Back to Journals » Nature and Science of Sleep » Volume 14
Effects of Obstructive Sleep Apnea on SARS-CoV-2 Antibody Response After Vaccination Against COVID-19 in Older Adults
Authors Tufik S, Andersen ML, Rosa DS , Tufik SB , Pires GN
Received 9 February 2022
Accepted for publication 15 June 2022
Published 28 June 2022 Volume 2022:14 Pages 1203—1211
DOI https://doi.org/10.2147/NSS.S361529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ahmed BaHammam
Sergio Tufik,1 Monica Levy Andersen,1 Daniela Santoro Rosa,2 Sergio Brasil Tufik,1 Gabriel Natan Pires1
1Departamento de Psicobiologia, Universidade Federal de São Paulo, São Paulo, Brazil; 2Departamento de Microbiologia, Imunologia e Parasitologia, Universidade Federal de São Paulo, São Paulo, Brazil
Correspondence: Gabriel Natan Pires, Departamento de Psicobiologia, Universidade Federal de São Paulo, Rua Napoleão de Barros, 925, São Paulo, CEP: 04024-002, Brazil, Email [email protected]; [email protected]
Introduction: Previous studies have linked sleep disturbances (including sleep deprivation and obstructive sleep apnea) to an impairment in immune response after vaccination for several diseases, although it has not yet been tested for COVID-19. This study sought to evaluate the effects of obstructive sleep apnea on anti-SARS-CoV-2 IgG levels after vaccination against COVID-19 among older adults.
Methods: The study was based on a convenience sample of inpatients who underwent full night type-I polysomnography. Inclusion criteria included being ≥ 60 years with full COVID-19 vaccination schedule. Exclusion criteria included previous COVID-19 diagnosis (assessed via self-report), less than 15 days between last dose and IgG testing, self-report of continuous positive air pressure (CPAP) use in the last three months, having undergone CPAP or split-night polysomnography, or incomplete/invalid data.
Results: Out of 122 included patients (no/mild OSA: 35; moderate: 31; severe: 56), 9.8% were considered seronegative for the IgG anti-SARS-CoV-2 test (IgG count< 50.0 AU/mL), and the median IgG levels for the whole sample was 273 AU/mL (IQR: 744), with no statistically significant differences among OSA severity groups. There was neither association between OSA severity and IgG serostatus nor correlation between IgG levels and apnea-hypopnea index. A linear regression model to predict IgG levels was built, produced an R2 value of 0.066 and the only significant predictor was time from vaccination to testing; while OSA severity was considered non-significant.
Discussion: Our results demonstrate that the severity of OSA is not correlated with a decrease in anti-SARS-CoV-2 IgG levels among older adults, and that the efficiency of COVID-19 vaccinations are not reduced from mild to severe OSA.
Keywords: sleep, sleep apnea syndrome, COVID-19, SARS-CoV-2, vaccination
Introduction
Since the beginning of the current pandemic, sleep disorders, sleep deprivation and poor sleep quality have been related to negative COVID-19 outcomes.1,2 Among all these sleep-related conditions, obstructive sleep apnea (OSA) stood out as an important risk factor, especially because it shares a common background profile with severe COVID-19 cases.3,4 This includes being older,5 overweight6 and having cardiometabolic problems.7 Initial theoretical discussions suggested that OSA may be related to COVID-19 in three different ways: 1. By increasing the vulnerability to SARS-CoV-2 infection, 2. By increasing the predisposition to severe cases and a poorer prognosis, and 3. By decreasing the efficacy of COVID-19 vaccination.2
Previous studies have already addressed the first two possibilities, rejecting OSA as a predisposing factor to SARS-CoV-2 infection, but confirming it as a risk factor for severe cases and poor outcomes in respect of COVID-19. The increased mortality of patients with OSA (11.7% vs 6.9% in non-OSA individuals) was observed in a cohort of 4688 PCR-confirmed COVID-19 cases.8 The researchers showed associations between OSA and adverse COVID-19 outcomes, including intensive care unit admission and the use of mechanical ventilation.8 In another study, which analysed 9405 COVID-19 patients, a higher OSA prevalence was reported in hospitalized patients (19.4% vs 4.5% among non-hospitalized patients).9 Recent meta-analyses corroborated these findings, demonstrating that OSA increased the chances of hospitalization, but not infection.10,11 These associations might be even more relevant among older adults,5 because they are at an increased risk of severe COVID-1912,13 and present a high prevalence of OSA (60.2% in those aged over 60, and 86.9% in those over 70).14
Some studies have speculated that sleep disturbances, particularly sleep deprivation and OSA, might decrease the efficacy of immunization against COVID-19,2,15,16 although it has not been properly investigated yet. This assumption is based on previous studies that indicated that sleep deprivation and short sleep duration decrease immune response after vaccination for H1N1, influenza, and hepatitis A;17–21 although a study found no impaired antibody response to influenza vaccination among OSA individuals.22 Benedict et al16 recently called for efforts to investigate and understand the role of sleep patterns on COVID-19 immune protection in order to assure and optimize vaccine efficacy. The influence of OSA on COVID-19 vaccine efficiency needs to be evaluated, especially among older adults who represent a risk group that has been prioritized in immunization programs.
Thus, we aimed to evaluate whether OSA is capable of decreasing the efficiency of immunization against COVID-19 among older adults by measuring anti-SARS-CoV-2 immunoglobulin G (IgG) antibody levels after vaccination.
Methods
Participants and Study Design
This was a cross-sectional evaluation based on a clinical convenience sample. The participants were included using the following criteria: minimum age of 60 years, full COVID-19 vaccination schedule, and having full night type-I polysomnography (PSG) performed at the Sleep Institute (Sao Paulo, Brazil) after January 1st, 2020. Participants were excluded based on the following criteria: Previous COVID-19 diagnosis (assessed via self-report), incomplete immunization (one dose for those that require two), less than 15 days between last dose and IgG testing, having undergone a continuous positive air pressure (CPAP) or split-night PSG at the Sleep Institute, self-report of CPAP use in the last three months, or incomplete/invalid data. No inclusion criteria regarding the type or brand of vaccines were applied. At the time of the recruitment, the following COVID-19 vaccines were available in Brazil: CoronaVac/Sinovac (inactivated virus), Oxford/AstraZeneca (adenovirus vector), Pfizer/BionTech (mRNA vaccine), and Janssen (adenovirus vector).
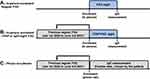
Eligible patients were recruited either in person or by phone, starting on June 4th, 2021. In person enrolment was valid for patients who underwent PSG from June 4th to July 24th, 2021. In this case, the patients were invited to participate by a research team member on the night before the PSG. Individuals who were undergoing a CPAP or a split-night PSG in this period were only considered eligible if a regular PSG had previously been performed at the Sleep Institute after January 1st, 2020 (then being assessed retrospectively in the database of the Sleep Institute). Phone enrolment was valid for patients who underwent PSG at the Sleep Institute from January 1st, 2020, to June 3rd, 2021. In this case, the patients were contacted and invited to participate by phone, their PSG results were assessed retrospectively in the database of the Sleep Institute and a date for the anti-SARS-CoV-2 IgG measurement was schedule in the following days. A schematic representation of the patient enrolment procedure is available in Figure 1.
Consent was obtained from all participants. When the participants were recruited in person, they read and signed an informed consent form, and when recruited by phone the form was read to them and they gave their oral consent. After giving their consent, the participants responded to a questionnaire regarding their COVID-19 diagnosis, vaccine status (type/brand, number of doses, and date of last dose), and any treatments for obstructive sleep apnea (OSA) in the last three months (including CPAP).
Polysomnography
All patients underwent a full-night type I PSG at the Sleep Institute, recorded using the Alice 6 LDxS system (Philips®). The following variables were monitored: six electroencephalogram channels (F4-M1, F3-M2, C4-M1, C3-M2, O2-M1, O1-M2); bilateral electro-oculogram; electromyogram (submentonian region and anterior tibialis muscle), one electrocardiogram channel (derivation D2 modified), two channels for airflow detection (thermistor and nasal pressure cannula), two channels for respiratory effort (thorax and abdomen, both by inductance plethysmography), percutaneous oxi-hemoglobin saturation (SpO2) and pulse rate via digital oximeter, snoring (microphone), and body position. Sleep staging and scoring was performed according to the American Academic of Sleep Medicine recommendations.23 Apneas correspond to a 90% reduction in respiratory flow for at least 10s, preferentially measured by the thermistor. Hypopneas correspond to a 30% reduction in respiratory flow for at least 10s, associated with an SpO2 desaturation of at least 3% or an arousal. Respiratory effort-related arousals (RERA) correspond to a flattening in the airflow curve for at least 10s, observed in the pressure cannula and associated with an arousal. The apnea-hypopnea index (AHI) was calculated as the average combined number of apneas and hypopneas per hour of sleep. The respiratory disturbance index (RDI) was calculated as the average combined number of apneas, hypopneas, and RERAs per hour of sleep. Based on the results of the PSG recordings, the participants were assigned to the following OSA severity groups, according to their AHI: No OSA (AHI<5); mild OSA (AHI≥5, but <15); moderate OSA (AHI≥15, but <30), and severe OSA (AHI≥ 30). As the number of participants with no OSA was small, they were merged with those with mild OSA for the analyses.
Anti-SARS-CoV-2 IgG Measurement
For participants recruited in-person, blood sampling for IgG testing took place on the morning after the PSG, in the sleep lab bedroom. For those recruited via phone, they could choose between blood sampling being performed either at their homes or in-lab (at the Centro de Diagnósticos Brasil (CDB), São Paulo).
The SARS-CoV-2 IgG serology was carried out in patients who have already been vaccinated with the complete vaccine schedule. Chemiluminescence assay (SARS-CoV-2 IgG II quant by Abbott®) was used to evaluate antibody titers based on the following cut-offs according to the manufacturer’s instructions: lower threshold: 6.8 arbitrary units (AU)/mL; higher threshold: 80.000 AU/mL; non-reagent: <50.0 AU/mL.
Sample Size Calculation and Statistical Analysis
The required sample size was 264 participants, calculated using GPower 3.1, considering three groups, five covariants, α<0.05, β<0.80, and an effect size of η2=0.06. However, the recruitment was ended on July 24th 2021, before the calculated sample size was reached, as preliminary data analysis showed that a significant effect of OSA on IgG levels was unlikely.
The main outcome considered in the analyses were anti-SARS-CoV-2 IgG levels (AU/mL) and the IgG reactive status (reactive or non-reactive). The following sleep-related respiratory parameters were used for the analyses: AHI, RDI, average and minimum SpO2 levels, and percentage of SpO2<90% out of the total sleep time (SpO2<90%).
The distribution of the continuous/numeric variables were tested using the Shapiro–Wilk’s test. The association between OSA severity groups and IgG reactive status (reactive or non-reactive) was evaluated by a X2 test. The correlation among anti-SARS-CoV-2 IgG levels, descriptive variables and sleep-related respiratory parameters were tested using a Spearman correlation test. The effect of confounding variables (sex and vaccine type) on IgG was tested using Student’s t test. Descriptive variables were compared among OSA severity groups using the Kruskal–Wallis test followed by Dwass-Steel-Critchlow-Fligner pairwise comparison. Anti-SARS-CoV-2 IgG levels among the OSA severity levels were compared using a one-way analysis of variance (ANOVA) with Welch’s correction. Finally, a linear regression model was composed considering anti-SARS-CoV-2 IgG levels as the outcome (dependent variable), OSA severity group as the main independent factor (no/mild OSA as reference level), and age and time from vaccination to testing as covariates. Numeric/continuous variables are presented as median and interquartile range (IQR) of the raw scale, but IgG were converted to logarithmic scales for the statistical analyses. Categorical data are presented as counts and percentage. All analyses were performed using Jamovi 1.6 (www.jamovi.org) and the significance threshold was established as p<0.05.
Ethical Approval
This study was reviewed and approved by the Universidade Federal de São Paulo Research Ethics Committee – CEP/UNIFESP (No: 0374/2020) and was performed in compliance with the Declaration of Helsinki. Signed informed consents of all participants were obtained.
Results
Out of 228 recruited participants, 122 were considered eligible. The flow diagram of the selection process is presented in Figure 2. The median AHI in the included sample was 26.6 (IQR: 35.8), which was not significantly different from those excluded due to self-reported previous diagnosis of COVID-19 (37.2 (IQR: 18), Mann–Whitney U: 978, p=0.707), therefore reducing the chances of selection bias due to the possibility of OSA increasing the likelihood of a positive COVID-19 history. Among the included participants, four (3.2%) had an AHI compatible with no OSA; 31 (25.4%) with mild OSA; 31 (25.4%) with moderate OSA, and 56 (45.9%) with severe OSA. As the number of participants with no OSA was small, they were merged with those with mild OSA for the analyses. Individuals with no OSA and with mild OSA had equivalent results on total sleep time (p=0.937), sleep efficiency (p=0.797), wake after sleep onset (p=0.580), arousal index (p=0.162) and percentages of N1 (p=0.205), N2 (p=0.773), N3 (p=0.707) and REM sleep (p=0.575), which supports the decision to merge these individuals in a single group.
 |
Figure 2 Flow diagram of patients inclusion. Abbreviations: CPAP, continuous positive air pressure; IgG, immunoglobulin G; OSA, obstructive sleep apnea; PSG, polysomnography. |
In our cohort, 91.0% (n=111) of the participants had received CoronaVac and 9.0% (n=11) the Oxford/AstraZeneca vaccine. Other types or brands were not reported by the participants. The median age of the sample was 72 years (IQR: 5.7), 65.5% were female (n=80) and the median time between vaccination and testing was 58.5 days (IQR: 30.8). The descriptive data for the full sample and for each OSA severity group are presented in Table 1.
 |
Table 1 Descriptive Variables and Comparisons Among Groups |
The median IgG count of the final sample was 273 AU/mL (IQR: 744). Only 12 participants (9.8%) were considered seronegative in the IgG anti-SARS-CoV-2 test (IgG count <50.0 AU/mL), while the remaining 110 (90.2%) were considered seropositive.
Exploratory analyses evaluating the effects or correlations of possible confounders on the IgG levels showed that only the time from vaccination to testing was statistically significant (Spearman’s rho: −0.254, p=0.005), while age (Spearman’s rho: 0.013, p=0.883), sex (Student’s t: 1.94, p=0.055), and vaccine type (Student’s t: −1.87, p=0.064) were not significant. IgG levels in the final sample were lower than among those excluded due to a previous self-reported positive diagnosis of COVID-19 (n=18, median: 2423 AU/mL, IQR: 10,812, Student’s t=6.16; p<0.001), confirming this as an important confounding factor and reinforcing the decision to exclude them from the sample.
We observed no statistically significant association between IgG serostatus and OSA severity (X2=0.912; p=0.634 – Figure 3A). The anti-SARS-CoV-2 IgG serum level was not significantly correlated with the AHI (Spearman’s rho: −0.169, p=0.063) or with other respiratory variables evaluated (Figure 3B) in the Spearman correlation test. No statistically significant difference was found in the IgG levels among the OSA severity groups (Figure 3C and D). The average IgG levels in the severe OSA group seem higher than in the other groups on a visual analysis, but this was impacted by two outliers, at 18,633.50 and 31,763.50 AU/mL (Figure 3D). A linear regression model to predict IgG levels was built, including OSA severity levels as an independent factor (no/mild OSA as the reference level) and age and time from vaccination to testing as covariates. The linear regression produced an R2 value of 0.066. The only statistically significant predictor was time from vaccination to testing (p=0.017); while OSA severity and age were considered non-significant.
Discussion
Our results demonstrate that anti-SARS-CoV-2 IgG levels among older adults after vaccination against COVID-19 does not decrease from mild to severe OSA. From a public health perspective, this is a very positive outcome as despite being at a higher risk for severe COVID-19, individuals with severe OSA are equally protected by the vaccination as those with mild or no OSA. Furthermore, when considering the whole sample, the seroconversion rate of IgG was of 90.2%, attesting the success and efficiency of COVID-19 immunization regardless of OSA presence. In comparison, in phase two clinical trials of CoronaVac, the seroconversion rates varied from 90.7% to 99.0% at 28 days after the second dose among individuals aged 60 and over.24 Our results are reasonably similar to these, suggesting that OSA has no effect on seroconversion and on anti-SARS-CoV-2 IgG levels among older adults.
Although quantitative serology tests indicate the levels of antibodies after being infected by SARS-CoV-2 or being vaccinated, the level of protection offered by the immunization does not solely rely on these levels. Results from current SARS-CoV-2 antibody tests face limitations in respect of evaluating the status of immunity against COVID-19.25 The humoral immune response triggered by vaccination has been shown to decline with aging and pronounced age-associated reduced immunogenicity to the COVID-19 mRNA vaccine was reported in a group of patients aged from 20 to 65 years.26 Another study with patients vaccinated with the Biontech/Pfizer vaccine also indicated lower levels of IgG in older subjects compared to the younger ones.27 Our data does not allow us to address the effect of age on IgG levels, as our sample was restricted to individuals aged 65 years or above, but seroconversion rates were satisfactory even considering the possible negative effect of aging on immune response.
Some limitation in respect of our results should be noted in order to promote a proper interpretation of our findings. First, as this study was based on a clinical convenience sample, we had a limited number of individuals with no OSA, consequently preventing us from evaluating the effects of OSA on IgG levels against a proper control group. However, we consider that the comparison against a mild OSA group is adequate, and that the seroconversion rate is sufficient to conclude that OSA has no effect on the anti-SARS-CoV-2 IgG levels among older adults. Second, according to the International Classification of Sleep Disorders - 3rd edition28 the correct diagnosis of mild OSA should encompass the presence of symptoms, which was not evaluated in this work. Thus, it is possible that some patients that were classified as mild OSA should be diagnosed with no OSA, which reinforces the decision to merge people with no and mild OSA into one group. As moderate and severe OSA are diagnosed based only on AHI, this limitation does not apply to the other groups. Third, as the previous history of positive COVID-19 diagnosis was based on self-report, it is possible that undiagnosed asymptomatic or mild cases have been included into our analyses. However, the incidence of self-reported COVID-19 cases among our entire recruited sample (8.3%, 19 out of 228 patients) was smaller than that observed for the Brazilian population (9.6%, as of August 2021). Fourth, evaluating co-morbidities, such as hypertension, diabetes and dyslipidaemia would have been interesting, as they might be considered as confounding factors on the effects of OSA. However, these data were not available in our data collection. Adding them to the regression model would allow a better explanation of the factors accounting for the IgG data distribution, but its actual effects on the explanation of the results attributed to OSA is uncertain.
The external validity and generalizability of our findings are still to be evaluated in other conditions. Further studies should, therefore, be performed to evaluate immune response in the same age group and/or in younger populations. The results are restricted to OSA syndrome and should not be extrapolated to other sleep-related conditions, such as insomnia, sleep deprivation or chronodisruption. The association with other factors that might influence vaccination efficacy should also be evaluated, including mental disorders,29 time of the day in which vaccines are taken30 and shift work.31
In our study, the sample comprised only two COVID-19 vaccine types/brands (CoronaVac and Oxford/AstraZeneca), which are the most frequently used vaccines in the Brazilian national immunization program. Two other vaccines against COVID-19 (Pfizer/BionTech and Janssen) are also approved for use in Brazil, but were not reported among our sample as they represented a small fraction of total vaccines applied at the time of enrolment. The results are likely to be reproduced with other vaccine types, although it would be helpful to perform a similar trial using different vaccine technologies, especially RNA vaccines. In conclusion, our results demonstrate that the increasing severity of OSA does lead to a decrease in anti-SARS-CoV-2 IgG levels among older adults, and, therefore, that the efficiency of COVID-19 vaccinations are not reduced from mild to severe OSA.
Acknowledgments
This work was supported by grants from the Associação Fundo de Incentivo à Pesquisa (AFIP), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). MLA, DSR and ST are CNPq fellowship recipients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
GNP is a shareholder at SleepUp™ (a Brazilian online CBTi company) but attests that this position has no relationship with the aims, preparation, or execution of this study and reports no other potential conflicts of interest in relation to this work. The other authors declare that they have no conflicts of interest in relation to this work. This article has not been submitted elsewhere for publication.
References
1. Li P, Zheng X, Ulsa MC, et al. Poor sleep behavior burden and risk of COVID-19 mortality and hospitalization. Sleep. 2021;44(8). doi:10.1093/sleep/zsab138
2. De Mello MT, Silva A, Guerreiro RC, et al. Sleep and COVID-19: considerations about immunity, pathophysiology and treatment. Sleep Sci. 2020;13(3):199.
3. Tufik S, Gozal D, Ishikura IA, Pires GN, Andersen ML. Does obstructive sleep apnea lead to increased risk of COVID-19 infection and severity? J Clin Sleep Med. 2020;16(8):1425–1426. doi:10.5664/jcsm.8596
4. Tufik S. Obstructive sleep apnea as a comorbidity to COVID-19. Sleep Sci. 2020;13(3):181.
5. Pires GN, Ishikura IA, Xavier SD, et al. Sleep in older adults and its possible relations with COVID-19. Front Aging Neurosci. 2021;13. doi:10.3389/fnagi.2021.647875
6. Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med. 2020;20(4):e109–e113. doi:10.7861/clinmed.2020-0239
7. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi:10.1016/j.jacc.2020.03.031
8. Cade BE, Dashti HS, Hassan SM, Redline S, Karlson EW. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020;202(10):1462–1464. doi:10.1164/rccm.202006-2252LE
9. Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2020;25:1155–1157. doi:10.1007/s11325-020-02203-0
10. Strausz S, Kiiskinen T, Broberg M, et al. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir Res. 2021;8(1). doi:10.1136/bmjresp-2020-000845
11. Hariyanto TI, Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021;82:47–53. doi:10.1016/j.sleep.2021.03.029
12. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi:10.1056/NEJMoa2002032
13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
14. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi:10.1016/j.sleep.2009.10.005
15. Zhu J, Zhang M, Sanford LD, Tang X. Advice for COVID-19 vaccination: get some sleep. Sleep Breath. 2021;25:2287–2288. doi:10.1007/s11325-021-02313-3
16. Benedict C, Cedernaes J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir Med. 2021;9:447–448. doi:10.1016/S2213-2600(21)00126-0
17. Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65(5):831–835. doi:10.1097/01.PSY.0000091382.61178.F1
18. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. doi:10.1001/jama.288.12.1469
19. Benedict C, Brytting M, Markström A, Broman JE, Schiöth HB. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol. 2012;13:1. doi:10.1186/1471-2172-13-1
20. Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35(8):1063–1069. doi:10.5665/sleep.1990
21. Prather AA, Pressman SD, Miller GE, Cohen S. Temporal links between self-reported sleep and antibody responses to the influenza vaccine. Int J Behav Med. 2021;28(1):151–158. doi:10.1007/s12529-020-09879-4
22. Dopp JM, Wiegert NA, Moran JJ, Muller D, Weber S, Hayney MS. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy. 2007;27(11):1483–1489. doi:10.1592/phco.27.11.1483
23. AASM. The AASM Manual for the Scoring of Sleep and Associated Events - Version 2.4. American Academy of Sleep Medicine; 2017.
24. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, Phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi:10.1016/S1473-3099(20)30987-7
25. Ismail AA. Serological tests for COVID-19 antibodies: limitations must be recognized. Ann Clin Biochem. 2020;57(4):274–276. doi:10.1177/0004563220927053
26. Jalkanen P, Kolehmainen P, Häkkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi:10.1038/s41467-021-24285-4
27. Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:2065–2072.
28. AASM. International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014.
29. Xiao K, Gillissie ES, Lui LMW, et al. Immune response to vaccination in adults with mental disorders: a systematic review. J Affect Disord. 2022;304:66–77. doi:10.1016/j.jad.2022.02.025
30. Wang W, Balfe P, Eyre DW, et al. Time of day of vaccination affects SARS-CoV-2 antibody responses in an observational study of health care workers. J Biol Rhythms. 2022;37(1):124–129. doi:10.1177/07487304211059315
31. Lammers-van der Holst HM, Lammers GJ, van der Horst GTJ, et al. Understanding the association between sleep, shift work and COVID-19 vaccine immune response efficacy: protocol of the S-CORE study. J Sleep Res. 2022;31(2):e13496. doi:10.1111/jsr.13496
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.