Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
New Onset of Severe Plaque Psoriasis Following COVID-19 Vaccination: A Case Report
Authors Hu YQ , Zhang JZ, Zhao Y
Received 28 May 2022
Accepted for publication 22 July 2022
Published 2 August 2022 Volume 2022:15 Pages 1485—1487
DOI https://doi.org/10.2147/CCID.S376555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yu-qing Hu, Jian-zhong Zhang, Yan Zhao
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Yan Zhao, Department of Dermatology, Peking University People’s Hospital, No. 11 South Avenue, Xi Zhi Men Street, Xicheng District, Beijing, People’s Republic of China, Tel +86-10-88325472, Fax +86-10-68318386, Email [email protected]
Abstract: A case of new onset of severe plaque psoriasis following COVID-19 vaccination was reported. A 63-year-old woman presented with multiple plaques for 2 months after the second dose of COVID-19 vaccination. Dermatological examination revealed diffuse erythematous papules and plaques on trunk and limbs. Her lesions responded well to the treatment of secukinumab 150 mg per week. In this case, we presented the potential association between COVID-19 vaccination and the onset of psoriasis. It is essential to recognize the possible adverse events as vaccination against COVID-19 continues worldwide.
Keywords: plaque psoriasis, COVID-19, vaccination
Introduction
Psoriasis is a chronic immune-mediated cutaneous inflammatory disease. Triggers of psoriasis included infection, stress, trauma, alcohol consumption and drug. A possible association of vaccination with the new onset or exacerbation of psoriasis has been reported.1 Here, to our knowledge, we reported a rare case of new-onset severe plaque psoriasis after COVID-19 vaccination.
Case Report
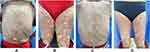
A 63-year-old woman presented with widespread erythema and plaques for 2 months, which occurred a day after the second dose of COVID-19 vaccination (SARS-Cov-2 Vaccine, inactivated, Beijing Institute of Biological Products Company, Beijing, China). The lesions began on her face and neck and spread to her trunk and extremities. Physical examination revealed diffuse erythematous plaques on her face, trunk and limbs (body surface area 72%, Psoriasis Area Severity Index 30.4, Figure 1A and B). Blood tests showed a normal blood count and she denied family history of psoriasis or any other putative triggers, such as drugs, previous infections and trauma. Based on these findings, she was diagnosed as acute plaque psoriasis triggered by COVID-19 vaccination. She was treated with secukinumab 150 mg per week because her weight was less than 60 kg. Her lesions responded well to the treatment of secukinumab after a month with significant improvement of scaly plaques on her back and extremities, leaving only some residual erythema and hyperpigmented patches (Figure 1C and D).
Discussion
With COVID-19 mass vaccination, cutaneous reactions to COVID-19 vaccines described are increasing.2 In a registry-based study including 414 cases by McMahon et al,3 injection site reactions, swelling, urticaria, erythromelalgia and flares of psoriasis after COVID-19 vaccination were reported. New onset or exacerbations of psoriasis following vaccination have been described in other different types of vaccines, including influenza, Bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide vaccines.4 Recently, new onset or exacerbations of psoriasis associated with COVID-19 vaccines have been reported, including guttate psoriasis and acute generalized pustular psoriasis.5 Wei et al1 reported six patients who experienced an exacerbation of known psoriasis and one patient with new-onset psoriasis following COVID-19 vaccination. Six of them presented with symptoms after the second dose of the vaccine. In our patient, psoriasis lesions occurred following the second dose of COVID-19 vaccination. And the lack of any other trigger factors (no infections or new medication) strongly suggests a causal association. However, most vaccination-related psoriasis were guttate variants and acute generalized pustular psoriasis.5 New-onset severe plaque psoriasis due to COVID-19 vaccines was scarce.
Psoriasis is an immune-mediated disorder characterized by Th1 and Th17 cells cytokines and a predominance of CD4+ T cells in the dermis. The onset of psoriasis can be induced by various medications, infections, vaccines and other triggers.6 Tumour necrosis factor (TNF)-α and interferon (IFN)-γ are two potent pro-inflammatory cytokines which can trigger inflammatory cascades in psoriasis.7 The mechanisms of new-onset psoriasis after vaccination are unknown. It has been elicited that IL-2, IL-12, TNF-α and IFN-γ produced by CD4+ T cells were increased after COVID-19 vaccination.8 IL-6-induce Th17 subset of CD4+ T cells in COVID-19 immunopathology and vaccine-induced immune enhancement was highlighted by recent studies.9 Therefore, it was speculated that COVID-19 vaccines may lead to activation of inflammatory pathways and thereby triggered psoriasis. However, further investigations are required.
As COVID-19 vaccination and booster vaccine shots continue worldwide in the future, it is necessary for dermatologists to be aware of the possibility of new-onset psoriasis secondary to COVID-19 vaccines. We should pay close attention to the possible adverse effects of COVID-19 vaccines and counteract the worsening of patient’s clinical condition.
Declaration of Patient Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Institutional approval is not required for this case study.
Funding
This work was supported by National Natural Science Foundation of China (82103711).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022; 19: 74–77. doi: 10.1016/j.jdcr.2021.11.016
2. Tachibana K, Kawakami Y, Tokuda M, Sato S, Sugihara S, Miyake T, et al. Flare-up of generalized pustular psoriasis following Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: two cases without mutations of IL36RN and CARD14 genes. J Dermatol. 2022. doi: 10.1111/1346-8138.16442
3. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after moderna and pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021; 85 (1): 46–55. doi: 10.1016/j.jaad.2021.03.092
4. Huang YW, Tsai TF. Exacerbation of psoriasis FOLLowing COVID-19 vaccination: report from a single center. Front Med-Lausanne. 2021. doi:10.3389/fmed.2021.812010
5. Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021; 17: 1–3. doi: 10.1016/j.jdcr.2021.08.035
6. Ouni N, Korbi M, Chahed F, et al. New-onset guttate psoriasis following coronavirus disease 2019 vaccination: about two cases. Dermatol Ther. 2022:15617. doi:10.1111/dth.15617
7. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017; 140 (3): 645–653. doi: 10.1016/j.jaci.2017.07.004
8. Tran TNA, Nguyen TTP, Pham NN, Pham NTU, Vu TTP, Nguyen HT. New onset of psoriasis following COVID-19 vaccination. Dermatol Ther. 2022; e15590. doi: 10.1111/dth.15590
9. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020; 53 (3): 368–370. doi: 10.1016/j.jmii.2020.03.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.