Back to Journals » Journal of Inflammation Research » Volume 15
Novel Signatures Based on the Lymphocyte-to-C-Reactive Protein Ratio Predict the Prognosis of Patients with Early Breast Cancer: A Retrospective Study
Authors Wang L, Zhang YL, Jiang C, Duan FF , Yuan ZY, Huang JJ, Bi XW
Received 27 February 2022
Accepted for publication 7 July 2022
Published 13 July 2022 Volume 2022:15 Pages 3957—3974
DOI https://doi.org/10.2147/JIR.S364284
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Li Wang,1,* Yu-Ling Zhang,2,* Chang Jiang,1,* Fang-Fang Duan,1 Zhong-Yu Yuan,1 Jia-Jia Huang,1 Xi-Wen Bi1
1Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 2Department of Endocrinology, Jiangxi Provincial People’s Hospital, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi-Wen Bi; Jia-Jia Huang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China, Email [email protected]; [email protected]
Background: The value of the lymphocyte-to-C-reactive protein (CRP) ratio (LCR) in early breast cancer (BC) is unclear. We explored the correlation between the LCR and survival of patients with early BC and established effective LCR-based prognostic signatures for predicting prognosis.
Methods: In this retrospective study, we randomized 623 patients with early-stage BC diagnosed in December 2010 to October 2012 at the Sun Yat-sen University Cancer Center to training and verification datasets. The median follow-up of all patients was 109 months. The survival differences were calculated by Kaplan–Meier method using the Log rank test. For overall survival (OS) and disease-free survival (DFS), the independent factors in the training dataset were identified using univariate and multivariate Cox analyses, in which two-tailed P-values < 0.05 were considered statistically significant. Based on this, we respectively constructed novel signatures for survival prediction and validated the efficiency of signatures through the concordance index (C-index), calibration and receiver operating characteristic (ROC) curves in both datasets.
Results: The LCR, lymphatic vessel invasion (LVI), progesterone receptor (PR) status, and Ki67 index were independent prognostic factors of OS. And the LCR and LVI are associated to DFS too. High LCR was associated with better OS and DFS. We constructed the prediction signatures based on those independent prognostic factors and calculated the risk scores. Patients in the training dataset with higher risk scores had significantly worse prognosis (P < 0.001). The signature had excellent discrimination capacity, with an OS C-index of 0.785 [95% confidence interval (CI): 0.713– 0.857] and 0.750 (95% CI: 0.669– 0.832) in the training and verification datasets, respectively. The time–ROC curves also suggest accurate prediction by the signature.
Conclusion: The LCR was a significant prognostic predictor of OS and DFS in early BC. The LCR-based prognostic signatures could be a useful tool for individualized therapeutic guidance.
Keywords: survival, nomogram, early breast cancer, LCR
Introduction
In women, breast cancer (BC) is the most common type of cancer.1 Although screening and diagnostic methods have been increasingly improved in the clinic, BC remains a threat to women’s health.2,3 In the clinic, clinicians typically choose treatments in terms of the molecular type of BC (estrogen receptor [ER]; progesterone receptor [PR]; human epidermal growth factor receptor 2 [Her2]; Ki67). Beside the molecular subtypes, various factors such as age, stage, and OncoType Dx test may affect the decision. There are several therapeutic regimens for each subtype.4–6 Despite this, BC recurrence and metastasis remain common clinical problems, resulting in poor curative effects and high mortality rates. Therefore, it is greatly necessary and important to search preferable prognostic factors for planning appropriate treatment. Currently, except for the tumor-node-metastasis (TNM) staging system,7 multifarious prognostic models have been proposed and trialed on a small scale.8–10 Nevertheless, owing to individual heterogeneity, accurate prediction of prognosis remains challenging worldwide. In addition, in many medium and small cities, people do not have access to Oncotype DX test, moreover, it’s costly too. Accordingly, identifying a reliable and robust indicator for patients with early BC is important and justified.
Inflammation is linked with cancer; specifically it plays decisive roles in almost all phases of tumor development, from initiation to metastasis.11,12 In recent years, inflammatory factor-related prognostic models have been reported in several cancers, for example, lung cancer and colorectal tumors.13–16 With regard to BC, the neutrophil-to-lymphocyte ratio (NLR),17,18 lymphocyte-to-monocyte ratio (LMR),19,20 and C-reactive protein (CRP)-to-albumin ratio (CAR) can be used as prognostic biomarkers.21 In infection, the lymphocyte count increases;22 meanwhile, the liver will generate more CRP.23 Therefore, the LCR, which combines the lymphocyte and CRP inflammatory indexes, might be a better reflection of the inflammatory condition than either index individually.
Nomograms integrating multifarious prognostic and decisive factors for forecasting event probability are now widely used in medical statistics.24,25 As far as we know, there remains a lack of studies on LCR as a precise prognostic factor of early BC. In the present article, we probed and tested the prognostic ability of LCR in patients with early BC. Based on the LCR, we established a graphic nomogram for predicting the survival outcomes of such patients, which might facilitate individualized survival predictions in the clinic.
Patients and Methods
Patient Selection
A total of 623 patients initially diagnosed as BC in December 2010 to October 2012 at the Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China) were randomly allocated to a training dataset and verification dataset in a 6:4 ratio. The inclusive criteria were: 1) age ≥ 18 years old; 2) pathological diagnosis as invasive BC; 3) without distant metastasis (lung, bone, liver, and brain); 4) available complete baseline laboratory data and specific follow-up data. We excluded patients who: 1) were pregnant or breastfeeding: 2) had a pathological diagnosis of ductal carcinoma in situ; 3) had synchronal malignancies; 4) had taken any medicine inducing an immune or inflammatory response in the most recent 3 months; 5) had any inflammatory disease (autoimmune diseases included).
In this study, all processes concerning human participants were conducted following the ethical principles of SYSUCC, the Helsinki declaration (1604) and its later revisions, and its analogical ethics. As this was a retrospective study, the requirement for signed informed consent by the patients was waived. In addition, all patients’ information was confidential.
Data Collection and Variable Categorization
All patient clinicopathological characteristics were manually extracted from the SYSUCC electronic medical records system. We extracted the patient age at diagnosis; postoperative pathological classification (PPC); lymphatic vessel invasion (LVI); T and N stage; ER, PR, and Her2 status; Ki67 index [stained with MIB1 monoclonal antibody (ZSGB-BIO, Beijing, China) and assessed by two professional pathologists]; and pathological grade (PG). Every participant underwent pretreatment assessments, including illness history inquiry, physical examination, and hematological and biochemical tests. The blood parameters CRP and lymphocyte before any anti-tumor treatment were also collected from the electronic medical records system.
BC specimens with >1% tumor nuclei positive for ER or PR via immunohistochemistry (IHC) testing were defined as ER- or PR-positive, respectively.26 For Her2 status, only tumor cells that scored 3+ on IHC or 2+ with ERBB2 gene amplification in Fluorescence in situ hybridization (FISH) were identified as Her2-positive.27 The continuous laboratory variable LCR was classified as a categorical variable based on its optimal cut-off value (0.33), calculated by maximally selected rank statistics analysis.
Outcome and Follow-Up
Overall survival (OS) time was specified as the interval from the time of diagnosis to death from any cause or to the last follow-up. Disease-free survival (DFS) time was referred to the time from randomization to disease recurrence or patient death from any cause. Patients were followed using outpatient examination and telephone interviews.
Statistical Analysis
The continuous variables and categorical variables were described by median values with interquartile ranges (IQR) and frequencies with percentages, respectively. Both variable comparisons and analysis of the relationship of LCR grade with other clinicopathological characteristics were conducted by chi-square test or Fisher’s exact test.
The LCR cut-off value was derived using the R package maxstat.28 Next, the LCR was classified as a categorical variable scored as 0 or 1. Cox proportional hazards matrixes were applied to conduct univariate and multivariate analyses. Only factors with two-tailed P-values < 0.2 in the univariate Cox regression analysis would be recruited to conduct the Proportional Hazards (PH) test. In multivariate analyses, two-tailed P-values < 0.05 were considered statistically significant. Based on the univariate and multivariate analyses results, nomograms with the terminal points of 5-year and 8-year OS and 5- and 8-year DFS were developed through the rms package in R software. Furthermore, the concordance index (C-index), calibration curves, and receiver operating characteristic (ROC) curves for OS and DFS were generated to evaluate the predictive performance and accuracy of the nomogram in both the training and validation datasets.
Furthermore, we counted the risk scores of all patients in the training and validation datasets using the corresponding regression coefficient of every recruited prognostic factor. The following formula was used: scores = e sum (every prognostic factor × corresponding coefficient). Then, based on the median risk scores, the patients in both datasets were divided into high- (total scores ≥ median scores) and low-risk (total scores < median scores) groups. Next, Kaplan-Meier survival analyses between the low- and high-risk groups in both datasets were performed using the survminer package and their significance was determined by the Log rank test.
All analyses were carried out in R software (version 4.1–0, Vanderbilt University, Nashville, TN, USA). P < 0.05 was considered significant except where specified otherwise.
Results
Patients’ Characteristics
We recruited 623 patients with early BC to the study. Table 1 shows their clinicopathological features. The whole cohort was randomized to a training and validation dataset in a 6:4 ratio (375 vs 248, respectively). Including LCR, all 12 clinicopathological variables and 4 treatment regimens were well balanced between the two datasets. The median age of all patients was 46 years (IQR: 39.0–55.0). Nearly half of the patients were T2 (53.5%) and N0 (49.6%). More than half of the patients (56.5%) had LVI. Most patients (91.8%) were diagnosed with invasive ductal carcinoma. The positive: negative ratio for hormone receptors (PR, ER) was approximately 2:1. Patients who were Her2-negative accounted for 70.8% (n = 441) of the whole cohort. Ki67 status was positive (>15%) in 418 patients (67.1%) and negative (≤15%) in 205 patients (32.9%). The majority of total patients received chemotherapy (85.1%) and more than half of patients received endocrine therapy (56.8%, 57.3% in training and validation datasets, respectively). There was no statistical difference between the LCR level and other clinicopathologic characteristics (P > 0.05) in the datasets (Table 2).
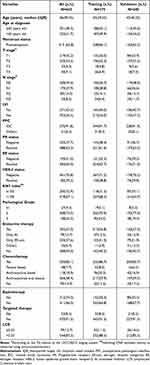 |
Table 1 Comparison of Baseline Clinicopathological Characteristics Between the Training and Validation Dataset |
 |
Table 2 The Relationship Between LCR Grade and Other Clinicopathological Characteristics in Training and Validation Sets |
Survival Analysis of Training Dataset Based on the LCR
In the training dataset, the LCR was correlated with OS and DFS. And the median follow-up of all patients in this group is 109 months. Compared to patients in the LCR-low (LCR ≤ 0.33) group, patients in the LCR-high (LCR > 0.33) group had better overall and disease-free survival (Figures 1 and 2, P < 0.001).
 |
Figure 1 Kaplan-Meier curves for the overall survival (OS) of patients based on lymphocyte/c-reactive protein ratio (LCR). |
 |
Figure 2 Kaplan-Meier curves for the overall survival (DFS) of patients based on lymphocyte/c-reactive protein ratio (LCR). |
Univariate and multivariate analyses for OS revealed that the LCR was a significant individual prognostic variable for OS (Table 3) and DFS (Table 4).
 |
Table 3 Univariate and Multivariate Cox Regression Analysis of OS in Training Set |
 |
Table 4 Univariate and Multivariate Cox Regression Analysis of DFS in Training Set |
Nomogram Establishment
We used the Cox proportional hazards regression model to respectively build prognostic signatures based on the stepwise regression method for OS (Figure 3A) and DFS (Figure 4A). Subsequently, combining the statistical results and clinical significance, we predicted 5- and 8-year of OS rates using the LVI, PR, Ki67, and LCR (Figure 3B). And the T stage, LVI and LCR were applied for predicting 5- and 8-year of DFS (Figure 4B).
Nomogram Verification
To validate the robustness of the OS prognostic model, which included four prognostic factors from the training set, we performed further verification in both datasets. The predictive nomogram exhibited a good predictive performance with a C-index of 0.785 (95% CI: 0.713–0.857) and 0.750 (95% CI: 0.669–0.832) in the training and validation dataset, respectively. The results also revealed an excellent area under the ROC curve (AUC) for OS of the nomogram in both the training dataset (5-year AUC: 0.809, 8-year AUC: 0.768) (Figure 5A) and validation dataset (5-year AUC: 0.770, 8-year AUC: 0.707) (Figure 5B). Furthermore, the calibration curves showed good consistency for the 5-and 8-year of OS between the actual observed and predicted survival rate in the training (Figure 5C and D) and validation datasets (Figure 5E and F). Similarly, we also verified the prediction model of DFS on the training and validation sets. The C-index of 0.815 (95% CI: 0.749–0.881) and 0.678 (95% CI: 0.582–0.774) in the training and validation dataset, respectively. The ROC curves for the DFS in training set and validation set are shown in the Figure 6A and B, respectively. In addition, the results of 5-and 8-year of DFS between the actual observed and predicted survival rate in the training (Figure 6C and D) and validation datasets (Figure 6E and F) also showed good consistency.
We counted the risk scores of every patient in both datasets (score = e sum (every prognostic factor × corresponding coefficient)) and then divided them into low- and high-risk groups according to the median risk score (Figures 7A, B and 8A, B). Survival analysis showed that patients in the high-risk group had poorer OS (Figure 7C and D) and DFS (Figure 8C and D) than those in the low-risk group. And in the high-risk group, the 5- and 8-year OS probability are respectively 88.6% and 84.6%. Correspondingly, the 5- and 8-year OS probability of patients in low-risk group achieve at 94.3% and 89.6%, respectively. Consistently, the high-risk group had higher mortality (Figure 7E and F) and high risk of recurrence or metastasis (Figure 8E and F).
Discussion
In this study, 623 BC patients were enlisted and randomized into training and validation datasets in a 6:4 ratio. According to the optimal LCR cut-off value of 0.33, the participants were grouped into LCR-low and LCR-high groups. The multivariate analysis results for the training dataset revealed that LVI, PR status, and the LCR were independent predictors of OS for patients with early-stage BC. And T stage, LVI, and the LCR were independently to predict DFS. The multivariate Cox regression analysis revealed no obvious statistical significance for the Ki67 index. Nevertheless, considering its clinical significance, we incorporated it into the nomogram construction for individualized prognostic prediction, which showed satisfactory discrimination and calibration. In addition, the model underwent verification in the validation dataset. The C-index, calibration curves, and ROC curve AUC also revealed good discriminative ability and satisfactory prognostic accuracy.
The relevance of inflammation to cancer was first proposed by Virchow in 1863.29 Subsequently, numerous studies have explored and reported on this relationship.30–32 At present, there is consensus that inflammation contributes to tumor development. Inflammation is involved in the construction of the tumor microenvironment and systemic changes for promoting tumor expansion. Additionally, inflammation can cause changes in the peripheral blood, such as achroacytosis and neutrophilia.33 Lymphocytes can inhibit tumor development by boosting immunosurveillance.34 Consequently, the increased lymphocyte number can indicate enhanced regulatory inhibition of tumor. Moreover, CRP accumulates massively in plasma when the body or tissue experiences infection or injury. It can activate complement and strengthen phagocytosis of phagocytes to regulate and eliminate pathogenic microorganisms.35 To summarize, as an inflammatory marker, lymphocytes have high specificity but low sensitivity, while CRP has high sensitivity but low specificity. Combining the two to complement each other is a good option. Moreover, the high LCR value linked to longer survival has been validated in other cancers.36–38
Second, it had been confirmed that the first step of metastatic dissemination involves local invasion of the adjacent organizations and entry to the microvasculature consisting of lymph and blood systems. Then, the tumor cells translocate to the micro-vessels of distant tissues and organs through the bloodstream. Therefore, LVI is an independent prognostic factor of poor OS and DFS.39,40
Third, PR is not only a gene target of ERα but also can modulate its behavior.41 In Erα-positive BC cells with PR expression, estrogen-mediated proliferation and ERα transcriptional activity is blocked.42 Blows explore the prognostic value of PR for patients with Erα-positive BC in a meta-analysis based on 10,159 patients, in which the mortality hazard ratio for PR status is showed to be time-dependent.43 In addition, high PR levels are associated with fewer metastatic events in early BC.44
Fourth, the Ki67 index, named for the expression level of the cell cycle antigen Ki67 in IHC staining, is calculated as follows: Ki67 index = positive-staining cells/total malignant cells in the tumor tissue. The Ki67 index is widely acknowledged as a proliferative marker and is used as an independent prognostic signature in early BC. High Ki67 levels are associated with higher mortality and shorter OS.45,46
Taking the individual patient’s condition into consideration, we believe that this prognostic signature is optimal for patients with early BC. Although several models based on NLR, LMR, CAR, or genes may predict patient prognosis,18,21,47 our model has its own characteristics. First, we used the C-index, calibration curves, and ROC curve survival analysis together to validate the robustness of our signature, and these methods are rarely used together. Moreover, our nomogram demonstrates a better AUC. Second, with regard to real-world feasibility, all information required in our model was obtained through basic characteristics inquiry, hematological and biochemical tests, and pathological examination, which is more convenient and economical than that required for gene-based prognosis models.48
Our study has some limitations. First, selection bias is common in retrospective studies, and our study is no exception. Second, this was a single-center study, which might have affected the stability of the results somewhat. Third, we focused only on the pre-treatment LCR and did not explore its dynamic change during therapy. Finally, our nomogram was not validated externally and might require further validation in a prospective, multicenter, and larger-sample cohort in the future.
Conclusion
We innovatively explored the clinical value of a novel inflammatory marker in patients with early-stage BC, LCR, and revealed that it is a significant prognostic predictor of OS and DFS. The LCR-based prognostic signature demonstrated good predictive probability and accuracy. Therefore, it can be used as a tool for promoting individualized survival prediction.
Abbreviations
CRP, C-reactive protein; LCR, lymphocyte-to-C-reactive protein ratio; BC, breast cancer; OS, overall survival; C-index, concordance index; ROC, receiver operating characteristic; LVI, lymphatic vessel invasion; PR, progesterone receptor; AUC, area under the curve; ER, estrogen receptor; Her2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; CAR, C-reactive protein-to-albumin ratio; SYSUCC, Sun Yat-sen University Cancer Center; PPC, postoperative pathological classification; PG, pathological grade; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; IQR, interquartile ranges; PH, proportional hazards.
Data Sharing Statement
The data analyzed in this study are available from the corresponding author (Xi-Wen Bi, E-mail: [email protected]) on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by ethics committee of Sun Yat-sen University Cancer Center (registration number: B2022-277- 01). Owing to we just retrospectively reviewed their medical data and did not impair their health, patient’s informed consent was waived.
Acknowledgments
The authors gratefully acknowledge contributions from the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Funding
This study is supported by the Natural Science Foundation of Guangdong Province (No.2019A151011781) and the Sci-Tech Project Foundation of Guangzhou City (No. 202002020033), without the involvement of commercial entities.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(11):1362–1389. doi:10.6004/jnccn.2018.0083
3. Schünemann HJ, Lerda D, Quinn C, et al. Breast cancer screening and diagnosis: a synopsis of the european breast guidelines. Ann Intern Med. 2020;172(1):46–56. doi:10.7326/M19-2125
4. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi:10.1093/annonc/mdr304
5. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi:10.1093/annonc/mdv221
6. Curigliano G, Criscitiello C. Maximizing the clinical benefit of anthracyclines in addition to taxanes in the adjuvant treatment of early breast cancer. J Clin Oncol. 2017;35(23):2600–2603. doi:10.1200/JCO.2017.72.5960
7. Zhang J, Zhao B, Jin F. The assessment of 8th edition AJCC prognostic staging system and a simplified staging system for breast cancer: the analytic results from the SEER database. Breast J. 2019;25(5):838–847. doi:10.1111/tbj.13347
8. Yu Y, Tan Y, Xie C, et al. Development and validation of a preoperative magnetic resonance imaging radiomics-based signature to predict axillary lymph node metastasis and disease-free survival in patients with early-stage breast cancer. JAMA Netw Open. 2020;3(12):e2028086. doi:10.1001/jamanetworkopen.2020.28086
9. Liu D, Wu J, Lin C. Breast subtypes and prognosis of breast cancer patients with initial bone metastasis: a population-based study. Front Oncol. 2020;10:580112. doi:10.3389/fonc.2020.580112
10. Cui X, Zhu H, Huang J. Nomogram for predicting lymph node involvement in triple-negative breast cancer. Front Oncol. 2020;10:608334. doi:10.3389/fonc.2020.608334
11. Kim S, Takahashi H, Lin -W-W, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi:10.1038/nature07623
12. Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi:10.1038/457036b
13. McMillan DC. The systemic inflammation-based glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.003
14. Zhang N, Ning F, Guo R, et al. Prognostic values of preoperative inflammatory and nutritional markers for colorectal cancer. Front Oncol. 2020;10:585083. doi:10.3389/fonc.2020.585083
15. Toyokawa T, Muguruma K, Yoshii M. Clinical significance of prognostic inflammation-based and/or nutritional markers in patients with stage III gastric cancer. BMC Cancer. 2020;20(1):517. doi:10.1186/s12885-020-07010-0
16. Sandfeld-Paulsen B, Meldgaard P, Sorensen BS. The prognostic role of inflammation-scores on overall survival in lung cancer patients. Acta Oncol. 2019;58(3):371–376. doi:10.1080/0284186X.2018.1546057
17. Moldoveanu D, Pravongviengkham V, Best G, et al. Dynamic neutrophil-to-lymphocyte ratio: a novel prognosis measure for triple-negative breast cancer. Ann Surg Oncol. 2020;27(10):4028–4034. doi:10.1245/s10434-020-08302-2
18. Yoon CI, Kim D, Ahn SG, et al. Radiotherapy-induced high neutrophil-to-lymphocyte ratio is a negative prognostic factor in patients with breast cancer. Cancers. 2020;12(7):1896. doi:10.3390/cancers12071896
19. Gilmore N, Mohile S, Lei L, et al. The longitudinal relationship between immune cell profiles and frailty in patients with breast cancer receiving chemotherapy. Breast Cancer Res. 2021;23(1):19.
20. Van Berckelaer C, Vermeiren I, Vercauteren L, et al. The evolution and prognostic role of tumour-infiltrating lymphocytes and peripheral blood-based biomarkers in inflammatory breast cancer patients treated with neoadjuvant chemotherapy. Cancers. 2021;13(18):4656. doi:10.3390/cancers13184656
21. Zhou L, Ma S, Balde AI, et al. A retrospective propensity score matched study of the preoperative c-reactive protein to albumin ratio and prognosis in patients with resectable non-metastatic breast cancer. Med Sci Monit. 2019;25:4342–4352.
22. Moro-García MA, Mayo JC, Sainz RM, et al. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol. 2018;9:339. doi:10.3389/fimmu.2018.00339
23. Sproston NR, Ashworth JJ. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
24. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi:10.1016/S1470-2045(14)71116-7
25. Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
26. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi:10.1200/JCO.19.02309
27. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi:10.1200/JCO.2018.77.8738
28. Kassambara A, Kosinski M, Biecek P, et al. Package “survminer”. Available from: https://cran.r-project.org/web/packages/survminer/survminer.pdf.
29. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
30. Garber K. First results for agents targeting cancer-related inflammation. J Natl Cancer Inst. 2009;101(16):1110–1112. doi:10.1093/jnci/djp266
31. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
32. Gkretsi V, Zacharia LC, Stylianopoulos T. Targeting inflammation to improve tumor drug delivery. Trends Cancer. 2017;3(9):621–630. doi:10.1016/j.trecan.2017.07.006
33. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
34. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi:10.1038/ni1102-991
35. Kuscuoglu D, Janciauskiene S, Hamesch K, et al. Liver - master and servant of serum proteome. J Hepatol. 2018;69(2):512–524. doi:10.1016/j.jhep.2018.04.018
36. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272(2):342–351. doi:10.1097/SLA.0000000000003239
37. Zhang H, Wang Y, Ni J, et al. Prognostic value of lymphocyte-C-Reactive protein ratio in patients undergoing radical cystectomy for bladder cancer: a population-based study. Front Oncol. 2021;11:760389. doi:10.3389/fonc.2021.760389
38. Okugawa Y, Toiyama Y, Fujikawa H, et al. Prognostic potential of lymphocyte-C-Reactive protein ratio in patients with rectal cancer receiving preoperative chemoradiotherapy. J Gastrointest Surg. 2021;25(2):492–502. doi:10.1007/s11605-019-04495-4
39. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi:10.1126/science.1203543
40. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi:10.1038/nrc2622
41. Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523(7560):313–317. doi:10.1038/nature14583
42. Zheng Z-Y, Bay B-H, Aw S-E, et al. A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J Biol Chem. 2005;280(17):17480–17487. doi:10.1074/jbc.M501261200
43. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi:10.1371/journal.pmed.1000279
44. Pichon MF, Pallud C, Brunet M, et al. Relationship of presence of progesterone receptors to prognosis in early breast cancer. Cancer Res. 1980;40(9):3357–3360.
45. Petrelli F, Viale G, Cabiddu M, et al. Prognost ic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–491. doi:10.1007/s10549-015-3559-0
46. De AE, Cardoso F, de Castro GJ, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007;96(10):1504–1513. doi:10.1038/sj.bjc.6603756
47. Peng Y, Chen R, Qu F, et al. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol Ther. 2020;21(2):189–196. doi:10.1080/15384047.2019.1680057
48. Sun L, Brentnall A, Patel S, et al. A cost-effectiveness analysis of multigene testing for all patients with breast cancer. AMA Oncol. 2019;5(12):1718–1730.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






