, Liu X, et al. Establishing and applying nomograms based on the 8th edition of the UICC/AJCC staging system to select patients with nasopharyngeal carcinoma who benefit from induction chemotherapy plus concurrent chemoradiotherapy. Oral Oncol. 2017;69:99–107. doi:10.1016/j.oraloncology.2017.04.015
24. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–1135. doi:10.1056/NEJMoa1905287
25. Lan XW, Xiao Y, Zou XB, Zhang XM, OuYang PY, Xie FY. Outcomes of adding induction chemotherapy to concurrent chemoradiotherapy for stage T3N0-1 nasopharyngeal carcinoma: a propensity-matched study. Onco Targets Ther. 2017;10:3853–3860. doi:10.2147/OTT.S133917
26. Li P, Zhang Q, Luo D, et al. Explore the value of adding induction chemotherapy to concurrent chemoradiotherapy in T3-4N0M0 nasopharyngeal carcinoma patients: a retrospective study. Cancer Manag Res. 2021;13:7067–7076. doi:10.2147/CMAR.S321471
27. Wang L, Wu Z, Xie D, Lv S, Xia L, Su Y. Can neoadjuvant chemotherapy improve survival in stage T3-4N1 nasopharyngeal carcinoma? A propensity matched analysis. Radiat Oncol. 2020;15(1):160. doi:10.1186/s13014-020-01594-4
28. Wu LR, Yu HL, Jiang N, et al. Prognostic value of chemotherapy in addition to concurrent chemoradiotherapy in T3-4N0-1 nasopharyngeal carcinoma: a propensity score matching study. Oncotarget. 2017;8(44):76807–76815. doi:10.18632/oncotarget.20014
29. Liu K, Lin S, Ke L, et al. Prognostic value and the potential role of treatment options for cervical lymph node necrosis in nasopharyngeal carcinoma. Oral Oncol. 2020;109:104864. doi:10.1016/j.oraloncology.2020.104864
30. Chen YP, Mao YP, Zhang WN, et al. Prognostic value of wait time in nasopharyngeal carcinoma treated with intensity modulated radiotherapy: a propensity matched analysis. Oncotarget. 2016;7(12):14973–14982. doi:10.18632/oncotarget.7789
31. Jiang YT, Chen KH, Yang J, et al. Prognostic significance of wait time for radical radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Head Neck. 2022;44(5):1182–1191. doi:10.1002/hed.27011
32. Liang H, Xiang YQ, Lv X, et al. Survival impact of waiting time for radical radiotherapy in nasopharyngeal carcinoma: a large institution-based cohort study from an endemic area. Eur J Cancer. 2017;73:48–60. doi:10.1016/j.ejca.2016.12.009
33. Yang K, Tian J, Zhang B, et al. A multidimensional nomogram combining overall stage, dose volume histogram parameters and radiomics to predict progression-free survival in patients with locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2019;98:85–91. doi:10.1016/j.oraloncology.2019.09.022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




