Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 16
Motor Dysfunctions in Fibromyalgia Patients: The Importance of Breathing
Received 27 September 2023
Accepted for publication 3 February 2024
Published 7 March 2024 Volume 2024:16 Pages 55—66
DOI https://doi.org/10.2147/OARRR.S442327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Chuan-Ju Liu
Bruno Bordoni,1 Allan R Escher2
1Dipartimento di Cardiologia, Fondazione Don Carlo Gnocchi IRCCS, Istituto di Ricovero e Cura, S Maria Nascente, Milano, 20100, Italia; 2Anesthesiology/Pain Medicine, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, 33612, USA
Correspondence: Bruno Bordoni, Email [email protected]; [email protected]
Abstract: The classification of fibromyalgia (FM) is not always immediate and simple, with the time from the first diagnosis, compared to the onset of symptoms, of a few years. Currently, we do not have instrumental or biochemical tests considered as gold standards; the clinician will make a diagnosis of FM based on the patient’s medical history and subjective assessment. The symptoms can involve physical, cognitive and psychological disorders, with the presence of pain of different origins and classifications: nociplastic, nociceptive and neuropathic pain. Among the symptoms highlighted, postural disorders and neuromotor uncoordination emerge, whose functional dysfunctions can increase the mortality and morbidity rate. An alteration of the diaphragm muscle could generate such functional motor problems. Considering that the current literature underestimates the importance of breathing in FM, the article aims to highlight the relationship between motor and diaphragmatic difficulties in the patient, soliciting new points of view for the clinical and therapeutic framework.
Keywords: fibromyalgia, diaphragm, breathing, pain, chronic widespread pain, fascia
Introduction
Fibromyalgia (FM) is the second most common rheumatologic disease after osteoarthritis.1 The etiology of FM remains elusive despite being a chronic syndrome and affecting approximately 6% of the world population.2,3 The clinical picture is not always immediate and simple, with a time from the first diagnosis to the onset of symptoms up to several years.4 FM affects the female sex with a higher percentage.5 The most characteristic symptoms are the presence of chronic widespread pain (for at least three consecutive months) of the musculoskeletal area, generalized fatigue, psychiatric alterations (anxiety and depression), cognitive decline, non-restorative sleep.4,6 There is no specific instrumental examination or biomarkers capable of predicting or diagnosing FM; the diagnosis is based on the medical history and clinical examinations.6
One of the great difficulties in correctly identifying FM is the overlap of multiple concomitant disorders and pathologies. Often the fibromyalgic patient presents with different clinical pictures and comorbidities, such as diabetes, obesity, allergic rhinitis, small-fiber neuropathy, kinesiophobia, other rheumatological pathologies (hypermobility spectrum disorder, Ehlers-Danlos syndrome), intestinal dysfunctions (irritable bowel syndrome, celiac disease), neuromotor imbalance, vestibulocochlear disorders, Raynaud’s phenomenon, orthostatic intolerance, pelvic floor dysfunction and sexual dysfunction, orofacial area dysfunction, loss of cervical lordosis.3,6–15
Another limit for the exhaustive clinical identification of the pathology could lie in categorizing a patient with fibromyalgia compared to a non-fibromyalgia person, as it is not an operation that always leads to taking into consideration all subgroups of patients with FM.16 For example, it is not always feasible to explain the origin of pain or when fibromyalgia starts.16 The pains classified from an algological point of view are essentially three. Neuropathic pain appears following a lesion of the nervous tissue, at a central or peripheral level, with or without inflammatory processes, with painful symptoms (acute, intermittent, paraesthesia); the approach is typically surgical or pharmacological.17 Nociceptive pain derives from a lesion with inflammation, and with a very precise localization by the patient and easy localization/stimulation of pain by the clinician; the therapy follows the pharmacological or surgical route.17 Nociplastic pain derives from apparently uninjured tissues, whose pain is stimulated by sensory stimuli (allodynia), with central and/or peripheral hypersensitization mechanisms, with top-down and/or bottom-up phenomena; the approach tends to be non-pharmacological.17 In fibromyalgia patients, allodynia, hypersensitization and top-down and/or bottom-up phenomena may coexist.16,18
There are different rating scales in force. Revised Fibromyalgia Impact Questionnaire (FIQR) (11-point numeric rating scale with scores from zero to ten), Fibromyalgia Assessment Status (FAS) (patient self-administered assessment for fatigue, disturbed sleep and pain, with starting from zero up to a maximum of forty-eight).19,20 Polysymptomatic Distress Scale (PDS) counts from zero to a maximum of thirty-one Nociplastic-based Fibromyalgia Features (NFF) 11-item assessment, taking into consideration multiple variables and emotional aspects.21,22 Symptom Severity Score (SSS) in conjunction with the use of the Extent of Somatic Symptoms (ESS), also take into consideration the cognitive aspect, with a score of zero-twelve and zero-three, respectively; Widespread Pain Index (WPI) is a self-report measure that highlights areas of the body with pain (19 body areas), which scale is usually administered with the SSS.18,23 Multidimensional health assessment questionnaire (MDHAQ) consists of multiple questions to which the patient is subjected to highlight possible rheumatic disorders (not only for FM); Fibromyalgia Rapid Screening Tool (FiRST) is a self-questionnaire to highlight the possibility that the patient has FM and no other rheumatic diseases.24,25 The evaluation scales try to propose a clinical discrimination that reflects as much as possible the indications of the American College of Rheumatology guidelines of 2016, whose key concept is that the diagnosis that leads to FM is not a diagnosis of exclusion.18,26 Despite these numerous evaluation scales for patient classification, not only is it difficult for the clinician to take a correct picture of the symptoms, but the clinician does not always take into consideration the modality/quality of the breathing in these patients.
The article reviews (narrative review) the information on some clinical motor aspects of the fibromyalgia patient, trying to make the relationship between the respiratory aspect and the motor alterations that the person with FM undergoes congruent. Considering that the non-pharmacological approach for nociplastic changes appears ideal for FM (containing symptoms and maintaining a well-managed symptom status), highlighting the extra-respiratory functions can prove to be a useful strategy for creating a more appropriate therapeutic procedure for the patient.
Motor Impairments of the Fibromyalgic Patient
Lack of motor balance during daily activities and increased risk of falling are part of the symptomatic picture of FM.10 Approximately 45% of patients suffer from balance disorders, with a reported number of falls of approximately 16 times per month compared to 6 times per month in healthy subjects. The possible causes are related to the presence of vertigo and visual problems, obesity, cognitive symptoms, depression and anxiety, the intake of muscle relaxants and anti-depressants, a sedentary lifestyle, kinesiophobia, axial alterations of the spine.2,10,11,27,28 Very often the patient is unaware of his lack of motor balance.11
Patients with FM may possibly tend to show less trunk stabilization due to a systemic decrease in proprioception.1 Currently, no certain cause has brought convincing answers to explain the postural alteration in these patients and the increased risk of falling.2
Although nearly half of people with FM experience a decline in motor control, no studies have looked at the influence of breathing on postural balance and motor control.
The Way of Breathing
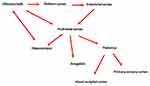
The central pattern generator (CPG) is the most important area of respiratory management, and includes the pons, midbrain, and medulla.29 Within this area we find the pre-Bötzinger complex (preBötC), the caudal ventral group (VRGc), the parabrachial/Kölliker-Fuse complex, the rostral ventral group (VRGr), and the nucleus of the solitary tract (NTS).29 The pre-inspiratory phase derives from the preBötC area, with management of the first respiratory muscle that is activated during the entry of air into the nose, ie, the dilator naris (innervated by the zygomatic branch of the facial nerve).30
When air enters the nares and reaches the upper airways, preBötC sends efferents to the hypoglossal nerve and the glossopharyngeal nerve (XII and IX cranial nerves); with these electrical impulses, the area of the lingual complex contracts in its lower portion, pushing the hyoid bone forward, dilating the upper airways.29
Passing Through the Nose
The flow of air entering the nose stimulates the epithelium (olfactory nerve) and the primary olfactory neurons (mitral neurons); via the neural mechanoreceptors of the olfactory bulb (olfactory G protein-coupled receptors/Piezo2 ion channels), efferents are activated towards the piriform cortex and the entorhinal cortex. From these last two cortical areas, further signals are sent to the prefrontal cortex, amygdala, thalamus, and hippocampus.31–33 The flow of air entering through the nose generates mechanical oscillations, which translate into rhythmic electric waves. The latter are information packets between different brain areas, near and far, with centrifugal directions.31 If the initial wave that reaches the olfactory bulb is measured in about 0.5–5 Hz, the latter can generate and modulate larger waves with frequencies up to 80–120 Hz.31 These neural oscillations modulate the cognitive aspect.32
Theta band (4–8 Hz) is most prevalent between the prefrontal cortex, olfactory bulb, and hippocampus, in a bi-directional fashion.32,34 Gamma band (25–120Hz) can be divided into slow waves (25–55Hz) and fast waves (>55Hz); both modes of oscillations can be found in the hippocampus and concomitantly with theta oscillations.34 Delta band (1–4 Hz) can be found during sleep and during brain activities that require attention and decision making, particularly in the primary motor cortex (M1) and primary sensory cortex (S1).35
Beta frequency (13–35 Hz) is found particularly in the M1 cortex and for cognitive tasks.36 Alpha band (7 to 13 Hz) is recognized in perception and working memory actions, originating from the thalamus, and involving S1 and the visual occipital cortex.37 The hippocampus is able to send very high frequency oscillations (150–300Hz) very quickly (sharp wave ripples). This occurs when the hippocampus tries to pass information to the neocortex for storage, in conjunction with breathing.34 Neural oscillations allow for the creation of a communication network between the entire cerebral and spinal system, improving function, reaction times, supporting existing connections and creating new neural links (synaptogenesis).38 Conversely, aberrant oscillations will cause morphological and functional alterations of the nervous system with the appearance of pathologies.39 Let us imagine Christmas lights (neurons) connected by wires (synapses) and wireless (oscillations); everything lights up if the electricity and internet are present with the correct frequency.
The pre-inspiratory phase modulates cognitive processes and the perception of proprioceptive sensations.40,41 This means that the nervous system through the pre-inspiration prepares the person for action (cognitive and motor), with respect to the need and the external environment. (Figure 1)
From the Nose to the Larynx
When the air from the nose is directed to the lumen of the larynx (we are still in the pre-inspiratory phase), the preBötC neurons stimulate the XII and IX cranial nerves, so that the contraction of the tongue pushes the hyoid bone towards the forward, widening the laryngeal lumen.42 Tongue movement activates some brain areas: M1, putamen, supplementary motor area, thalamus, cerebellum and insula, anterior cingulate cortex.43 The M1 area is fundamental for the motor commands to the skeletal muscles, the putamen (caudate putamen) is activated for the preparation of the motor action and for some phases of cognitive learning.42,44 Supplementary motor area (dorsomedial frontal cortex) is responsible for planning and processing complex movements, as well as cognitive tasks.45
The thalamic somatosensory area, and in particular, the contralateral medial area of the ventroposteromedial thalamus, is stimulated via the lingual nerve, to process proprioceptive information (emotions, movement, and pain), as well as processes related to memory and cognition.46–48 The hypoglossal nucleus and the trigeminal motor nucleus send afferents to the cerebellum, in order to adapt and prepare motor and emotional behavior for the respiration that will be required.49 The insula (insular oral region) is stimulated by the tongue to receive and process somatovisceral information; the insula translates this information into empathy, pain management and self-awareness.50–52 Anterior cingulate cortex has a crucial role in processing voluntary movements, handling nociceptive information and influencing emotion-based motor behavior.53–55
This pre-inhale phase prepares the person to act with respect to self-awareness, that is, it induces a specific motor and emotional behavior not only with respect to the need, but also weighing one’s own adequacy in dealing with the need exhaustively.
The Movement of the Diaphragm
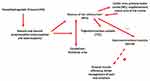
In the inspiratory phase, the diaphragm begins to move downward and forward thanks to the action of the area preBötC, which stimulates the phrenic nerves.42 In this very first phase of inspiration, the contraction of the diaphragm stimulates the low-threshold cutaneous receptors (Ruffini, Merkel), which afferents will go towards the S1 area.56,57 S1 receives these afferents (including visual ones) and processes possible results of the motor action that will derive from area M1; predicts and anticipates possible motor behaviors.58 S1 sends signals to the insula and M1, so that the body prepares to better handle all the incoming proprioceptive information.59
The oscillatory activity of the preBötC area is influenced by central (cerebellum, cortical and subcortical area) and peripheral (proprioception) neural circuits. Approximately 10–20% of the neurons constituting preBötC can send rhythmic and constant afferents autonomously, independently of the electrical efferents arriving in this area.60,61 This oscillatory mechanism is called respiratory pacemakers and is directed towards the limbic area, such as the amygdala, the periaqueductal gray area, the thalamus, the hypothalamus.60,61
Not only is the preBötC area sufficient to produce an inspiratory action by itself, as it constitutes a spinal and cerebral neural network towards other respiratory centres, but it always solicits the limbic area.41,49 The limbic area consists of several neural components, which are fundamental for the interaction of the person in the social, emotional, and motivational aspects, processing proprioceptive information (exteroception and interoception). Motor behavior, movement or non-action will arise from the management of sensory information62 (Figure 2).
The completion of the diaphragmatic movement will generate a pressure called transdiaphragmatic (Pdi), which is the difference between intrapleural and gastric pressure.63 These pressure alterations and body fluid redistribution will activate different receptors that are part of the proprioceptive system.64 Phrenic motor neurons receive excitatory efferents from the area VRGc, VRGc, NTS and the dorsal respiratory group (DRG).65,66 The phrenic nerves themselves send proprioceptive information via myelinated (type Ia, Ib, III–IV) and unmyelinated (type C) fibers.67,68 About 95% of the proprioceptive information stimulated by the diaphragmatic movement will be received by the NTS, while the remaining information package will go towards the trigeminal nucleus caudalis (TCC).42,69–72 The preferential route is the spinothalamic tract and spinomesencephalic.71 The afferents collected by the TCC can be exchanged with the NTS in a bi-directional manner, especially if the information is of the nociceptive type.73–76 TCC will also send proprioceptive information to the paramedian lobule of the cerebellum and the vestibular area.77 The vagal system can be seen as a synchronizer of the peripheral and nervous systems, and, in particular, the NTS can be seen as a key center for visceral and somatic information.77,78
Nucleus of the Solitary Tract
The information arriving at the NTS is sent to the cerebellum (all cerebellar nuclei) and the vestibular area, to return to the NTS.79,80 The re-elaborations of the NTS data will be directed towards the entire limbic area, M1 and towards the supplementary motor area of the cortex.42,70,81–84 NTS afferents will serve to regulate body homeostasis, from a motor, emotional and pain perception point of view, as well as for systemic metabolic homeostasis.
The same cortical and limbic areas will respond via efferents to NTS. New information from higher centers returning to the NTS will stimulate the latter to release inhibitory efferents to the caudal ventrolateral medulla area, resulting in inhibition for the rostroventrolateral medulla area (RVLM).42,70,82,85,86 In this medullary area we find the pre-ganglionic sympathetic neurons, which last inhibited by NTS will decrease the release of efferents, with elevation of the activity of the parasympathetic system.42,70 In this context, the neuromotor expression of the limbs and trunk improves, such as strength and balance.42,87,88
Passive expiration occurs thanks to the sending of inhibitory efferents from the Bötzinger area to the preBötC area; Bötzinger neurons are excited by the parabrachial nuclei/Kölliker-Fuse complex.41
The Postural Action of the Diaphragm
Completing the inhalation generates exhaustive intra-abdominal pressure (IAP). When the diaphragm is correctly contracted, the abdominal muscles thanks to the control by CPG, it expresses a tension that allows the physiological oblique descent of the diaphragm.41 The pelvic floor muscle complex tends to decrease the tone, so as to increase the diaphragmatic movement space and create a “suction” which allows to increase the IAP.41 IAP causes a hydraulic effect around the dorsolumbar spine and a reduction in the electrical activity of the deep spinal musculature.89,90 This mechanism allows the skeletal muscles of the limbs and trunk to express correct movements, and to maintain posture.41
Furthermore, one third of the diaphragm musculature is always contracted when the body is standing or sitting, and its contraction increases if the need to move increases in parallel.91,92 The diaphragm is the inspiratory muscle par excellence, but it is also the postural muscle par excellence. Before the person voluntarily moves a limb, the diaphragm is already contracted (about 20 milliseconds before) to the extent that the creation of an adequate IAP is necessary; this behavior goes beyond the respiratory function alone93,94 (Figure 3).
The contractile action of the diaphragm precedes the movement of the skeletal muscles and allows the adequate execution of the gesture and the maintenance of posture.41
Clinical Summary
NTS receives a lot of somatic and visceral information, and in the presence of peripheral and central neural alterations, it may not have the ability to correctly manage the afferents; it could be speculated that this fall under the concept of dysautonomia and increase pain perception in FM.95 This last concept could be one of the central motivations of dysautonomia. The diaphragm sends multiple information to NTS, but a contractile functional decrease could alter NTS function and/or sending inconsistent afferents.95 This loop may not allow NTS to act as an inhibitor of the sympathetic system, which could be speculated to create the premises for chronic pain.
It has been suggested that patients with FM have higher sympathetic system activity than healthy subjects and that shallow breathing increases the response of the same autonomic system.12,96,97 A correct inspiration stimulates the action of the vagus nerve (parasympathetic), while a non-exhaustive breath stimulates the type IV diaphragmatic afferents; a constant activation of the latter afferents could be speculated to cause the increase of the sympathetic system activity.97,98
In patients with FM the vagus nerve volume (cross-sectional area) is decreased compared to healthy subjects (although not all literature states this information).99 On the basis of this speculation (the vagus nerve represents about 80–90% of the total of all parasympathetic afferents), we hypothesize that this adaptation in relation to non-optimal respiration could be linked to the genesis of FM.99 It is no coincidence that among the indications that the clinician issues to the fibromyalgia patient, there is that of performing respiratory exercises.9,12
In FM, we hypothesize, based on preliminary studies, that the previously illustrated neural connectivity is affected, particularly for the insular area and somatosensory cortex; there is possibly a constant loss of bodily representation (proprioception).100 The oscillatory synchronizations that allow different areas to communicate effectively are possibly affected. We hypothesize, that this event occurs due to a reduction of the afferents reaching these cortical areas and to a concomitant excess of sympathetic activity.100
In the presence of fibromyalgia, it has been theorized, that the thalamus undergoes a reduction in the informational relationship with the somatosensory cortex, with an imbalance in the gamma aminobutyric acid (GABA)/glutamate ratio.101 We hypothesize that all cortical and subcortical areas involved in the previously suggested respiratory network are reduced in quantity (gray matter) in the presence of FM; the olfactory nerve demonstrates non-physiological neural activity, and there is a decrease in the volume of the olfactory bulb.102–106 We have no information on the entorhinal and piriformis cortex in the context of FM. Trigeminal nucleus caudalis appears to undergo functional alterations in FM, probably further altering the information it sends to the NTS.106–108
Finally, there is a relationship with the presence of allergic rhinitis (AR) and a dysfunction of the diaphragm, but the causes are elusive.7 AR induces a reduction in the movement of the rib cage and is considered as one of the causes that lead to important respiratory pathologies. AR causes diaphragm muscle dysfunction with a decrease in diaphragm contractile force.109 Patients with FM have reduced movement of the diaphragm, with reduced thoracic expansion.110 AR alters the functionality of the olfactory bulb (probably due to a constant neuro-inflammation), with cognitive impairment and “loss of energy” compared to active physical activity.111–113 Further studies are necessary.
The patient with FM may suffer from neuromotor uncoordination of the tongue, not necessarily related to neurological disorders.114 Probably, these motor disturbances of the lingual muscle complex would explain the finding of obstructive sleep apnea syndrome (OSAS) in patients with FM (about 65.9%).115 OSAS leads to morphological and functional dysfunctions of the diaphragm muscle.116
The patient with FM has dysfunctions involving breathing, from a neural and morpho-functional point of view, from the nose to the diaphragm.
Discussion
To understand the motivations underlying the breath network, we should imagine a hominid in the jungle with a stone in hand. The hominid must understand and choose what to do with the stone, with respect to the environment in which he finds himself, that is, he must decide whether to attack or run away with the aim of surviving. Air enters the nose, stimulating areas such as the hippocampus, amygdala, prefrontal cortex, thalamus and prepares the person for action (cognitive and motor), with respect to need and the external environment.32,33
The hominid begins at a sub-conscious level to “guess” what the stone is for and how to use it. The initial movement of the tongue, in the continuation of the respiratory act, stimulates some cerebral areas, such as M1, putamen, supplementary motor area, thalamus, cerebellum and the insula, anterior cingulate cortex.43 This phase helps the hominid to understand if he is fit to carry out certain actions (in the presence of emotions, pain, and movement), therefore if he can face the succession of events with confidence or fear. The evolution of man over millions of years has created the respiratory mode as we know it, allowing man to interact correctly with the external environment with the perfect relationship of the diaphragm and the stimulation of the proprioceptive pathways; the alteration of the diaphragm leads to dysfunctions which probably occur in the fibromyalgia patient.
The very first movement of the diaphragm stimulates the S1 area, in order to prepare the body for action, with respect to the multiple proprioceptive information that will arrive (emotions, pain, muscle-joint status).59 The completion of the diaphragmatic movement will allow the final action to be expressed concretely and effectively, attacking to feed, or running away to avoid being eaten. The parasympathetic system will be more incisive to have greater strength and precision (run away or attack), better management of pain to perform the action (must survive), and better management of emotions such that the action performed is not compromised (too much fear or too much security).42,87,88
If the breathing network is faulty, motor, emotional and nociceptive problems will take over. Sensory perception is vital to successfully integrate into a social environment.117 The breath allows us to translate proprioceptive solicitations (interoception and exteroception) into motor and emotional behaviour: we are as we breathe, and we breathe as we are. Movement and emotions (including pain) are the same coin.118
Fibromyalgia patients with neuromotor incoordination show no signs of neurological damage, and neuromotor instability is a predictor of FM severity.2 Motor instability results from a decrease in the recognition of sensory afferents (proprioception).1,5,119 There appears to be a decrease in the ability to handle and decipher sensory information, with an impaired behavioral return.120 Patients with FM show neuromotor dysfunction in the control of spinal posture due to postural perception decline.121
As discussed in previous sections, it is the contraction of the diaphragm that informs the central nervous system of body position and postural needs (by sending information from body receptors); all receptor afferents are directed towards the cortex by the activity of the diaphragm.90,91 The result is improved neuromotor expression.42 The IAP created by an optimal contraction is fundamental for an exhaustive postural control of the spine; if this is not done correctly, postural and motor balance disturbances will occur.87,89
Although patients with FM can suffer from dyspnea, regardless of whether they have pulmonary or cardiac disease, and although only a few studies have considered breath stimulation (meditation) in this clinical setting, no studies have considered diaphragm function and of the respiratory network with neuromotor expression in the patient.122–124
The clinician should pay more attention to the diaphragm in the context of fibromyalgia, as well as research should put more effort into highlighting the neuromotor functions of breathing and FM.
Conclusions
There is currently no pharmacological or non-pharmacological approach that is able to resolve the pathology exhaustively or that is recognized as the best treatment. There is no specific instrumental examination or biomarkers capable of predicting or diagnosing FM; the diagnosis is based on the history and clinical objectivity. The patient lives with a variety of co-morbidities, including impaired neuro-coordination and postural imbalances.
The article reviewed the neural connections that support the act of breathing, from the entry of air through the nose, up to the movement of the diaphragm during inspiration. The inspiration positively influences the motor actions, through the stimulation of the parasympathetic system. Currently, the panorama of scientific research underestimates the importance of breathing with respect to neuromotor expression. Greater efforts must be put in place to better understand the therapeutic procedure in the patient with FM, also considering the neural relationships of the breath.
Funding
The article has been funded by the Italian Ministry of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lomas-Vega R, Rodríguez-Almagro D, Peinado-Rubia AB, et al. Joint assessment of equilibrium and neuromotor function: a validation study in patients with fibromyalgia. Diagnostics. 2020;10(12):1057. doi:10.3390/diagnostics10121057
2. Peinado-Rubia A, Osuna-Pérez MC, Rodríguez-Almagro D, Zagalaz-Anula N, López-Ruiz MC, Lomas-Vega R. Impaired balance in patients with fibromyalgia syndrome: predictors of the impact of this disorder and balance confidence. Int J Environ Res Public Health. 2020;17(9):3160. doi:10.3390/ijerph17093160
3. Leon-Llamas JL, Murillo-Garcia A, Villafaina S, Domínguez-Muñoz FJ, Morenas J, Gusi N. Relationship between kinesiophobia and mobility, impact of the disease, and fear of falling in women with and without fibromyalgia: a cross-sectional study. Int J Environ Res Public Health. 2022;19(14):8257. doi:10.3390/ijerph19148257
4. Demir-Göçmen D, Altan L, Korkmaz N, Arabacı R. Effect of supervised exercise program including balance exercises on the balance status and clinical signs in patients with fibromyalgia. Rheumatol Int. 2013;33(3):743–750. doi:10.1007/s00296-012-2444-y
5. Reddy RS, Tedla JS, Dixit S, et al. Cervical joint position sense and its correlations with postural stability in subjects with fibromyalgia syndrome. Life. 2022;12(11):1817. doi:10.3390/life12111817
6. Arnold LM, Bennett RM, Crofford LJ, et al. AAPT Diagnostic Criteria for Fibromyalgia. J Pain. 2019;20(6):611–628. doi:10.1016/j.jpain.2018.10.008
7. Gultuna S, Tezel N, Ozalp Ates FS. Fibromyalgia in the patients with allergic rhinitis: its prevalence and impact on the quality of life. Am J Rhinol Allergy. 2019;33(6):716–722. doi:10.1177/1945892419864526
8. Viceconti A, Geri T, De Luca S, et al. Neuropathic pain and symptoms of potential small-fiber neuropathy in fibromyalgic patients: a national on-line survey. Joint Bone Spine. 2021;88(4):105153. doi:10.1016/j.jbspin.2021.105153
9. Vrouva S, Sopidou V, Koutsioumpa E, et al. Can exercise affect the pain characteristics in patients with fibromyalgia? A randomized controlled trial. Healthcare. 2022;10(12):2426. doi:10.3390/healthcare10122426
10. Chiaramonte R, Bonfiglio M, Chisari S. Multidisciplinary protocol for the management of fibromyalgia associated with imbalance. Our experience and literature review. Rev Assoc Med Bras. 2019;65(10):1265–1274. doi:10.1590/1806-9282.65.10.1265
11. Kibar S, Yıldız HE, Ay S, Evcik D, Ergin ES. New Approach in fibromyalgia exercise program: a preliminary study regarding the effectiveness of balance training. Arch Phys Med Rehabil. 2015;96(9):1576–1582. doi:10.1016/j.apmr.2015.05.004
12. Zetterman T, Markkula R, Miettinen T, Kalso E. Heart rate variability responses to cognitive stress in fibromyalgia are characterised by inadequate autonomous system stress responses: a clinical trial. Sci Rep. 2023;13(1):700. doi:10.1038/s41598-023-27581-9
13. Dos Santos GB, Sato TO, Miwa-Cerqueira T, Bifani BE, Rocha APR, Carvalho C. Pelvic floor dysfunctions in women with fibromyalgia: a cross-sectional study. Eur J Obstet Gynecol Reprod Biol. 2023;282:1–6. doi:10.1016/j.ejogrb.2022.12.030
14. Salaffi F, Di Carlo M, Farah S, Giorgi V, Mosca N, Sarzi-Puttini P. Overactive bladder syndrome and sexual dysfunction in women with fibromyalgia and their relationship with disease severity. Clin Exp Rheumatol. 2022;40(6):1091–1101. doi:10.55563/clinexprheumatol/9mbbpb
15. Katz RS, Leavitt F, Cherny K, Small AK, Small BJ. The vast majority of patients with fibromyalgia have a straight neck observed on a lateral view radiograph of the cervical spine: an aid in the diagnosis of fibromyalgia and a possible clue to the etiology. J Clin Rheumatol. 2023;29(2):91–94. doi:10.1097/RHU.0000000000001912
16. Wolfe F, Michaud K, Klooster PMT, Rasker JJ. Looking at fibromyalgia differently - An observational study of the meaning and consequences of fibromyalgia as a dimensional disorder. Semin Arthritis Rheum. 2023;58:152145. doi:10.1016/j.semarthrit.2022.152145
17. Murphy AE, Minhas D, Clauw DJ, Lee YC. Identifying and managing nociplastic pain in individuals with rheumatic diseases: a narrative review. Arthritis Care Res. 2023. doi:10.1002/acr.25104
18. Berwick R, Barker C, Goebel A; guideline development group. The diagnosis of fibromyalgia syndrome. Clin Med. 2022;22(6):570–574. doi:10.7861/clinmed.2022-0402
19. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. doi:10.1186/ar2783
20. Salaffi F, Sarzi-Puttini P, Girolimetti R, Gasparini S, Atzeni F, Grassi W. Development and validation of the self-administered Fibromyalgia Assessment Status: a disease-specific composite measure for evaluating treatment effect. Arthritis Res Ther. 2009;11(4):R125. doi:10.1186/ar2792
21. Wolfe F, Walitt BT, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of Fibromyalgia (FM) and FM Severity. J Rheumatol. 2015;42(8):1494–1501. doi:10.3899/jrheum.141519
22. Ghavidel-Parsa B, Bidari A, Atrkarroushan Z, Khosousi MJ. Implication of the nociplastic features for clinical diagnosis of fibromyalgia: development of the preliminary Nociplastic-Based Fibromyalgia Features (NFF) Tool. ACR Open Rheumatol. 2022;4(3):260–268. doi:10.1002/acr2.11390
23. Maffei ME. Fibromyalgia: recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int J Mol Sci. 2020;21(21):7877. doi:10.3390/ijms21217877
24. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42(10):2220–2230. doi:10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5
25. Fan A, Tournadre A, Pereira B, et al. Performance of Fibromyalgia Rapid Screening Tool (FiRST) to detect fibromyalgia syndrome in rheumatic diseases. Rheumatology. 2016;55(10):1746–1750. doi:10.1093/rheumatology/kew244
26. Giorgi V, Sirotti S, Romano ME, et al. Fibromyalgia: one year in review 2022. Clin Exp Rheumatol. 2022;40(6):1065–1072. doi:10.55563/clinexprheumatol/if9gk2
27. Jones KD, Horak FB, Winters-Stone K, Irvine JM, Bennett RM. Fibromyalgia is associated with impaired balance and falls. J Clin Rheumatol. 2009;15(1):16–21. doi:10.1097/RHU.0b013e318190f991
28. Ahbouch A, Moustafa IM, Shousha T, Arumugam A, Oakley P, Harrison DE. An Investigation of the Association between 3D Spinal Alignment and Fibromyalgia. J Clin Med. 2022;12(1):218. doi:10.3390/jcm12010218
29. Bordoni B, Walkowski S, Escher A, Ducoux B. The importance of the posterolateral area of the diaphragm muscle for palpation and for the treatment of manual osteopathic medicine. Complement Med Res. 2022;29(1):74–82. doi:10.1159/000517507
30. Patel-Khurana N, Fregosi RF. Motor unit number in a small facial muscle, dilator naris. Exp Brain Res. 2015;233(10):2897–2902. doi:10.1007/s00221-015-4359-9
31. Wang J, Hamill OP. Piezo2-peripheral baroreceptor channel expressed in select neurons of the mouse brain: a putative mechanism for synchronizing neural networks by transducing intracranial pressure pulses. J Integr Neurosci. 2021;20(4):825–837. doi:10.31083/j.jin2004085
32. Mofleh R, Kocsis B. Delta-range coupling between prefrontal cortex and hippocampus supported by respiratory rhythmic input from the olfactory bulb in freely behaving rats. Sci Rep. 2021;11(1):8100. doi:10.1038/s41598-021-87562-8
33. Zelano C, Jiang H, Zhou G, et al. Nasal Respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci. 2016;36(49):12448–12467. doi:10.1523/JNEUROSCI.2586-16.2016
34. Heck DH, Kozma R, Kay LM. The rhythm of memory: how breathing shapes memory function. J Neurophysiol. 2019;122(2):563–571. doi:10.1152/jn.00200.2019
35. Nácher V, Ledberg A, Deco G, Romo R. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc Natl Acad Sci U S A. 2013;110(37):15085–15090. doi:10.1073/pnas.1314681110
36. Chandrasekaran C, Bray IE, Shenoy KV. Frequency shifts and depth dependence of premotor beta band activity during perceptual decision-making. J Neurosci. 2019;39(8):1420–1435. doi:10.1523/JNEUROSCI.1066-18.2018
37. Halgren M, Ulbert I, Bastuji H, et al. The generation and propagation of the human alpha rhythm. Proc Natl Acad Sci U S A. 2019;116(47):23772–23782. doi:10.1073/pnas.1913092116
38. Warm D, Schroer J, Sinning A. Gabaergic Interneurons in early brain development: conducting and orchestrated by cortical network activity. Front Mol Neurosci. 2022;14:807969. doi:10.3389/fnmol.2021.807969
39. Vila-Merkle H, González-Martínez A, Campos-Jiménez R, et al. Sex differences in amygdalohippocampal oscillations and neuronal activation in a rodent anxiety model and in response to infralimbic deep brain stimulation. Front Behav Neurosci. 2023;17:1122163. doi:10.3389/fnbeh.2023.1122163
40. Arshamian A, Iravani B, Majid A, Lundström JN. Respiration modulates olfactory memory consolidation in humans. J Neurosci. 2018;38(48):10286–10294. doi:10.1523/JNEUROSCI.3360-17.2018
41. Bordoni B, Purgol S, Bizzarri A, Modica M, Morabito B. The influence of breathing on the central nervous system. Cureus. 2018;10(6):e2724. doi:10.7759/cureus.2724
42. Bordoni B, Escher AR. Functional evaluation of the diaphragm with a noninvasive test. J Osteopath Med. 2021;121(11):835–842. doi:10.1515/jom-2021-0101
43. Groenendijk IM, Luijten SPR, de Zeeuw CI, et al. Whole brain 7T-fMRI during pelvic floor muscle contraction in male subjects. Neurourol Urodyn. 2020;39(1):382–392. doi:10.1002/nau.24218
44. Zhuo W, Lundquist AJ, Donahue EK, et al. A mind in motion: exercise improves cognitive flexibility, impulsivity and alters dopamine receptor gene expression in a Parkinsonian rat model. Curr Res Neurobiol. 2022;3:100039. doi:10.1016/j.crneur.2022.100039
45. Cañas A, Juncadella M, Lau R, Gabarrós A, Hernández M. Working Memory deficits after lesions involving the supplementary motor area. Front Psychol. 2018;9:765. doi:10.3389/fpsyg.2018.00765
46. El-Boustani S, Sermet BS, Foustoukos G, Oram TB, Yizhar O, Petersen CCH. Anatomically and functionally distinct thalamocortical inputs to primary and secondary mouse whisker somatosensory cortices. Nat Commun. 2020;11(1):3342. doi:10.1038/s41467-020-17087-7
47. Shimohata M, Watanabe Y, Tanaka H. Numbness in the tip of the tongue and lower lip caused by thalamic hemorrhage. J Stroke Cerebrovasc Dis. 2014;23(3):557–559. doi:10.1016/j.jstrokecerebrovasdis.2013.03.021
48. Angulo Salavarria MM, Dell’Amico C, D’Agostino A, Conti L, Onorati M. Cortico-thalamic development and disease: from cells, to circuits, to schizophrenia. Front Neuroanat. 2023;17:1130797. doi:10.3389/fnana.2023.1130797
49. Krohn F, Novello M, van der Giessen RS, De Zeeuw CI, Pel JJM, Bosman LWJ. The integrated brain network that controls respiration. Elife. 2023;12:e83654. doi:10.7554/eLife.83654
50. Soyman E, Bruls R, Ioumpa K, et al. Intracranial human recordings reveal association between neural activity and perceived intensity for the pain of others in the insula. Elife. 2022:
51. Wu X, Lu X, Zhang H, et al. Sex difference in trait empathy is encoded in the human anterior insula. Cereb Cortex. 2022;bhac398. doi:10.1093/cercor/bhac398
52. Terneusen A, Winkens I, van Heugten C, et al. Neural Correlates of impaired self-awareness of deficits after acquired brain injury: a systematic review. Neuropsychol Rev. 2023;33(1):222–237. doi:10.1007/s11065-022-09535-6
53. Hoffstaedter F, Grefkes C, Caspers S, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. 2014;35(6):2741–2753. doi:10.1002/hbm.22363
54. Lee JA, Chen Q, Zhuo M. Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines. 2022;10(11):2745. doi:10.3390/biomedicines10112745
55. Vogt BA. Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28–46. doi:10.1016/j.jchemneu.2016.01.010
56. Bordoni B, Marelli F, Morabito B, Sacconi B. Manual evaluation of the diaphragm muscle. Int J Chron Obstruct Pulmon Dis. 2016;11:1949–1956. doi:10.2147/COPD.S111634
57. Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105(4):1106–1113. doi:10.1152/japplphysiol.90722.2008
58. Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA. Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation. Neuropsychologia. 2015;79(Pt B):246–255. doi:10.1016/j.neuropsychologia.2015.07.007
59. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi:10.1016/s0165-0173(96)00011-2
60. Morgado-Valle C, Beltran-Parrazal L. Respiratory rhythm generation: the whole is greater than the sum of the parts. Adv Exp Med Biol. 2017;1015:147–161. doi:10.1007/978-3-319-62817-2_9
61. Anderson TM, Ramirez JM. Respiratory rhythm generation: triple oscillator hypothesis. F1000Res. 2017;6:139. doi:10.12688/f1000research.10193.1
62. Sokolowski K, Corbin JG. Wired for behaviors: from development to function of innate limbic system circuitry. Front Mol Neurosci. 2012;5:55. doi:10.3389/fnmol.2012.00055
63. Schepens T, Fard S, Goligher EC. Assessing diaphragmatic function. Respir Care. 2020;65(6):807–819. doi:10.4187/respcare.07410
64. Bordoni B, Marelli F, Morabito B, Sacconi B. Depression and anxiety in patients with chronic heart failure. Future Cardiol. 2018;14(2):115–119. doi:10.2217/fca-2017-0073
65. Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol. 2001;532(Pt 2):483–497. doi:10.1111/j.1469-7793.2001.0483f.x
66. Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179(1):71–79. doi:10.1016/j.resp.2011.02.014
67. Nair J, Streeter KA, Turner SMF, et al. Anatomy and physiology of phrenic afferent neurons. J Neurophysiol. 2017;118(6):2975–2990. doi:10.1152/jn.00484.2017
68. Dempsey JA. Respiratory Determinants of exercise limitation: focus on phrenic afferents and the lung vasculature. Clin Chest Med. 2019;40(2):331–342. doi:10.1016/j.ccm.2019.02.002
69. Streeter KA, Sunshine MD, Davenport PW, Fuller DD. Phrenic afferent activation modulates cardiorespiratory output in the adult rat. J Neurophysiol. 2021;126(6):2091–2103. doi:10.1152/jn.00433.2021
70. Hilz MJ. Transcutaneous vagus nerve stimulation - A brief introduction and overview. Auton Neurosci. 2022;243:103038. doi:10.1016/j.autneu.2022.103038
71. Al-Chalabi M, Reddy V, Gupta S. Neuroanatomy, Spinothalamic Tract. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
72. Young RL, Page AJ, Cooper NJ, Frisby CL, Blackshaw LA. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology. 2010;138(3):
73. Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in guinea pigs. Front Physiol. 2015;6:378. doi:10.3389/fphys.2015.00378
74. Driessen AK, Farrell MJ, Dutschmann M, Stanic D, McGovern AE, Mazzone SB. Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Struct Funct. 2018;223(9):4005–4022. doi:10.1007/s00429-018-1732-z
75. Neuhuber WL, Berthoud HR. Functional anatomy of the vagus system - Emphasis on the somato-visceral interface. Auton Neurosci. 2021;236:102887. doi:10.1016/j.autneu.2021.102887
76. Peng KP, May A. Noninvasive vagus nerve stimulation modulates trigeminal but not extracephalic somatosensory perception: functional evidence for a trigemino-vagal system in humans. Pain. 2022;163(10):1978–1986. doi:10.1097/j.pain.0000000000002595
77. Neuhuber WL, Berthoud HR. Functional anatomy of the vagus system: how does the polyvagal theory comply? Biol Psychol. 2022;174:108425. doi:10.1016/j.biopsycho.2022.108425
78. Gidron Y, Deschepper R, De Couck M, Thayer JF, Velkeniers B. The vagus nerve can predict and possibly modulate non-communicable chronic diseases: introducing a neuroimmunological paradigm to public health. J Clin Med. 2018;7(10):371. doi:10.3390/jcm7100371
79. Novello M, Bosman LWJ, De Zeeuw CI. A systematic review of direct outputs from the cerebellum to the brainstem and diencephalon in mammals. Cerebellum. 2022. doi:10.1007/s12311-022-01499-w
80. Yates BJ, Billig I, Cotter LA, Mori RL, Card JP. Role of the vestibular system in regulating respiratory muscle activity during movement. Clin Exp Pharmacol Physiol. 2002;29(1–2):112–117. doi:10.1046/j.1440-1681.2002.03612.x
81. Shi MY, Ding LF, Guo YH, Cheng YX, Bi GQ, Lau PM. Long-range GABAergic projections from the nucleus of the solitary tract. Mol Brain. 2021;14(1):38. doi:10.1186/s13041-021-00751-4
82. Laviolette L, Niérat MC, Hudson AL, Raux M, Allard E, Similowski T. The supplementary motor area exerts a tonic excitatory influence on corticospinal projections to phrenic motoneurons in awake humans. PLoS One. 2013;8(4):e62258. doi:10.1371/journal.pone.0062258
83. Green AL, Paterson DJ. Using deep brain stimulation to unravel the mysteries of cardiorespiratory control. Compr Physiol. 2020;10(3):1085–1104. doi:10.1002/cphy.c190039
84. Bassi JK, Connelly AA, Butler AG, et al. Analysis of the distribution of vagal afferent projections from different peripheral organs to the nucleus of the solitary tract in rats. J Comp Neurol. 2022;530(17):3072–3103. doi:10.1002/cne.25398
85. Belyakov VI, Merkulova NA, Inyushkin AN. Respiratory effects of sensorimotor cortex and their mechanisms in rats. Bull Exp Biol Med. 2002;133(4):314–317. doi:10.1023/a:1016265130557
86. Ozaki I, Kurata K. The effects of voluntary control of respiration on the excitability of the primary motor hand area, evaluated by end-tidal CO2 monitoring. Clin Neurophysiol. 2015;126(11):2162–2169. doi:10.1016/j.clinph.2014.12.032
87. Siedlecki P, Ivanova TD, Shoemaker JK, Garland SJ. The effects of slow breathing on postural muscles during standing perturbations in young adults. Exp Brain Res. 2022;240(10):2623–2631. doi:10.1007/s00221-022-06437-0
88. Bordoni B, Marelli F, Morabito B, Sacconi B. Depression, anxiety and chronic pain in patients with chronic obstructive pulmonary disease: the influence of breath. Monaldi Arch Chest Dis. 2017;87(1):811. doi:10.4081/monaldi.2017.811
89. Bordoni B, Marelli F, Morabito B, Sacconi B, Caiazzo P, Castagna R. Low back pain and gastroesophageal reflux in patients with COPD: the disease in the breath. Int J Chron Obstruct Pulmon Dis. 2018;13:325–334. doi:10.2147/COPD.S150401
90. Kolar P, Sulc J, Kyncl M, et al. Stabilizing function of the diaphragm: dynamic MRI and synchronized spirometric assessment. J Appl Physiol. 2010;109(4):1064–1071. doi:10.1152/japplphysiol.01216.2009
91. Sembera M, Busch A, Kobesova A, Hanychova B, Sulc J, Kolar P. Postural-respiratory function of the diaphragm assessed by M-mode ultrasonography. PLoS One. 2022;17(10):e0275389. doi:10.1371/journal.pone.0275389
92. Illidi CR, Romer LM. Stabilising function of the human diaphragm in response to involuntary augmented breaths induced with or without lower-limb movements. Exp Physiol. 2022;107(12):1477–1492. doi:10.1113/EP090605
93. Hodges PW, Gandevia SC. Activation of the human diaphragm during a repetitive postural task. J Physiol. 2000;522(Pt 1):165–175. doi:10.1111/j.1469-7793.2000.t01-1-00165.xm
94. Gandevia SC, Butler JE, Hodges PW, Taylor JL. Balancing acts: respiratory sensations, motor control and human posture. Clin Exp Pharmacol Physiol. 2002;29(1–2):118–121. doi:10.1046/j.1440-1681.2002.03611.x
95. Zamunér AR, Forti M, Andrade CP, Avila MA, da Silva E. Respiratory sinus arrhythmia and its association with pain in women with fibromyalgia syndrome. Pain Pract. 2016;16(6):704–711. doi:10.1111/papr.12321
96. Zamunér AR, Barbic F, Dipaola F, et al. Relationship between sympathetic activity and pain intensity in fibromyalgia. Clin Exp Rheumatol. 2015;33(1 Suppl 88):S53–7.
97. Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev. 2017;22(2):191–207. doi:10.1007/s10741-016-9549-4
98. Marmerstein JT, McCallum GA, Durand DM. Direct measurement of vagal tone in rats does not show correlation to HRV. Sci Rep. 2021;11(1):1210. doi:10.1038/s41598-020-79808-8
99. Papadopoulou M, Papapostolou A, Bakola E, et al. Neurophysiological and ultrasonographic comparative study of autonomous nervous system in patients suffering from fibromyalgia and generalized anxiety disorder. Neurol Sci. 2022;43(4):2813–2821. doi:10.1007/s10072-021-05606-3
100. Pujol J, Blanco-Hinojo L, Doreste A, et al. Distinctive alterations in the functional anatomy of the cerebral cortex in pain-sensitized osteoarthritis and fibromyalgia patients. Arthritis Res Ther. 2022;24(1):252. doi:10.1186/s13075-022-02942-3
101. Demori I, Giordano G, Mucci V, et al. Thalamocortical bistable switch as a theoretical model of fibromyalgia pathogenesis inferred from a literature survey. J Comput Neurosci. 2022;50(4):471–484. doi:10.1007/s10827-022-00826-8
102. Sawaddiruk P, Paiboonworachat S, Chattipakorn N, Chattipakorn SC. Alterations of brain activity in fibromyalgia patients. J Clin Neurosci. 2017;38:13–22. doi:10.1016/j.jocn.2016.12.014
103. Dorris ER, Maccarthy J, Simpson K, McCarthy GM. Sensory perception quotient reveals visual, scent and touch sensory hypersensitivity in people with fibromyalgia syndrome. Front Pain Res. 2022;3:926331. doi:10.3389/fpain.2022.926331
104. Özsoy-ünübol T, Kullakçi H, Ilhan İ, Yilmaz F. Evaluation of olfactory and gustatory functions in patients with fibromyalgia syndrome: its relationship with anxiety, depression, and alexithymia. Arch Rheumatol. 2020;35(4):584–591. doi:10.46497/ArchRheumatol.2020.7833
105. Sayılır S, Çullu N. Decreased olfactory bulb volumes in patients with fibromyalgia syndrome. Clin Rheumatol. 2017;36(12):2821–2824. doi:10.1007/s10067-017-3772-9
106. Boadas-Vaello P, Homs J, Reina F, Carrera A, Verdú E. Neuroplasticity of Supraspinal structures associated with pathological pain. Anat Rec. 2017;300(8):1481–1501. doi:10.1002/ar.23587
107. Nazeri M, Zarei MR, Pourzare AR, Ghahreh-Chahi HR, Abareghi F, Shabani M. Evidence of altered trigeminal nociception in an animal model of fibromyalgia. Pain Med. 2018;19(2):328–335. doi:10.1093/pm/pnx114
108. Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255(3):439–450. doi:10.1002/cne.902550310
109. Silva PFDS, Dornelas de Andrade A, de Andrade LC, et al. Impact of moderate-severe persistent allergic rhinitis on thoraco-abdominal kinematics and respiratory muscle function. J Asthma. 2020;57(1):21–27. doi:10.1080/02770903.2018.1543433
110. Jonsson K, Peterson M. Peak expiratory flow rate and thoracic mobility in people with fibromyalgia. A cross sectional study. Scand J Pain. 2019;19(4):755–763. doi:10.1515/sjpain-2019-0044
111. Liu P, Qin D, Lv H, et al. Activation of dopamine D2 receptor alleviates neuroinflammation in a mouse model of allergic rhinitis with olfactory dysfunction. Allergy Asthma Immunol Res. 2021;13(6):882–895. doi:10.4168/aair.2021.13.6.882
112. Robles-Figueroa M, Bedolla-Barajas M, Morales-Romero J, Pulido-Guillén NA, Bustos-Gutiérrez LRM. Allergic rhinitis is associated with loss of energy and concentration difficulty: a cross-sectional study. Am J Rhinol Allergy. 2020;34(1):108–114. doi:10.1177/1945892419877554
113. Wang Y, Song XY, Wei SZ, et al. Brain response in allergic rhinitis: profile and proposal. J Neurosci Res. 2023;101(4):480–491. doi:10.1002/jnr.25159
114. Fasano A, Tinazzi M. Functional facial and tongue movement disorders. Handb Clin Neurol. 2016;139:353–365. doi:10.1016/B978-0-12-801772-2.00031-X
115. Altıntop Geçkil A, Aydoğan Baykara R. Coexistence of obstructive sleep apnea syndrome and fibromyalgia. Tuberk Toraks. 2022;70(1):37–43. doi:10.5578/tt.20229905
116. Bordoni B, Escher AR, Toccafondi A, Mapelli L, Banfi P. Obstructive Sleep Apnea and Role of the Diaphragm. Cureus. 2022;14(9):e29004. doi:10.7759/cureus.29004
117. Hilber P. The role of the cerebellar and vestibular networks in anxiety disorders and depression: the internal model hypothesis. Cerebellum. 2022;21(5):791–800. doi:10.1007/s12311-022-01400-9
118. Hilber P, Cendelin J, Le Gall A, Machado ML, Tuma J, Besnard S. Cooperation of the vestibular and cerebellar networks in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:310–321. doi:10.1016/j.pnpbp.2018.10.004
119. Gucmen B, Kocyigit BF, Nacitarhan V, Berk E, Koca TT, Akyol A. The relationship between cervical proprioception and balance in patients with fibromyalgia syndrome. Rheumatol Int. 2022;42(2):311–318. doi:10.1007/s00296-021-05081-1
120. Brun C, McCabe CS, Mercier C. The contribution of motor commands to the perturbations induced by sensorimotor conflicts in fibromyalgia. Neuroscience. 2020;434:55–65. doi:10.1016/j.neuroscience.2020.03.017
121. Toprak Celenay S, Mete O, Coban O, Oskay D, Erten S. Trunk position sense, postural stability, and spine posture in fibromyalgia. Rheumatol Int. 2019;39(12):2087–2094. doi:10.1007/s00296-019-04399-1
122. Caidahl K, Lurie M, Bake B, Johansson G, Wetterqvist H. Dyspnoea in chronic primary fibromyalgia. J Intern Med. 1989;226(4):265–270. doi:10.1111/j.1365-2796.1989.tb01392.x
123. ABuranruk O. randomized clinical trial of self-stretching with and without mindful breathing - immediate effect on pressure pain and range of motion in myofascial pain syndrome. J Bodyw Mov Ther. 2022;32:29–35. doi:10.1016/j.jbmt.2022.05.016
124. Paccione CE, Stubhaug A, Diep LM, Rosseland LA, Jacobsen HB. Meditative-based diaphragmatic breathing vs. vagus nerve stimulation in the treatment of fibromyalgia-A randomized controlled trial: body vs. machine. Front Neurol. 2022;13:1030927. doi:10.3389/fneur.2022.1030927
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.