Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Importance of the Diaphragm in Neuromotor Function in the Patient with Chronic Obstructive Pulmonary Disease
Authors Bordoni B , Escher A , Compalati E, Mapelli L , Toccafondi A
Received 9 January 2023
Accepted for publication 13 April 2023
Published 11 May 2023 Volume 2023:18 Pages 837—848
DOI https://doi.org/10.2147/COPD.S404190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Bruno Bordoni,1 Allan Escher,2 Elena Compalati,1 Luca Mapelli,1 Anastasia Toccafondi1
1Department of Cardiology, Institute of Hospitalization and Care with Scientific Address, Foundation Don Carlo Gnocchi IRCCS S Maria Nascente, Milano, Italy; 2Anesthesiology/Pain Medicine, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Correspondence: Bruno Bordoni, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a constant and chronic narrowing of the respiratory airways, with numerous associated symptoms, not always related to the pathological adaptation of the lungs. Statistical projections show that COPD could become the third leading cause of death globally by 2030, with a significant increase in deaths by 2060. Skeletal muscle dysfunction, including the diaphragm, is one of the causes linked to the increase in mortality and hospitalization. Little emphasis is given by the scientific literature to the importance of the diaphragm towards functional neuromotor pathological expressions. The article reviews the adaptation of the skeletal muscles, with greater attention to the adaptations of the diaphragm, thereby highlighting the non-physiological variations that the main respiratory muscle undergoes and the neuromotor impairment found in COPD. The text could be an important reflection from a clinical and rehabilitation point of view, to direct greater attention to the function and adaptation of the diaphragm muscle.
Keywords: diaphragm, COPD, physiotherapy, rehabilitation, pain, fascia, ageing, osteopathic
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex and constantly evolving pathology, which is characterized by a progressive and constant limitation of the available air volume (airflow obstruction).1 COPD could become the third leading cause of death for the population by 2030.1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) identifies COPD as:
a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.2
GOLD estimates that the number of patients who will die of COPD in 2060 will be approximately 5.4 million deaths annually, compared to 3.2 million deaths in 2015 annually.3 In the USA, the incidence of the disease involves 10.2–20.9% of the population, in Europe, despite a trend of reduction in mortality and a decreased difference between men and women, the percentage of findings remains high (3–26.1%).3 Some data from Africa, few and not covering all geographical areas, show COPD detection rates of 1.6–23.8%; in Latin America, the percentage is between 30% and 31.1%.3 In China, the percentage is about 8.6% (data from 2018), and highly variable and incomplete values for other Asian regions, with an average of 3.5–6.7%.3
COPD is considered as the fifth cause of burden on the economy of the various states due to recurring hospitalizations and pharmacological treatments.4 Probably, this global economic aggravation derives from the fact that this pathology does not involve only the pulmonary area but develops many dysfunctions and physiological alterations involving the whole-body system.4 Although emphysema and chronic bronchitis are responsible for triggering the chronicity of the disease, the patient does not always go to hospital for respiratory reasons, but for important cardiac problems, fractures, the onset of lung tumours, mood disorders, muscle complex that prevent independence and severe metabolic alterations.1,5 Furthermore, it is not always possible to correctly assess the severity of COPD, as there is only a moderate relationship between pulmonary adaptation and the reduction in the patient’s quality of life.6 COPD is a systemic disease, and as such it is expressed with multiple symptoms, such as insomnia and drowsiness, dry mouth, anorexia, pain (local or from multiple involved areas), nausea, constipation, gastric reflux, dysphagia, sleep apnea, cough, fatigue and shortness of breath, wheezing and dyspnea, temporomandibular disorders and urinary incontinence.2,5,7–10 Some symptoms related to the presence of COPD and which have a negative impact on the mortality and morbidity of patients, are the finding of anxiety and depression, with values ranging from 8% up to a maximum of 80% of patients.1,5,11 Functional and structural alterations of the skeletal muscles (almost all patients), lack of coordination and an increase in accidental falls cause a decrease in active movement, leading to a decline in quality of life, an increase in mortality and in the number of hospitalizations.12–14
The article reviews the adaptation of the skeletal muscles, with greater attention to the adaptations of the diaphragm, trying to highlight the relationship between the non-physiological variations that the main respiratory muscle undergoes and the neuromotor impairment found in COPD. The text could be an important reflection from a clinical and rehabilitation point of view to direct greater care to the function and adaptation of the diaphragm muscle.
Peripheral Skeletal Muscle Adaptation in the Presence of COPD
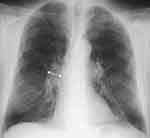
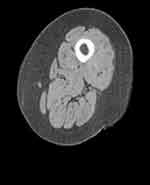
Patients with COPD experience skeletal muscle alterations, causing an increase in the rate of morbidity and mortality, as well as in disease exacerbation events and in the number of hospitalizations.15 Respiratory disease does not always perfectly reflect peripheral muscle adaptation.13,14 The following figures highlight, for the same patient, a slower pathological adaptation of the lungs of an average degree, compared to an accentuated sarcopenic adaptation (Figures 1 and 2).
Muscle functional impairment is found more with emphysematous patients than with chronic bronchitis, and for about 55% of patients with stable COPD; this process probably occurs faster than the pathological adaptation of the lungs.13,14 There are different causes, single or superimposable, which can induce skeletal muscle dysfunction. Cigarette smoke, the presence of diabetes, malnutrition, advanced age, polluted air and a sedentary lifestyle combine to produce non-physiological muscular behaviors.16,17 COPD is a further cause of muscle dysfunction. We can find systemic inflammation, oxidation, multiple drug intake, nocturnal and diurnal hypoxia, hypercapnia. The patient often presents co-morbidities such as polyneuropathy, osteoporosis, cardiovascular pathologies, bronchiectasis, obstructive sleep apnea, chronic kidney disease, anxiety and depression, nonalcoholic fatty liver disease, nonspecific pain and gastroesophageal reflux.5,9,11,18–20 These co-morbidities negatively affect muscle adaptation.
Changes in Muscles in COPD: Males versus Females
Females with COPD suffer a loss of lean mass in a greater percentage than males, and with a lower value of the expressed strength.21 Limb musculature undergoes a phenotypic transition in patients, with a tendency for loss of number and volume of aerobic fibers or type I fibers, and an increase in anaerobic fibers or type II fibers, compared to age-matched healthy subjects.21 Women have slightly fewer type IIx fibers (more glycolytic muscle cells than type II fibers), and a higher number of hybrid fibers. The latter information highlights a difficulty of the musculature in women to implement a correct regeneration and with more dilated times (compared to men).21 Women lose more muscle mass and are weaker than men. In patients with COPD, we can find sarcopenia, decrease in mass and strength, and concomitant atrophy, weakness and disappearance of lean material.22 Skeletal muscle areas, particularly of the extremities, have reduced capillary architecture, and increased mitochondrial and ribosomal dysfunction.14,23
Stable Disease vs Exacerbation
Exacerbation of respiratory symptoms causes an increase in non-physiological adaptations of skeletal muscles. The expression of muscle strength after 3 days of hospitalization, compared to stable patients, is lower; after 5 days of hospitalization, muscle strength is reduced by 5%, compared to outpatients.24 Hospitalization reduces muscle strength not only in the lower limbs, but also in the upper limbs; this decrease is greater for patients subject to multiple exacerbations during the year.24 The acute phase of the disease causes a reduction in the lean mass of the parasternal/intercostal muscles, which in parallel reflect the decrease in leg volumes; chest muscle weakness corresponds to spirometric severity.25,26 The severity of the presence of sarcopenia/atrophy in patients with COPD determines a worse prognosis, with increased mortality.16,25,27 We do not know the detailed pathological reasons that cause such muscular adaptations, and further studies will be necessary to better understand the therapeutic procedure.
Balance Impairment
Non-physiological muscle adaptation in patients with COPD, as measured by surface electromyography (sEMG) and electromyographic activity (EMG) under stress, determines a decrease in neuromotor coordination and early fatigue.28–32 This impaired neuro-coordination of the limbs and trunk during active motor activity of patients causes accidental falls and increased fear of falling, creating a behavioral framework that further degenerates into motor dysfunction, physical deconditioning and increased mortality.33 Patients with COPD have an approximately 51% higher rate of falling than healthy people of the same age.34 Females are more prone to accidental falls than males.34 During the acute phase, with hospitalization, the percentage of falls increases up to 55% of patients, and always with a higher finding value than in non-COPD and hospitalized elderly subjects (35%).34 The risk of falls in COPD is not related to forced expiratory volume in the 1st second (FEV1).34 Impaired neuro-coordination causes a disturbance of body balance during daily activities, a decrease in limb and trunk control during walking, regardless of the degree of severity of lung function.34,35
There are several reasons given in order to understand the problem of lack of balance. The same causes that lead to muscle dysfunction, mentioned above, are pointed out as important genesis of balance deterioration.35 Other causes leading to an increase in the incidence of falls could be related to the presence of depression and anxiety, the use of portable oxygen devices, dyspnoea, altered biomechanics of the chest, decreased muscle elasticity, reduced neurocognitive function, obesity.16,34–38 A possible decline in the components that help maintain balance, the vestibular area and the proprioceptive system, can increase the number of accidental falls.16,39 Visual dysfunctions in COPD patients would not result in a balance disorder.40
Patients with COPD and neuromuscular dysfunctions show a slower recovery in restoring the center of body balance, in the presence of external stresses, and with accentuated trunk rigidity.41 There is greater body sway during limb movements, a slower pace than in people of the same age but not with COPD: the pattern of movement and gait in patients is altered.41,42
Another possible cause that would determine a dysfunction of balance, placing COPD among the chronic pathologies with the highest incidence of accidental falls (behind only osteoarthritis), comes from the non-physiological adaptation of the diaphragm muscle.40,42,43
Diaphragm Muscle Adaptation in the Presence of COPD
Currently, we do not have sufficient clinical attention directed to the diaphragm muscle in COPD patients; diaphragmatic dysfunction is present at every stage of the disease.3
Effect of Dynamic Hyperinflation
The maximal inspiratory pressure (PImax) is lower than in healthy subjects, as is the transdiaphragmatic pressure (Pdi) generated by the diaphragm.40,44 The contractile fibers are shortened, giving an inspiratory attitude and a flatter morphology.44 This morphological dysfunction corresponds to a lower functionality and expressed strength; the latter reflects the presence of hyperinflation and dyspnoea.44 Figure 3 highlights a diaphragm of a COPD patient in an inspiratory attitude, with flattening of the diaphragm; in the healthy subject, the right area is about 1.9 centimeters higher.45
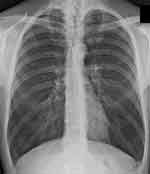 |
Figure 3 Chest x-ray of a COPD patient showing pulmonary hyperinflation characterized by parenchymal hyperdiaphania and flattening of the diaphragm. |
In COPD patients, the elasticity of the lung parenchyma decreases exponentially with the chronicity of the disease. In obese patients, in particular (especially in the android form), lipofibroblasts can accumulate in the lung tissue, secreting pro-inflammatory substances (adipocytokines) and transform into myofibroblasts, increasing pulmonary fibrosis.46 As the disease progresses, the chest expands to a lesser extent, the intercostal musculature will become stiffer, and the respiratory accessory musculature will be more ineffective.47 The diaphragm will be forced to work harder to overcome the resistances of the more rigid chest, with consequent morphological change (more “flat”), structural change (shorter fibers), positional change (inspiratory attitude), phenotypic change (phenotypic shift) and functional (greater stiffness).
The diaphragm undergoes a flattening with mechanical disadvantages. There is a reduction of its area of apposition, a decline in the coordination between the diaphragm itself and the rib muscles, an increase in the radius of curvature (less ability to maintain the tension produced).3,44,48 The phrenic nerve undergoes myelin damage, with a slowing of the conduction velocity, with higher neuropathy values for subjects with hyperinflation and, in particular, for the left phrenic area.48,49 Neuropathic adaptations correlate positively with FEV1.49 Such neuropathy is a direct indicator of the risk of accidental falls.50
Change in Contractile Function and Fiber Phenotype
The diaphragm undergoes an unphysiological phenotypic adaptation, with an increase in type I fibers and a decline in type II fibers. Such metabolic change appears to occur faster than the phenotypic change of limb musculature.44
Contractile fibers show signs of atrophy, myolysis, sclerosis and fibrosis.44,47 We can find a decline in endoplasmic reticulum function, which dysfunction causes an accumulation of intracellular calcium (Ca2+).51 The accumulation of Ca2+ in the sarcoplasm is cytotoxic for the cell, stimulating biochemical reactions that can lead to apoptosis.52 Furthermore, Ca2+ can bind to inorganic phosphorus (Pi), slowing and preventing the formation of actomyosin bridges, causing a weaker diaphragmatic contraction.44,53 The force expressed by the diaphragm is about 35% lower in patients, in particular, in patients with severe COPD.3
The amount of myosin decreased by about 50% less than in healthy subjects, with a decreased Ca2+ binding sensitivity, with further decline in contractile strength.44 The amount of the nebulin protein is reduced, making the muscle fiber more fragile to mechanical stress.44,54 Another sarcomeric protein, titin, does not seem to decrease in quantity, but undergoes a decline in elastic capacity, negatively altering the mechanotransductive capacity of the fiber (less regenerative capacity and greater stiffness).44
The volume of fibers, both glycolytic and oxidative, has a reduced volume (atrophy) of about 40–60% compared to healthy subjects, with increases in the ubiquitin-proteasome pathway. There is an accumulation of sarcomere area Z proteins, a misalignment of the sarcomeres and loss of serial sarcomeres (about 10–15% of the total), typical of a myopathy.3,14,44,55 There are several pathways that disrupt diaphragm fiber structure and function in the presence of oxidative stress and inflammation. Activation of the canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, the activation of the non-canonical NF-κB pathway, and activation of the myostatin-mothers against decapentaplegic homolog 3 (Smad3) pathway.56
The fiber is shortened by about 28% compared to healthy subjects, with an increase in collagenous tissue.3,57 The regeneration capacity of the fibers is reduced (increased myostatin values), as is the ability of the satellite cells to repair the various muscle components.3,44 FEV1 is inversely related to diaphragm hypotrophy.44
Changes in Blood and Lymph Supply
We do not know the adaptation of the lymphatic vessels of the diaphragm in the presence of COPD. Generally, with inflammation involving the body system, from the chest to the abdomen, the diaphragm undergoes lymphangiogenesis.58
The blood volume and capillary scaffolding affecting the diaphragm musculature appear to be preserved and, despite the increase in the number of type I fibers, the diaphragm shows reduced endurance capacity.44 It appears that the patient stops physical activity due to diaphragmatic weakness, despite an increase in phrenic electrical activity; dyspnea is the response to diaphragm fatigue and not necessarily to pathological pulmonary adaptation.3,59
Diaphragm weakness, hypotrophy (diaphragm thickening fraction), reduced range of motion, are correlated with symptom exacerbation and re-hospitalization.60 The diaphragm predicts the possible exacerbation of symptoms in COPD patients, and it becomes essential to evaluate the diaphragm for an in-depth clinical assessment.60
Another step forward that the clinician should take is to consider the importance of the diaphragm as a determinant for the neuromotor expression of the patient with COPD.
Role of the Diaphragm in Neuromotor Function and Balance
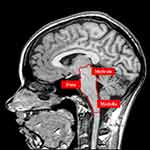
The diaphragm is an important muscle not only in the respiratory field, but also in the neuromotor field.61 The areas that manage the breath and non-respiratory actions of the diaphragm, and which serve as an informational crossroads between the brain areas and the spinal cord, are located in the pons, midbrain and medulla, ie, the central pattern generator (CPG).61 Within the CPG, we find the pre-Bötzinger complex (preBötC), the caudal ventral group (VRGc), the rostral ventral group (VRGr), the parabrachial/Kölliker-Fuse complex and the nucleus of the solitary tract (NTS) of the vagus nerve (Figures 4).61
Proprioceptive Response from the Diaphragm
The movement of the diaphragm generates a Pdi, which is the difference between gastric and intrapleural pressure.62 These pressure changes and redistribution of body fluids activate multiple body receptors (mechanoreceptors, visceroceptors, chemoreceptors, baroreceptors, etc.), which are part of the exteroceptive and interoceptive system; the latter two fall within the definition of proprioception.63
The diaphragm itself carries phrenic afferents from mechanoreceptors (30–45% sensory-type fibers), which are activated by specific stimuli (animal studies). Type IA fibers are activated during the exhalation phase (lengthening of muscle fibers) and during constant contraction and in the presence of fatigue.64 Type Ib fibers (Golgi tendon organs and simil-Pacinian corpuscles) send information during an increase in the contractile stress of the diaphragm (physical activity), during inspiration. Type III–IV fibers, myelinated and unmyelinated (type C fibers), respectively, and with a smaller diameter than the previous fibers (type IA), are activated by unphysiological breathing patterns and by the presence of metabolites related to contractile fatigue.64
The unmyelinated fibers, the most representative of the diaphragm, can have components of a sympathetic type, and on an animal model, they send information in the presence of diaphragmatic fatigue with vasoconstrictive functions; type III fibers are not activated by diaphragmatic contractile fatigue.64,65 Studies on cadavers show that the right phrenic nerve has catecholaminergic axons and has a larger diameter, while the left phrenic nerve lacks them and has a smaller overall diameter; the reasons are not known.66
There are only a few dozen spindles of the diaphragm but, despite this evident diversity compared to the skeletal muscles of the limbs and trunk, they are sufficient to exert an adequate afferential influence towards the central nervous system.67 In a healthy subject, before the diaphragm performs a complete movement of inspiration, low-threshold cutaneous receptors (Merkel, Ruffini) are stimulated.68,69 These receptors send afferents to the cortex (somatosensory opercular area or primary somatosensory cortex or area S1).61 The S1 area is important to prepare the body system to adequately manage proprioceptive stimulations, activating the insula and the anterior cingulate cortex.70 In particular, the right insula is fundamental for the conception of the self, that is, the correct elaboration of the bodily stimuli for the expression of the final movement (and the emotional aspect) with respect to our adequacy in executing this movement in the context in which where we are, at a given moment.71 When the diaphragm contracts to complete the inspiration, all the stimulated body receptors and the few diaphragmatic receptors will send information to the midbrain area (about 95% of the information) and to the spinal trigeminal nucleus, via spinothalamus pathways (laminae I–X).61,72,73 The nucleus of the solitary tract (NTS) in the midbrain will receive the major afferential information, while only a small portion of the receptor inputs will go to the spinal trigeminal nucleus or trigeminal nucleus caudalis.61,74 Likewise, the afferents of the vagus nerve from the crural area of the diaphragm (and from the phrenoesophageal ligaments), stimulated by the respiratory movement, will arrive at the NTS.75 The NTS will reciprocally exchange the information received with the cerebellum (from all cerebellar nuclei) and with the vestibular area.76,77 The trigeminal nucleus caudalis will exchange information with the vagal system (NTS), with the cerebellum (paramedian lobule) and with the vestibular area.78–81 The processing of the data obtained from this neurological network will be sent by the NTS towards the limbic area (periaqueductal gray area, amygdala, thalamus, pituitary) and towards the primary motor cortex or M1 (motor coordination) and the supplementary motor area of the cortex o SMA (movement planning and learning).61,73,82–84
Descending excitatory information will be sent from the limbic area and motor cortex M1-SMA to the NTS.61,83,85,86 Finally, NTS sends inhibitory information to the rostral ventrolateral medullary area or premotor area of the sympathetic system.61,79,87 NTS will involve the CPG and phrenic neurons.66 Inhibition of the sympathetic area will produce an increase in the activity of the parasympathetic system, affecting neuromotor expression, with increases in strength and coordination (in particular, with slow and deep breaths).61,86,88
Intra-Abdominal Pressure and Posture
The diaphragm is essential not only for the force expressed, but also for maintaining body posture during daily activities, controlling the position of the lumbodorsal area allows for better control of limb movement.66 The inspiration causes an increase in intra-abdominal pressure (IAP), thanks also to the activation of the abdominal muscles (in particular, the transversus muscle), and the descent of the pelvic floor.61,89 The contraction of the diaphragm precedes the movements of the limbs, that is, for the anticipatory postural regulation.89 This pre-contraction allows the various spinal and cortical centers to have information before deciding what movement to make. The greater the demand for muscle strength in the limbs (lifting a load or pedaling), the wider the excursion of the diaphragm will be; in this way, there will be a better stabilization of the trunk (greater IAP) and an optimization of the coordination of the limbs.89,90 If the IAP is insufficient, for example, due to shallower breathing, postural control problems, balance alterations and limb dysfunctions will occur.89,91 IAP creates a hydraulic effect for the stabilization of the lumbodorsal column with a reduction of the electrical activity of the deep back muscles; the latter event occurs because the posterior spinal musculature is not used to maintain posture.9,91 The movement of the diaphragm during postural tasks is not correlated with the contraction function for respiration. The diaphragm is a structure that encompasses two identities and with non-homogeneous movements.91 If the need to create balance (a demanding bodily action) increases, this situation will decrease the ability of the diaphragm to express itself as a respiratory muscle.91 Likewise, the diaphragmatic contraction that precedes voluntary (about 20 milliseconds before muscle activity of the limbs), and non-voluntary movements, is independent of respiratory function.92,93
It is the contraction of the diaphragm that informs the central nervous system of body position and postural needs (by sending information from body receptors); this proprioceptive information is conveyed towards the cortex by the activity of the diaphragm.92,93
The Postural Diaphragm
The parasympathetic system plays an important role in the proper functioning of the diaphragm and neuro coordination.61 COPD patients show an increase in the sympathetic system (sympathoexcitation), and this increase predicts a decrease in exercise tolerance and a poor prognosis.94,95 This chronic sympathoexcitation is inversely related to PImax.95 During an exacerbation, there is a discrepancy between the activity of the sympathetic and parasympathetic systems.
Lung filling/emptying stimulates mechanoreceptors, such as rapid-adapting pulmonary stretch receptors (RARs), slow-adapting pulmonary stretch receptors (SARs), and C-type fibers. In particular, RARs send signals via the vagus nerve to the NTS to favor diaphragm activation.96 Lung hyperinflation and emphysema stimulate more RARs and type C fibers.97,98 Overstimulation of these receptors could cause a release of local pro-inflammatory substances, causing bronchoconstriction and perpetuating a pathological pulmonary environment.95,99 During an exacerbation, the parasympathetic system increases.
Generally, vagal hyperactivation is measured indirectly by the decrease in heart rate variability (HRV).100 In reality, HRV does not correctly reflect vagus nerve involvement.101 The parasympathetic system is more active in acute phases at the pulmonary level, but not at the systemic level. In fact, the parasympathetic system in the acute phases fails to activate the diaphragm more.60 Data confirm that in the exacerbation phases, the COPD patient is more at risk of falling.34 There is a relationship between diaphragm weakness and the risk of falls.
We know that electromyography of the spinal muscles and that of the external obliques and rectus abdominis is increased compared to healthy subjects; this should be an indirect clue to the dysfunction of the diaphragm as a postural muscle.41 We know that the ability to manage proprioceptive information in the COPD patient is impaired, and this alteration is connected to diaphragmatic dysfunction in the role of postural muscle.40,87 The diaphragm remains in a shortened condition, and with reduced excursion capacity in COPD patients.3,25 Another relationship between diaphragm weakness and fall risk.
The diaphragm is unable to adequately solicit the spino-cortical and cortico-spinal neurological pathways, as its contractile capacity is decreased; the result is a balance impairment, increased risk of accidental falls and increased mortality/morbidity. We can strongly speculate that the impaired postural balance, the neuro-motor incoordination of the trunk and limbs, could be related to the diaphragm.
We know that rehabilitation inspiratory training improves postural balance in patients.33 The same literature is very sparse on the relationship between diaphragm training and neuro-coordination response in COPD patients.33
In light of the above, the clinician and rehabilitation field and scientific research should place greater emphasis on the assessment and training of the diaphragm, not only with the goal of slowing the progression of the disease or helping the patient in the acute phases, but as a target to prevent the occurrence of accidental falls.
Conclusions
Chronic obstructive pulmonary disease (COPD) causes deterioration of the airways, with persistent and non-reversible airflow limitation. COPD could become the third leading cause of death for the population by 2030. There are several causes that lead to structural and functional alterations of the lungs, many of which could be counteracted before developing the disease, such as lifestyle and increased attention in avoiding some daily habits, such as physical activity and cigarette smoking, respectively.
The skeletal muscles of the trunk, the limbs and the diaphragm muscle undergo non-physiological adaptations over time, which worsen the patient’s clinical picture and lead to an increase in accidental falls. The article reviewed the literature concerning the pathological adaptation of the diaphragm, placing emphasis on the spino-cortical and cortico-spinal neurological relationships that influence the management of proprioceptive information, and how diaphragmatic dysfunction can alter the neuro-coordination of the COPD patient. A greater interest of the clinician and the physiotherapist should be directed towards the diaphragm to counteract the genesis of unwanted falls.
Ethics Statement
The people involved in the figures to accompany the article have consented to the publication of the images.
Funding
The article has been funded by the Italian Ministry of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tang T, Li Z, Lu X, Du J. Development and validation of a risk prediction model for anxiety or depression among patients with chronic obstructive pulmonary disease between 2018 and 2020. Ann Med. 2022;54(1):2181–2190. doi:10.1080/07853890.2022.2105394
2. Feizi H, Alizadeh M, Nejadghaderi SA, et al. The burden of chronic obstructive pulmonary disease and its attributable risk factors in the Middle East and North Africa region, 1990–2019. Respir Res. 2022;23(1):319. doi:10.1186/s12931-022-02242-z
3. Fei F, Siegert R, Zhang X, Gao W, Koffman J. Symptom clusters, associated factors and health-related quality of life in patients with chronic obstructive pulmonary disease: a structural equation modelling analysis. J Clin Nurs. 2023;32(1–2):298–310. doi:10.1111/jocn.16234
4. Cao Y, Li P, Wang Y, Liu X, Wu W. Diaphragm dysfunction and rehabilitation strategy in patients with chronic obstructive pulmonary disease. Front Physiol. 2022;13:872277. doi:10.3389/fphys.2022.872277
5. Zhang L, Sun Y. Muscle-bone crosstalk in chronic obstructive pulmonary disease. Front Endocrinol. 2021;12:724911. doi:10.3389/fendo.2021.724911
6. de Jong C, van Boven JFM, de Boer MR, Kocks JWH, Berger MY, van der Molen T. Improved health status of severe COPD patients after being included in an integrated primary care service: a prospective cohort study. Eur J Gen Pract. 2022;28(1):66–74. doi:10.1080/13814788.2022.2059070
7. Bordoni B. Costen’s syndrome and COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:457–460. doi:10.2147/COPD.S200787
8. Bordoni B, Marelli F, Morabito B, Castagna R. Chest pain in patients with COPD: the fascia’s subtle silence. Int J Chron Obstruct Pulmon Dis. 2018;13:1157–1165. doi:10.2147/COPD.S156729
9. Bordoni B, Marelli F, Morabito B, Sacconi B, Caiazzo P, Castagna R. Low back pain and gastroesophageal reflux in patients with COPD: the disease in the breath. Int J Chron Obstruct Pulmon Dis. 2018;13:325–334. doi:10.2147/COPD.S150401
10. Button BM, Holland AE, Sherburn MS, Chase J, Wilson JW, Burge AT. Prevalence, impact and specialised treatment of urinary incontinence in women with chronic lung disease. Physiotherapy. 2019;105(1):114–119. doi:10.1016/j.physio.2018.07.006
11. Bordoni B, Marelli F, Morabito B, Sacconi B. Depression, anxiety and chronic pain in patients with chronic obstructive pulmonary disease: the influence of breath. Monaldi Arch Chest Dis. 2017;87(1):811. doi:10.4081/monaldi.2017.811
12. Oliveira CC, Lee AL, McGinley J, et al. Balance and falls in acute exacerbation of chronic obstructive pulmonary disease: a prospective study. COPD. 2017;14(5):518–525. doi:10.1080/15412555.2017.1342232
13. Zhang L, Li D, Chang C, Sun Y. Myostatin/HIF2α-mediated ferroptosis is involved in skeletal muscle dysfunction in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:2383–2399. doi:10.2147/COPD.S377226
14. Zhao H, Li P, Wang J. The role of muscle-specific MicroRNAs in patients with chronic obstructive pulmonary disease and skeletal muscle dysfunction. Front Physiol. 2022;13:954364. doi:10.3389/fphys.2022.954364
15. Kaygusuz MH, Oral Tapan O, Tapan U, Genc S. Balance impairment and cognitive dysfunction in patients with chronic obstructive pulmonary disease under 65 years. Clin Respir J. 2022;16(3):200–207. doi:10.1111/crj.13469
16. Voica AS, Oancea C, Tudorache E, et al. Chronic obstructive pulmonary disease phenotypes and balance impairment. Int J Chron Obstruct Pulmon Dis. 2016;11:919–925. doi:10.2147/COPD.S101128
17. Luo H, Zhang Q, Niu Y, Kan H, Chen R. Fine particulate matter and cardiorespiratory health in China: a systematic review and meta-analysis of epidemiological studies. J Environ Sci. 2023;123:306–316. doi:10.1016/j.jes.2022.04.026
18. Lu HY, Liao KM. The incidence of bronchiectasis in chronic obstructive pulmonary disease. Open Med. 2022;17(1):1927–1934. doi:10.1515/med-2022-0599
19. Cirovic A, Denic A, Clarke BL, Vassallo R, Cirovic A, Landry GM. A hypoxia-driven occurrence of chronic kidney disease and osteoporosis in COPD individuals: new insights into environmental cadmium exposure. Toxicology. 2022;482:153355. doi:10.1016/j.tox.2022.153355
20. Wagih Shaltout S, Abd El-Maksoud M, Abdel Rahman A, Yousef AM, El Sherbiny W. Clinical spectrum of nonalcoholic fatty liver disease in patients with chronic obstructive pulmonary disease. Turk Thorac J. 2022;23(6):420–425. doi:10.5152/TurkThoracJ.2022.22002
21. Sharanya A, Ciano M, Withana S, Kemp PR, Polkey MI, Sathyapala SA. Sex differences in COPD-related quadriceps muscle dysfunction and fibre abnormalities. Chron Respir Dis. 2019;16:1479973119843650. doi:10.1177/1479973119843650
22. Lage VKDS, de Paula FA, Lima LP, et al. Plasma levels of myokines and inflammatory markers are related with functional and respiratory performance in older adults with COPD and sarcopenia. Exp Gerontol. 2022;164:111834. doi:10.1016/j.exger.2022.111834
23. Attaway AH, Bellar A, Mishra S, et al. Adaptive exhaustion during prolonged intermittent hypoxia causes dysregulated skeletal muscle protein homeostasis. J Physiol. 2022. doi:10.1113/JP283700
24. Gayan-Ramirez G, Decramer M. Mechanisms of striated muscle dysfunction during acute exacerbations of COPD. J Appl Physiol. 2013;114(9):1291–1299. doi:10.1152/japplphysiol.00847.2012
25. Alqahtani JS, Oyelade T, Sreedharan J, et al. Diagnostic and clinical values of non-cardiac ultrasound in COPD: a systematic review. BMJ Open Respir Res. 2020;7(1):e000717. doi:10.1136/bmjresp-2020-000717
26. Wallbridge P, Parry SM, Das S, et al. Parasternal intercostal muscle ultrasound in chronic obstructive pulmonary disease correlates with spirometric severity. Sci Rep. 2018;8(1):15274. doi:10.1038/s41598-018-33666-7
27. Jin J, Li F, Fan C, Wu Y, He C. Elevated mir-145-5p is associated with skeletal muscle dysfunction and triggers apoptotic cell death in C2C12 myotubes. J Muscle Res Cell Motil. 2022;43(3):135–145. doi:10.1007/s10974-022-09624-2
28. Calatayud J, Torres-Castro R, Vera-Uribe R, et al. Neuromuscular and acute symptoms responses to progressive elastic resistance exercise in patients with chronic obstructive pulmonary disease: cross-sectional study. Front Med. 2022;9:934410. doi:10.3389/fmed.2022.934410
29. Gephine S, Mucci P, Bielmann M, et al. Quadriceps physiological response during the 1-min sit-to-stand test in people with severe COPD and healthy controls. Sci Rep. 2022;12(1):794. doi:10.1038/s41598-022-04820-z
30. Valle MS, Casabona A, Di Fazio E, et al. Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system. Sci Rep. 2021;11(1):18077. doi:10.1038/s41598-021-97621-9
31. Casabona A, Valle MS, Laudani L, et al. Is the power spectrum of electromyography signal a feasible tool to estimate muscle fiber composition in patients with COPD? J Clin Med. 2021;10(17):3815. doi:10.3390/jcm10173815
32. Frazão M, Santos ADC, Araújo AA, et al. Neuromuscular efficiency is impaired during exercise in COPD patients. Respir Physiol Neurobiol. 2021;290:103673. doi:10.1016/j.resp.2021.103673
33. Tounsi B, Acheche A, Lelard T, Tabka Z, Trabelsi Y, Ahmaidi S. Effects of specific inspiratory muscle training combined with whole-body endurance training program on balance in COPD patients: randomized controlled trial. PLoS One. 2021;16(9):e0257595. doi:10.1371/journal.pone.0257595
34. Oliveira CC, Annoni R, Lee AL, McGinley J, Irving LB, Denehy L. Falls prevalence and risk factors in people with chronic obstructive pulmonary disease: a systematic review. Respir Med. 2021;176:106284. doi:10.1016/j.rmed.2020.106284
35. Loughran KJ, Atkinson G, Beauchamp MK, et al. Balance impairment in individuals with COPD: a systematic review with meta-analysis. Thorax. 2020;75(7):539–546. doi:10.1136/thoraxjnl-2019-213608
36. Mętel S, Kostrzon M, Adamiak J. Dynamic balance and chest mobility of older adults after speleotherapy combined with pulmonary rehabilitation, endurance and strength training-a prospective study in chronic respiratory diseases. Int J Environ Res Public Health. 2022;19(18):11760. doi:10.3390/ijerph191811760
37. McCrum C, Vaes AW, Delbressine JM, et al. A pilot study on the feasibility and effectiveness of treadmill-based perturbations for assessing and improving walking stability in chronic obstructive pulmonary disease. Clin Biomech. 2022;91:105538. doi:10.1016/j.clinbiomech.2021.105538
38. Porto EF, Castro AAM, Fausto DM, et al. Balance impairment and lower limbs strength in patients with COPD who fell in the previous year. Monaldi Arch Chest Dis. 2022;92(4). doi:10.4081/monaldi.2022.1204
39. de Castro LA, Ribeiro LR, Mesquita R, et al. Static and functional balance in individuals with COPD: comparison with healthy controls and differences according to sex and disease severity. Respir Care. 2016;61(11):1488–1496. doi:10.4187/respcare.04749
40. Janssens L, Brumagne S, McConnell AK, et al. Proprioceptive changes impair balance control in individuals with chronic obstructive pulmonary disease. PLoS One. 2013;8(3):e57949. doi:10.1371/journal.pone.0057949
41. Smith MD, Chang AT, Hodges PW. Balance recovery is compromised and trunk muscle activity is increased in chronic obstructive pulmonary disease. Gait Posture. 2016;43:101–107. doi:10.1016/j.gaitpost.2015.09.004
42. Jirange P, Vaishali K, Sinha MK, Bairapareddy KC, Alaparthi GK. A cross-sectional study on balance deficits and gait deviations in COPD patients. Can Respir J. 2021;2021:6675088. doi:10.1155/2021/6675088
43. Rodrigues GD, Gurgel JL, da Nobrega ACL, Soares PPDS. Orthostatic intolerance: a handicap of aging or physical deconditioning? Eur J Appl Physiol. 2022;122(9):2005–2018. doi:10.1007/s00421-022-04978-4
44. Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respir Res. 2008;9(1):12. doi:10.1186/1465-9921-9-12
45. Bordoni B, Walkowski S, Escher A, Ducoux B. The importance of the posterolateral area of the diaphragm muscle for palpation and for the treatment of manual osteopathic medicine. Complement Med Res. 2022;29(1):74–82. doi:10.1159/000517507
46. Palma G, Sorice GP, Genchi VA, et al. Adipose tissue inflammation and pulmonary dysfunction in obesity. Int J Mol Sci. 2022;23(13):7349. doi:10.3390/ijms23137349
47. Chen Y, Li J, Dong B, Zhu Z, Lyu G. Two-dimensional shear wave elastography: a new tool for evaluating respiratory muscle stiffness in chronic obstructive pulmonary disease patients. BMC Pulm Med. 2022;22(1):441. doi:10.1186/s12890-022-02231-4
48. Marino S, Bettini P, Pini L, et al. Effects of chronic and acute pulmonary hyperinflation on phrenic nerve conduction in patients with COPD. COPD. 2020;17(4):378–383. doi:10.1080/15412555.2020.1779680
49. Elnemr R, Sweed RA, Shafiek H. Diaphragmatic motor cortex hyperexcitability in patients with chronic obstructive pulmonary disease. PLoS One. 2019;14(12):e0217886. doi:10.1371/journal.pone.0217886
50. Kahnert K, Föhrenbach M, Lucke T, et al. The impact of COPD on polyneuropathy: results from the German COPD cohort COSYCONET. Respir Res. 2020;21(1):28. doi:10.1186/s12931-020-1293-6
51. Barreiro E, Salazar-Degracia A, Sancho-Muñoz A, Aguiló R, Rodríguez-Fuster A, Gea J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J Appl Physiol. 2019;126(6):1572–1586. doi:10.1152/japplphysiol.00670.2018
52. Reggiani C, Marcucci L. A controversial issue: can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers? J Gen Physiol. 2022;154(9):e202213167. doi:10.1085/jgp.202213167
53. Bordoni B. Comment on: the effect of pedal pump lymphatic technique versus passive recovery following maximal exercise: a randomized cross-over trial. Sports Med Open. 2022;8(1):51. doi:10.1186/s40798-022-00443-w
54. Wang Z, Grange M, Pospich S, et al. Structures from intact myofibrils reveal mechanism of thin filament regulation through nebulin. Science. 2022;375(6582):eabn1934. doi:10.1126/science.abn1934
55. Zhang B, Li P, Li J, Liu X, Wu W. Effect of oxidative stress on diaphragm dysfunction and exercise intervention in chronic obstructive pulmonary disease. Front Physiol. 2021;12:684453. doi:10.3389/fphys.2021.684453
56. Bordoni B. Updated Perspectives on the Role of Biomechanics in COPD: considerations for the Clinician [Letter]. Int J Chron Obstruct Pulmon Dis. 2022;17:3017–3018. doi:10.2147/COPD.S395493
57. Wada S, Matsuoka S, Mimura H. Inspiratory and expiratory CT analyses of the diaphragmatic crus in chronic obstructive pulmonary disease. Jpn J Radiol. 2022;40(12):1257–1262. doi:10.1007/s11604-022-01314-w
58. Ngamsnae P, Okazaki T, Ren Y, et al. Anatomy and pathology of lymphatic vessels under physiological and inflammatory conditions in the mouse diaphragm. Microvasc Res. 2023;145:104438. doi:10.1016/j.mvr.2022.104438
59. James MD, Phillips DB, Vincent SG, et al. Canadian Respiratory Research Network. Exertional dyspnoea in patients with mild-to-severe chronic obstructive pulmonary disease: neuromechanical mechanisms. J Physiol. 2022;600(18):4227–4245. doi:10.1113/JP283252
60. Wei S, Lu R, Zhang Z, et al. MRI-assessed diaphragmatic function can predict frequent acute exacerbation of COPD: a prospective observational study based on telehealth-based monitoring system. BMC Pulm Med. 2022;22(1):438. doi:10.1186/s12890-022-02254-x
61. Bordoni B, Escher AR. Functional evaluation of the diaphragm with a noninvasive test. J Osteopath Med. 2021;121(11):835–842. doi:10.1515/jom-2021-0101
62. Schepens T, Fard S, Goligher EC. Assessing Diaphragmatic Function. Respir Care. 2020;65(6):807–819. doi:10.4187/respcare.07410
63. Bordoni B, Marelli F, Morabito B, Sacconi B. Depression and anxiety in patients with chronic heart failure. Future Cardiol. 2018;14(2):115–119. doi:10.2217/fca-2017-0073
64. Nair J, Streeter KA, Turner SMF, et al. Anatomy and physiology of phrenic afferent neurons. J Neurophysiol. 2017;118(6):2975–2990. doi:10.1152/jn.00484.2017
65. Dempsey JA. Respiratory determinants of exercise limitation: focus on phrenic afferents and the lung vasculature. Clin Chest Med. 2019;40(2):331–342. doi:10.1016/j.ccm.2019.02.002
66. Fuller DD, Rana S, Smuder AJ, Dale EA. The phrenic neuromuscular system. Handb Clin Neurol. 2022;188:393–408. doi:10.1016/B978-0-323-91534-2.00012-6
67. Pickering M, Jones JF. The diaphragm: two physiological muscles in one. J Anat. 2002;201(4):305–312. doi:10.1046/j.1469-7580.2002.00095.x
68. Chun KY, Son YJ, Seo S, Lee HJ, Han CS. Nonlinearly frequency-adaptive, self-powered, proton-driven somatosensor inspired by a human mechanoreceptor. ACS Sens. 2020;5(3):845–852. doi:10.1021/acssensors.0c00119
69. Zeveke AV, Efes ED, Polevaya SA. An integrative framework of the skin receptors activation: mechanoreceptors activity patterns versus “labeled lines”. J Integr Neurosci. 2013;12(1):47–56. doi:10.1142/S0219635213500052
70. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi:10.1016/S0165-0173(96)00011-2
71. Scalabrini A, Wolman A, Northoff G. The self and its right insula-differential topography and dynamic of right vs. left insula. Brain Sci. 2021;11(10):1312. doi:10.3390/brainsci11101312
72. Streeter KA, Sunshine MD, Davenport PW, Fuller DD. Phrenic afferent activation modulates cardiorespiratory output in the adult rat. J Neurophysiol. 2021;126(6):2091–2103. doi:10.1152/jn.00433.2021
73. Hilz MJ. Transcutaneous vagus nerve stimulation - A brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
74. Al-Chalabi M, Reddy V, Gupta S. Neuroanatomy, spinothalamic tract. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
75. Young RL, Page AJ, Cooper NJ, Frisby CL, Blackshaw LA. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology. 2010;138(3):1091–101.e1–e5. doi:10.1053/j.gastro.2009.08.053
76. Novello M, Bosman LWJ, De Zeeuw CI. A systematic review of direct outputs from the cerebellum to the brainstem and diencephalon in mammals. Cerebellum. 2022. doi:10.1007/s12311-022-01499-w
77. Yates BJ, Billig I, Cotter LA, Mori RL, Card JP. Role of the vestibular system in regulating respiratory muscle activity during movement. Clin Exp Pharmacol Physiol. 2002;29(1–2):112–117. doi:10.1046/j.1440-1681.2002.03612.x
78. Neuhuber WL, Berthoud HR. Functional anatomy of the vagus system: how does the polyvagal theory comply? Biol Psychol. 2022;174:108425. doi:10.1016/j.biopsycho.2022.108425
79. Peng KP, May A. Noninvasive vagus nerve stimulation modulates trigeminal but not extracephalic somatosensory perception: functional evidence for a trigemino-vagal system in humans. Pain. 2022;163(10):1978–1986. doi:10.1097/j.pain.0000000000002595
80. Ni RJ, Huang ZH, Luo PH, Ma XH, Li T, Zhou JN. The tree shrew cerebellum atlas: systematic nomenclature, neurochemical characterization, and afferent projections. J Comp Neurol. 2018;526(17):2744–2775. doi:10.1002/cne.24526
81. Zhang Y, Zhang Y, Tian K, et al. Calcitonin gene-related peptide facilitates sensitization of the vestibular nucleus in a rat model of chronic migraine. J Headache Pain. 2020;21(1):72. doi:10.1186/s10194-020-01145-y
82. Shi MY, Ding LF, Guo YH, Cheng YX, Bi GQ, Lau PM. Long-range GABAergic projections from the nucleus of the solitary tract. Mol Brain. 2021;14(1):38. doi:10.1186/s13041-021-00751-4
83. Laviolette L, Niérat MC, Hudson AL, Raux M, Allard E, Similowski T. The supplementary motor area exerts a tonic excitatory influence on corticospinal projections to phrenic motoneurons in awake humans. PLoS One. 2013;8(4):e62258. doi:10.1371/journal.pone.0062258
84. Green AL, Paterson DJ. Using deep brain stimulation to unravel the mysteries of cardiorespiratory control. Compr Physiol. 2020;10(3):1085–1104. doi:10.1002/cphy.c190039
85. Belyakov VI, Merkulova NA, Inyushkin AN. Respiratory effects of sensorimotor cortex and their mechanisms in rats. Bull Exp Biol Med. 2002;133(4):314–317. doi:10.1023/A:1016265130557
86. Ozaki I, Kurata K. The effects of voluntary control of respiration on the excitability of the primary motor hand area, evaluated by end-tidal CO2 monitoring. Clin Neurophysiol. 2015;126(11):2162–2169. doi:10.1016/j.clinph.2014.12.032
87. Teixeira AL, Vianna LC. The exercise pressor reflex: an update. Clin Auton Res. 2022;32(4):271–290. doi:10.1007/s10286-022-00872-3
88. Siedlecki P, Ivanova TD, Shoemaker JK, Garland SJ. The effects of slow breathing on postural muscles during standing perturbations in young adults. Exp Brain Res. 2022;240(10):2623–2631. doi:10.1007/s00221-022-06437-0
89. Sembera M, Busch A, Kobesova A, Hanychova B, Sulc J, Kolar P. Postural-respiratory function of the diaphragm assessed by M-mode ultrasonography. PLoS One. 2022;17(10):e0275389. doi:10.1371/journal.pone.0275389
90. Illidi CR, Romer LM. Stabilising function of the human diaphragm in response to involuntary augmented breaths induced with or without lower-limb movements. Exp Physiol. 2022;107(12):1477–1492. doi:10.1113/EP090605
91. Kolar P, Sulc J, Kyncl M, et al. Stabilizing function of the diaphragm: dynamic MRI and synchronized spirometric assessment. J Appl Physiol. 2010;109(4):1064–1071. doi:10.1152/japplphysiol.01216.2009
92. Hodges PW, Gandevia SC. Activation of the human diaphragm during a repetitive postural task. J Physiol. 2000;522(Pt1):165–175. doi:10.1111/j.1469-7793.2000.t01-1-00165.xm
93. Gandevia SC, Butler JE, Hodges PW, Taylor JL. Balancing acts: respiratory sensations, motor control and human posture. Clin Exp Pharmacol Physiol. 2002;29(1–2):118–121. doi:10.1046/j.1440-1681.2002.03611.x
94. Gidron Y, Deschepper R, De Couck M, Thayer JF, Velkeniers B. The vagus nerve can predict and possibly modulate non-communicable chronic diseases: introducing a neuroimmunological paradigm to public health. J Clin Med. 2018;7(10):371. doi:10.3390/jcm7100371
95. Spiesshoefer J, Regmi B, Ottaviani MM, et al. Sympathetic and vagal nerve activity in COPD: pathophysiology, presumed determinants and underappreciated therapeutic potential. Front Physiol. 2022;13:919422. doi:10.3389/fphys.2022.919422
96. Yu J. A historical perspective of pulmonary rapidly adapting receptors. Respir Physiol Neurobiol. 2021;287:103595. doi:10.1016/j.resp.2020.103595
97. Dallak MA, Pirie LJ, Davies A. The influence of pulmonary receptors on respiratory drive in a rabbit model of pulmonary emphysema. Respir Physiol Neurobiol. 2007;156(1):33–39. doi:10.1016/j.resp.2006.08.001
98. Matsumoto S, Yoshida S, Ikeda M, Nishikawa T, Saiki C, Takeda M. Effects of potassium channel blockers on hyperinflation-induced rapidly adapting pulmonary stretch receptor stimulation in the rabbit. Life Sci. 2001;70(5):491–501. doi:10.1016/s0024-3205(01)01424-2
99. Pelleg A, Xu F, Zhuang J, Undem B, Burnstock G. DT-0111: a novel drug-candidate for the treatment of COPD and chronic cough. Ther Adv Respir Dis. 2019;13:1753466619877960. doi:10.1177/1753466619877960
100. Kabbach EZ, Mazzuco A, Borghi-Silva A, et al. Increased parasympathetic cardiac modulation in patients with acute exacerbation of COPD: how should we interpret it? Int J Chron Obstruct Pulmon Dis. 2017;12:2221–2230. doi:10.2147/COPD.S134498
101. Marmerstein JT, McCallum GA, Durand DM. Direct measurement of vagal tone in rats does not show correlation to HRV. Sci Rep. 2021;11(1):1210. doi:10.1038/s41598-020-79808-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.