Back to Journals » International Journal of General Medicine » Volume 15
AC099850.3/NCAPG Axis Predicts Poor Prognosis and is Associated with Resistance to EGFR Tyrosine-Kinase Inhibitors in Lung Adenocarcinoma
Authors Bao J, Wu Y, Zhang K, Qi H
Received 15 March 2022
Accepted for publication 22 August 2022
Published 29 August 2022 Volume 2022:15 Pages 6917—6930
DOI https://doi.org/10.2147/IJGM.S365695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jiaqi Bao,1,* Yanlong Wu,2,* Kun Zhang,3 Huijuan Qi4
1Department of Thoracic Surgery, Affiliated Hospital of Chifeng University, Chifeng, People’s Republic of China; 2Department of Urology, Affiliated Hospital of Chifeng University, Chifeng, People’s Republic of China; 3Department of Radiology, Huhhot First Hospital, Huhhot, People’s Republic of China; 4Department of Gynecology, Affiliated Hospital of Chifeng University, Chifeng, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huijuan Qi, Department of Gynecology, Affiliated Hospital of Chifeng University, Chifeng, People’s Republic of China, Email [email protected]
Background: TKI-acquired resistance markedly interferes with treatment of lung cancer patients with EGFR mutant features. Long non-coding RNAs (lncRNAs) modify EGFR-TKI resistance during tumor progression. Non-structural maintenance of chromosomes condensin I complex subunit G (NCAPG) is a mitosis-related protein that is involved in tumorigenesis. We investigated the potential regulatory lncRNAs of NCAPG in lung adenocarcinoma (LUAD) and assessed their roles in EGFR-TKI resistance.
Methods: Data for 1678 lung cancer patients were retrieved from TCGA and GEO databases and used to evaluate NCAPG and lncRNAs expressions, as well as their prognostic significance in LUAD. Protein levels of NCAPG in LUAD were validated by immuno-histochemistry. To assess the relationship between NCAPG levels and EGFR-TKIs sensitivity, a cohort of 57 LUAD patients administered with EGFR-TKIs was used.
Results: Both NCAPG and lncRNA AC099850.3 were over-expressed in LUAD tissues, and correlated with tumor progression and poor prognosis in LUAD. LncRNA AC099850.3 was identified as a potential regulator of NCAPG expressions. The AC099850.3/NCAGP axis was markedly correlated with EGFR mutations and IC50 of EGFR-TKIs. Besides, elevated NCAPG levels were associated with EGFR-TKIs resistance in 57 LUAD patients undergoing TKIs treatment. Gene set enrichment analysis revealed that both AC099850.3 and NCAGP were abundant in the cell cycle and the p53 signaling pathway.
Conclusion: The AC099850.3/NCAPG axis is a potential prognostic predictor and therapeutic biomarker for EGFR-TKIs in LUAD.
Keywords: lung cancer, biomarker, NCAPG, lncRNA, EGFR
Introduction
Globally, lung cancer is the leading cause of tumor-associated mortality.1 Intriguingly, non-small cell lung cancer (NSCLC) constitutes more than 85% of all lung cancer cases.2,3 This group of cancers mainly include lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC). Currently, surgery, targeted chemotherapy, immunotherapy and radiotherapy among other intervention methods are the main therapeutic approaches for NSCLC.4–6 Despite recent advances in early detection, diagnosis, targeted and immune therapies, the mortality rate due to NSCLC is high.7–9 Therefore, elucidation of molecular mechanisms and identification of new therapeutic targets is vital for clinical management of NSCLC.
The epidermal growth factor receptor (EGFR) signaling cascade has a critical role in cell proliferation and NSCLC survival.10 Drugs, for instance the EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib have been shown to reduce tumor sizes in NSCLC patients with EGFR-activating mutations.11 However, due to drug resistance, the overall clinical efficacy from these drugs in NSCLC individuals is low.12 Therefore, it is important to identify novel biologic signatures to complement the initial EGFR mutation status for enhanced prediction of EGFR-TKIs responsivity and elucidate on molecular mechanisms of EGFR-TKIs acquired resistance.
NCAPG is a mitosis-related protein involved in chromosomal condensation during mitosis.13–15 As an oncogene, NCAPG affects the proliferation and migration of hepatocellular carcinoma cells and is correlated with poor prognostic outcomes.16–22 Through pan-cancer analyses of the Cancer Genome Atlas (TCGA) dataset, Xiao et al23 established that NCAPG is over-expressed in 16 cancer types. This phenomenon is remarkably correlated with oncogenesis and progression of various tumors, such as gastric,24 prostate cancer,25 endometrial26 and clear cell renal cell carcinomas.27 Recently, several researchers have found that NCAPG is required for tumorigenesis and progression of LUAD.28–30 Thus, NCAPG is possibly a novel biomarker for LUAD early diagnosis, prognosis prediction, and drug development. However, whether NCAPG plays a role in EGFR-TKI resistance has never been clarified.
Long non-coding RNAs (lncRNAs) are >200 nucleotides in length. They play vital roles in cancer cell proliferation, metastasis and EGFR-TKI resistance.31–34 Studies have evaluated the transcription landscape of NCAPG to establish its significance in tumorigenesis. However, these studies mainly focused on protein-coding genes. Elucidation of its regulatory network, especially at the lncRNA levels, will be beneficial for understanding cancer pathogenesis and for informing the development of appropriate prevention and treatment strategies.
Using TCGA and GEO databases, NCAPG levels in NSCLC, the clinical value and cancer-related functions of NCAPG in interacting with lncRNA AC099850.3 and potential mechanisms of the AC099850.3/NCAPG axis were evaluated. Besides, the significance of the AC099850.3/NCAPG axis in EGFR-TKIs resistance in LUAD was delineated.
Materials and Methods
Data Mining from the TCGA Database
Level 3 mRNA-seq (HTSeq-FPKM) data for 535 primary LUAD tissues and 59 non-malignant tissue samples were abstracted from the TCGA database. Clinical data from 522 patients with primary LUAD were also obtained from the TCGA-LUAD dataset. After excluding patients without complete survival data and those with a survival time of less than 30 days, 477 LUAD patients were included for subsequent analyses. The TCGA-LUSC dataset contained data for 502 patients with primary LUSC and 49 non-malignant tissue samples. Finally, 467 LUSC patients with complete NCAPG mRNA-seq and clinical data were selected for further analyses. Strawberry-Perl-5.30.0.1 and R software (v3.6.3) were used to collate and normalize the original data. DNA methylation and copy number variations (CNVs) of NCAPG were assessed using cBioPortal of Cancer Genomics,35 whereas the relationship between levels of NCAPG with TP53 and associated mutations were evaluated using the TCGA-LUAD dataset. Characteristics of LUAD and LUSC patients from TCGA databases are presented in Table S1.
Oncomine Analysis
Oncomine is a frequently-used cancer microarray data resource and integrated data-mining platform that provides published transcriptomic data on multiple cancer types.36 We used it to assess NCAPG mRNA expressions in tumor and non-malignant LUAD as well as LUSC tissues. Search parameters were: Gene: NCAPG mRNA, Cancer Type: Lung cancer, Threshold by Fold-change>1, p<0.001, Gene rank = top 10%.
Data Mining from Gene Expression Omnibus (GEO) Database
Sequencing and clinical data for lung cancer patients were downloaded from the GEO database. The downloaded three datasets (GSE31210,37 GSE3021938 and GSE7209439) were independently analyzed to validate our results. The characteristics of LUAD and LUSC patients from the GEO database are shown in Table S2.
LUAD Patient Samples
To assess NCAPG protein levels, between Jan. 2018 and Oct. 2018, twenty-two cancerous tissue samples and their matched non-malignant lung tissue samples located > 5 cm at the edge of the cancerous tissue were selected from 22 patients in our hospital. All patients were randomly selected and pathologically confirmed with LUAD. Patients who had received radiotherapy and chemotherapy, or had other malignant tumors, coronary heart disease, or diabetes were excluded. Among the 22 LUAD patients (13 male and 9 female; median age 58.5 years, range 41–77 years), 5, 5, 1, 9 and 2 patients were respectively classified into IA, IB, IIA, IIB and IIIA LUAD stages, according to the AJCC 7th staging system. This study was approved by the Research Ethics Committee of the Affiliated Hospital of Chifeng University and written informed consents were obtained from all participants. All the procedures in the study complied with the 1964 Declaration of Helsinki and its subsequent amendments.
Clinical Patients and Therapeutic Evaluation of EGFR-TKIs
To assess the relationship between NCAPG levels and EGFR-TKIs sensitivity, a retrospective analysis was conducted on a cohort of 57 LUAD patients that had been treated with EGFR-TKIs in our hospital from Feb. 2018 to Jun. 2019. Among the 57 LUAD patients (30 male and 27 female; median age 63.4 years, range 40–83 years), 2, 5, 25, 9 and 16 patients were respectively classified into IIA, IIB, IIIA, IIIB and IV LUAD stages. Participants were only administered with erlotinib or gefitinib during the research period. Therapeutic efficacies were evaluated at 1–3 months after EGFR-TKIs administration using CT following the RECIST 1.1 criteria, as previously described.40,41 In cases of clinical progression, patients were switched to other treatment options, such as radiotherapy and chemotherapy.
Immunohistochemistry Staining Assays
Immunohistochemistry (IHC) was performed on samples from LUAD patients as previously described.42 Briefly, after deparaffinization, hydration and antigen retrieval, tissue sections were incubated overnight at 4°C with anti-NCAPG primary antibodies (Rabbit Anti-NCAPG antibody ab251864, 1:100; Abcam Corp., Cambridge, UK). After washing, they were incubated with secondary antibodies (Goat Anti-Rabbit IgG H, L ab205718; 1:200,00; Abcam Corp., Cambridge, UK), stained and independently assessed by two experienced pathologists. An overall protein expression score, ideally ranging from 0 to 12, was computed as previously documented.42,43
Prediction of NCAPG-Related lncRNAs
Based on lncRNA information in the Ensembl database, lncRNA expression profiles were extracted from TCGA-LUAD RNA-Seq data in Perl software. Then, differentially expressed lncRNAs (DElncRNAs) between non-malignant groups and disease groups in the TCGA-LUAD dataset were respectively analyzed with thresholds of |fold Change (FC)| > 1.0 and p < 0.05 using “limma” in R. Co-expression relationships between DElncRNAs and NCAPG were computed by Spearman correlation coefficients. The DElncRNAs that were markedly correlated with NCAPG were considered potential NCAPG-related lncRNAs in LUAD (Spearman correlation coefficients> 0.6 and p <0.001).44,45
Prediction of EGFR-Targeted Therapy Responses
Responses to EGFR-TKIs by LUAD patients were analyzed using the pharmacogenomics database; Genomics of Drug Sensitivity in Cancer (GDSC; https://www.cancerrxgene.org). The estimated IC50 (half maximal inhibitory concentrations) of EGFR-TKIs were computed by “pRRophetic” in R software (3.6.3).46,47
Gene Set Enrichment Analysis (GSEA)
KEGG enrichment analysis was performed by GSEA to determine which cascade AC099850.3 and NCAPG are enriched in. Median expression levels of AC099850.3 and NCAPG mRNA were used as cut-off points for high and low expressions of the protein in 535 TCGA-LUAD samples. AC099850.3 and NCAPG associated signaling cascades in cancer were identified using GSEA 4.1 (http://software.broadinstitute.org/gsea/).48 Corresponding cascades were screened based on Gene size≥50 and false discovery rate (FDR) q-value<0.05.
Statistical Analysis
The IBM SPSS Statistics 23.0 (SPSS, Chicago, IL, USA) and GraphPad 8.02 softwares were used for statistical analyses. Based on median NCAPG expression values, samples were stratified into high- NCAPG and low- NCAPG expression groups. Data for NCAPG mRNA levels in non-malignant samples were analyzed using the Shapiro–Wilk test. The Mann–Whitney U or Student’s t-test were used to compare NCAPG levels between the groups. Overall survival (OS) and progress-free survival (PFS) outcomes were analyzed using Kaplan-Meier (K-M) curve method with a Log rank test. Cox regression analysis was performed to determine the independent prognostic ability of NCAPG. Then, a nomogram was constructed on the basis of the results of Cox multivariate analyses in terms of OS using “rms” in R software. p < 0.05 was the threshold for statistical significance.
Results
NCAPG Was Highly Expressed in Lung Cancer
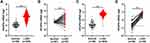
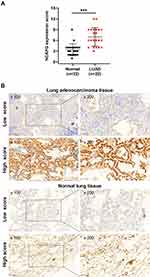
By evaluating the transcription data from TCGA-LUAD (Figure 1A and B) and TCGA-LUSC datasets (Figure 1C and D), in tandem with seven analyses of the Oncomine database (Table 1), NCAPG mRNA was established to be markedly over-expressed in both LUAD and LUSC tissues, relative to non-malignant lung tissues. Further IHC analysis revealed that NCAPG protein levels were over-expressed in LUAD tissues (Figure 2A, t=6.500, p<0.0001). NCAPG was abundant in cytoplasms of LUAD cells and neighboring non-malignant lung tissue cells (Figure 2B).
 |
Table 1 Comparative Expression of NCAPG mRNA in 6 GEO Oncomine Datasets |
Over-Expressed NCAPG Was Associated with LUAD Progression and Poor Prognosis
NCAPG mRNA levels were markedly associated with TNM (Figure 3A, p=0.0023) and N (Figure 3B, p=0.0011) stages of LUAD. These results were validated in 2 independent GEO LUAD datasets. Evaluation of the GSE31210 LUAD dataset revealed that NCAPG mRNA levels were positively associated with TNM stage (Figure 3C, p=0.0002), relapse status (Figure 3D, p<0.0001) and smoking status (Figure 3E, p=0.0001). Moreover, analyses of the GSE30219 dataset revealed that elevated NCAPG mRNA levels were markedly associated with T stage (Figure 3F, p<0.0001), N stage (Figure 3G, p=0.0211), and relapse status (Figure 3H, p<0.0001) in LUAD patients. Based on analyses of the TCGA-LUSC dataset, NCAPG mRNA levels were significantly correlated with N stage (Figure S1A), but not with TNM stage, T stage and distant metastasis in LUSC patients (Figure S1B, C, D). These relationships were not found in LUSC patients in the GSE30219 dataset (Figure S1E-G).
LUAD patients in TCGA-LUAD (Figure 4A), GEO31210 (Figure 4B and C), GSE30219 (Figure 4D) and GSE72094 (Figure 4E-G) datasets with elevated NCAPG levels had markedly worse OS outcomes, especially early-stage LUAD patients. Besides, elevated NCAPG mRNA levels markedly correlated with shorter PFS for LUAD patients in GEO31210 (Figure 4H and I) and GSE30219 (Figure 4J) datasets. However, NCAPG levels were not markedly correlated with OS and PFS for LUSC patients in TCGA-LUSC (Figure S2A) and GSE30219-LUSC datasets (Figure S2B, C). These findings imply that over-expression of NCAPG is associated with poorer survival outcomes in LUAD patients, but not LUSC patients.
NCAPG Was an Independent Prognostic Predictor for OS and PFS in LUAD
Univariate and multivariate Cox regression analyses revealed that NCAPG is an independent predictor of OS for LUAD patients in TCGA-LUAD, GEO31210, GSE30219 and GSE72094 datasets (Figure 5A and B). In addition, NCAPG levels were established to independent predictors of PFS for LUAD patients in GEO31210 and GSE30219 LUAD datasets (Figure 5A and B).
To validate the prognostic significance of NCAPG, a nomogram was created based on TNM stage, N stage and NCAPG mRNA levels, which had been revealed to be independent indicators for OS via multivariate analyses of the TCGA-LUAD dataset. Using the nomogram, we predicted the one-year, three-year and five-year survival rates for LUAD patients (Figures 5C and S3). Based on these results, a higher total point of the nomogram implies poorer prognostic outcomes for LUAD patients.
NCAPG mRNA Levels Were Regulated by DNA Copy Number Alterations and Methylation
Then, we determined the association between NCAPG mRNA levels with its CNAs and DNA methylation to establish the potential dysregulation mechanisms of NCAPG in LUAD. By analyzing NCAPG CNAs in 512 LUAD patients, we found that, although low copy gain and amplification (gain and amplification, n=89, 17.38%) was not frequent, it was still associated with markedly-increased NCAPG levels, relative to diploid patients (Figure 6A). Spearman linear regression analysis revealed that NCAPG mRNA levels were markedly associated with DNA methylation levels (Figure 6B, r=−0.556, p<0.001), suggesting that methylation might also be involved in up-regulation of NCAPG in LUAD.
LncRNA AC099850.3 is a Potential Regulator of NCAPG Expressions and Correlates with Prognosis in LUAD
Among the acquired lncRNAs, 1638 DElncRNAs (1405 upregulated and 233 downregulated lncRNAs) were selected (Figure S4). Besides, assessment of potential regulatory lncRNAs of NCAPG using the approaches mentioned above revealed AC099850.3, TMPO-AS1, and AC091057.1 as candidates for further validation (Figure 7A). Notably, lncRNA AC099850.3 had the strongest correlation with NCAPG levels in LUAD (Figure 7A, r = 0.8765, p<0.0001). Thus, lncRNA AC099850.3 was selected for further studies. Compared to non-malignant lung tissues, AC099850.3 was markedly upregulated in LUAD tissues (Figure 7B, p<0.001). Then, clinicopathological and prognostic analyses were performed to verify the significance of AC099850.3 in LUAD. Overexpressed AC099850.3 levels were markedly associated with advanced TNM and N stages (Figure 7C, p<0.001). Besides, Kaplan-Meier analyses indicated that elevated AC099850.3 expressions were associated with unfavorable OS in LUAD (Figure 7D. p<0.05).
AC099850.3/NCAPG Expressions Correlated with EGFR-TKIs Resistance in LUAD
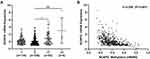
Given that targeted therapy is effective for LUAD, we accessed the GDSC database to assess the relationship between NCAPG levels and responses to EGFR-TKIs. For erlotinib, a commonly used EGFR-TKIs in LUAD, patients in the high NCAPG expression group had higher IC50 values (Figure 8A, p<0.001). Biologically, EGFR mutations in LUAD are associated with the efficacy of EGFR-TKIs, thus, we performed a chi-square test to compare differences in mutation frequencies of EGFR between high and low NCAPG expression groups. In the TCGA-LUAD dataset, mutation frequencies of EGFR in low NCAPG expression patients were markedly higher than for those in high NCAPG expression patients (Figure 8B, χ2=12.681, p<0.001). Comparable associations were found between AC099850.3 and responses to EGFR-TKIs (Figure 8C and D).
To assess the relationship between NCAPG levels and EGFR-TKIs sensitivity in 57 LUAD patients administered with erlotinib or gefitinib, we evaluated NCAPG levels via the IHC assay and followed up with them to establish clinical responses. NCAPG levels in EGFR-TKIs resistant LUAD patients were markedly elevated, relative to those in EGFR-TKIs sensitive LUAD patients (Figure 8E, t=3.651, p=0.0006). These observations suggested that AC099850.3 and NCAGP are involved in resistance to EGFR-TKIs.
AC099850.3/NCAPG Associated Signaling Cascades in LUAD
KEGG pathway analyses were performed to assess the biological significance of NCAPG over-expression in LUAD. “cell cycle (q<0.0001)”, “p53 signaling cascade (q=0.0002)”, “RNA degradation (q=0.0003)”, “pancreatic cancer (q<0.0081)”, “small cell lung cancer (q=0.0088)” and “cancer cascades (q=0.0255)” pathways were significantly enriched (Figure 9A). Assessment of the relationship between NCAPG levels and TP53 mutations in TCGA-LUAD samples (Figure 9B, left panel) revealed markedly elevated NCAPG levels in the TP53 mutation group (Mann–Whitney U =30,383, p=0.0028). Besides, there were significant negative correlations between TP53 and NCAPG levels (Figure 9B, right panel, Mann–Whitney U=16201, p<0.0001). Survival analyses showed that elevated NCAPG levels and TP53 mutations were associated with poor OS in LUAD (Figure 9C, p<0.05), whereas elevated NCAPG and low TP53 levels correlated with poor OS in LUAD patients (Figure 9D, p<0.05).
To determine the biological functions of AC099850.3, GSEA was performed on the TCGA-LUAD dataset (Figure S5). Elevated AC099850.3 levels were enriched in “cell cycle (q<0.0001)”, “ubiquitin mediated proteolysis (q<0.0001)”, “RNA degradation (q<0.0001)”, “p53 signaling cascade (q<0.0001)”, “renal cell carcinoma (q<0.0001)” and “small cell lung cancer (q=0.0006)” signaling cascades.
Discussion
The biological functions and prognostic significance of various biomarkers in cancers can be evaluated through data mining in TCGA and GEO datasets. Through systematic bioinformatic analyses, we found that NCAPG mRNA levels were elevated in both LUAD and LUSC tissues. Further analysis of the 22 LUAD tissue samples by IHC assays validated that NCAPG protein levels were elevated in LUAD patients. Assessment of potential mechanisms involved in NCAPG up-regulation in LUAD revealed that DNA copy gain and methylation might contribute to this phenomenon. Further analyses indicated that overexpressed NCAPG were markedly associated with rapid tumor progression, early recurrence as well as poor OS and PFS outcomes in LUAD patients. Importantly, COX analyses showed that NCAPG is an independent prognostic factor in LUAD. The established nomogram was shown to accurately predict the survival outcomes for LUAD patients. LUAD and LUSC differ with regards to disease pathology, molecular mechanisms, EGFR mutational status and patient outcomes.49,50 Correlations between NCAPG tumor aggressiveness and survival outcomes in LUSC were insignificant. These findings imply differences in the significance of NCAPG in development and progression of LUAD and LUSC, and that NCAPG may be a promising molecular target in LUAD, but not in LUSC.
Consistent with our findings, studies have reported that NCAPG is a progression and poor prognosis factor in LUAD, providing a novel therapeutic target for LUAD.28–30 Furthermore, overexpressed NCAPG protein levels were established in 16 tumor types and were significantly associated with poor survival outcomes in various cancer types, such as lung, breast, HCC or ovarian cancer in TCGA datasets.23 However, there are differences between our study and these studies. These studies did not involve upstream lncRNA regulating NCAPG and its relationship with EGFR-TKI resistance.
Given the significance of lncRNAs on gene expressions and tumor development, we attempted to identify the potential regulatory lncRNAs of NCAPG.32,51 Screening revealed three NCAPG-related DElncRNAs, including AC099850.3, TMPO-AS1, and AC091057.1. Among them, only AC099850.3, a previously uncharacterized lncRNA, was associated with tumor progression and poor prognostic outcomes in LUAD. Consistent with our findings, AC099850.3 was found to be upregulated in hepatocellular carcinoma patients.52 Bioinformatics analyses revealed AC099850.3 as an autophagy-related lncRNA, and elevated AC099850.3 levels were markedly associated with poor survival outcomes in tongue cancer and hepatocellular carcinoma patients.53,54 These findings indicate that lncRNA AC099850.3 may be a potential regulator of NCAPG in LUAD.
We established that NCAPG was overexpressed in cell cycle and p53 signaling cascades, and abnormalities in TP53 gene and over-expressions of NCAPG were commonly found in TCGA LUAD patients, implying potential crosstalks between lncRNA AC099850.3, NCAPG and p53. This result is further supported by findings of GSEA that AC099850.3 is primarily abundant in regulation of cell cycle and p53 signaling cascades. In addition, combined alterations of p53 and NCAPG correlated with LUAD prognosis. These findings indicate that AC099850.3/NCAGP axis can induce tumorigenesis and progression by activating the p53 signaling cascade. Consistent with our findings, NCAPG has been correlated with the p53 signaling cascade in hepatocellular carcinoma.17,20
LUAD patients whose tumors have activating mutations within EGFR significantly benefit from treatment using EGFR-TKIs. Unfortunately, acquired therapeutic resistance to EGFR-TKIs develops after a certain treatment period.55 We found that elevated NCAGP and AC099850.3 levels were associated with increased estimated IC50 for erlotinib. Increased expressions of AC099850.3 and NCAGP were markedly associated with elevated mutation frequencies of EGFR. Moreover, higher expression levels of NCAPG were associated with EGFR-TKIs resistance in 57 LUAD patients undergoing TKIs treatment. For the first time, we found that EGFR-TKIs‑treated LUAD patients with suppressed NCAPG levels had markedly improved clinical responses, relative to those with high NCAPG expressions. Thus, the AC099850.3/NCAGP axis may be a novel mechanism for understanding EGFR-TKIs resistance in LUAD and may provide a new strategy for developing AC099850.3/NCAGP related therapeutic approaches to overcome LUAD’s EGFR-TKIs resistance.
Despite the above findings, our study has limitations: (1) The prognostic effect of NCAPG content might be dependent on the respective therapy regimen, and the predictive effect of NCAPG might differ depending on the respective treatment modality. (2) Due to bioinformatics analyses and limited data, we can only conclude that NCAPG and lncRNA AC099850.3 are the important factors affecting LUAD progression and prognosis. Further investigations are warranted to clarify the exact roles of NCAPG and lncRNA AC099850.3 in LUAD, and whether they interact. (3) The sample size of hospital LUAD patients was small and only LUAD patients were included, thus, the association between NCAPG and its prognostic value in LUSC should be confirmed and verified in a larger population. (4) Although the sample size for the EGFR-TKIs treated cohort was small and we were unable to conduct survival analysis due to incomplete follow-up data, we established that elevated NCAPG expressions are associated with EGFR-TKIs resistance. These findings should be confirmed in the laboratory to establish if the AC099850.3/NCAGP axis is involved in resistance to EGFR-TKIs in LUAD.
Conclusion
In conclusion, NCAPG levels are upregulated in LUAD tissues. Overexpressed NCAPG is associated with tumor aggressiveness and predicted poor outcomes in LUAD patients. LncRNA AC099850.3 may be a potential regulator of NCAPG expressions and correlates with prognostic outcomes for LUAD patients. Moreover, AC099850.3/NCAGP axis is associated with EGFR mutations and resistance to EGFR-TKIs. These results show the clinical significance of the AC099850.3/NCAPG axis in LUAD, suggesting it could be a prognostic predictor and therapeutic biomarker for the efficacies of EGFR-TKIs in LUAD.
Ethics Approval and Consent to Participate
The experiments were approved by Ethics Committee of Affiliated Hospital of Chifeng University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
2. Inamura K. Lung Cancer: understanding Its Molecular Pathology and the 2015 WHO Classification. Front Oncol. 2017;7:548.
3. Wang Y, Wo Y, Lu T, et al. Circ-AASDH functions as the progression of early stage lung adenocarcinoma by targeting miR-140-3p to activate E2F7 expression. Translational Lung Cancer Res. 2021;10(1):57–70.
4. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non–small cell lung cancer. JAMA. 2019;322(8):764.
5. Andrews Wright NM, Goss GD. Third-generation epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Translational Lung Cancer Res. 2019;8(S3):S247–S264.
6. Mountzios G, Linardou H, Kosmidis P. Immunotherapy in non-small cell lung cancer: the clinical impact of immune response and targeting. Ann Translational Med. 2016;4(14):268.
7. Zhu C, Zhuang W, Chen L, Yang W, Ou W. Frontiers of ctDNA, targeted therapies, and immunotherapy in non-small-cell lung cancer. Translational Lung Cancer Res. 2020;9(1):111–138.
8. Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Brit J Cancer. 2019;121(9):725–737.
9. Tsiara A, Liontos M, Kaparelou M, et al. Implementation of immunotherapy in the treatment of advanced non-small cell lung cancer (NSCLC). Ann Translational Med. 2018;6(8):144.
10. Dong Z, Wang Y, Ding V, et al. GLI1 activation is a key mechanism of erlotinib resistance in human non-small cell lung cancer. Oncol Lett. 2020;20(4):76.
11. Leighl NB, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49–57.
12. Xu Y, Liu H, Chen J, Zhou Q. Acquired resistance of lung adenocarcinoma to EGFR-tyrosine kinase inhibitors gefitinib and erlotinib. Cancer Biol Ther. 2010;9(8):572–582.
13. Murphy LA, Sarge KD. Phosphorylation of CAP-G is required for its chromosomal DNA localization during mitosis. Biochem Bioph Res Co. 2008;377(3):1007–1011.
14. Sutani T, Sakata T, Nakato R, et al. Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation. Nat Commun. 2015;6:1.
15. Eberlein A, Takasuga A, Setoguchi K, et al. Dissection of Genetic Factors Modulating Fetal Growth in Cattle Indicates a Substantial Role of the Non-SMC Condensin I Complex, Subunit G (NCAPG) Gene. Genetics. 2009;183(3):951–964.
16. Bayo J, Fiore EJ, Dominguez LM, et al. A comprehensive study of epigenetic alterations in hepatocellular carcinoma identifies potential therapeutic targets. J Hepatol. 2019;71(1):78–90.
17. Wang Y, Gao B, Tan PY, et al. Genome-wide CRISPR knockout screens identify NCAPG as an essential oncogene for hepatocellular carcinoma tumor growth. THE FASEB Journal. 2019;33(8):8759–8770.
18. Ai J, Gong C, Wu J, et al. MicroRNA-181c suppresses growth and metastasis of hepatocellular carcinoma by modulating NCAPG. Cancer Manag Res. 2019;11:3455–3467.
19. Zhang Q, Su R, Shan C, Gao C, Wu P. Non-SMC Condensin I Complex, Subunit G (NCAPG) is a Novel Mitotic Gene Required for Hepatocellular Cancer Cell Proliferation and Migration. Oncol Res Featuring Preclinical Clin Cancer Therapeutics. 2018;26(2):269–276.
20. Liu K, Li Y, Yu B, et al. Silencing non-SMC chromosome-associated polypeptide G inhibits proliferation and induces apoptosis in hepatocellular carcinoma cells. Can J Physiol Pharm. 2018;96(12):1246–1254.
21. Fu Q, Yang F, Zhao J, et al. Bioinformatical identification of key pathways and genes in human hepatocellular carcinoma after CSN5 depletion. Cell Signal. 2018;49:79–86.
22. Liu W, Liang B, Liu H, et al. Overexpression of non-SMC condensin I complex subunit G serves as a promising prognostic marker and therapeutic target for hepatocellular carcinoma. Int J Mol Med. 2017;40(3):731–738.
23. Xiao C, Gong J, Jie Y, et al. NCAPG Is a Promising Therapeutic Target Across Different Tumor Types. Front Pharmacol. 2020;11:665.
24. Song B, Du J, Song D, Ren J, Feng Y. Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and miR-1179 may contribute to the progression of gastric cancer. Biol Res. 2018;51:1.
25. Arai T, Okato A, Yamada Y, et al. Regulation of NCAPG by miR-99a-3p (passenger strand) inhibits cancer cell aggressiveness and is involved in CRPC. Cancer Med. 2018;7(5):1988–2002.
26. Zhang W, Gao L, Wang C, et al. Combining Bioinformatics and Experiments to Identify and Verify Key Genes with Prognostic Values in Endometrial Carcinoma. J Cancer. 2020;11(3):716–732.
27. Zhang H, Zou J, Yin Y, et al. Bioinformatic analysis identifies potentially key differentially expressed genes in oncogenesis and progression of clear cell renal cell carcinoma. PeerJ. 2019;7:e8096.
28. Wu Y, Lin Y, Pan J, et al. NCAPG promotes the progression of lung adenocarcinoma via the TGF-β signaling pathway. Cancer Cell Int. 2021;21(1):1–443.
29. Sun H, Zhang H, Yan Y, et al. NCAPG promotes the oncogenesis and progression of non-small cell lung cancer cells through upregulating LGALS1 expression. Mol Cancer. 2022;21(1):55.
30. Wang X, Tian X, Sui X, et al. Increased expression of NCAPG (Non-SMC condensing I complex subunit G) is associated with progression and poor prognosis of lung adenocarcinoma. Bioengineered. 2022;13(3):6113–6125.
31. Guo Y, Qu Z, Li D, et al. Identification of a prognostic ferroptosis-related lncRNA signature in the tumor microenvironment of lung adenocarcinoma. Cell Death Discov. 2021;7(1):190.
32. Zhang Y, Huang Y, Wang D, et al. LncRNA DSCAM-AS1 interacts with YBX1 to promote cancer progression by forming a positive feedback loop that activates FOXA1 transcription network. Theranostics. 2020;10(23):10823–10837.
33. Li Y, Jiang T, Zhou W, et al. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat Commun. 2020;11:1.
34. Huang J, Pan B, Xia G, et al. LncRNA SNHG15 regulates EGFR-TKI acquired resistance in lung adenocarcinoma through sponging miR-451 to upregulate MDR-1. Cell Death Dis. 2020;11:7.
35. Cerami E, Gao J, Dogrusoz U, et al. The cBio Cancer Genomics Portal: an Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2(5):401–404.
36. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, Pathways, and Networks in a Collection of 18,000 Cancer Gene Expression Profiles. Neoplasia. 2007;9(2):166–180.
37. Okayama H, Kohno T, Ishii Y, et al. Identification of Genes Upregulated in ALK-Positive and EGFR/KRAS/ALK-Negative Lung Adenocarcinomas. Cancer Res. 2012;72(1):100–111.
38. Rousseaux S, Debernardi A, Jacquiau B, et al. Ectopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancers. Sci Transl Med. 2013;5(186):186ra66.
39. Schabath MB, Welsh EA, Fulp WJ, et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene. 2016;35(24):3209–3216.
40. Shen H, Guan D, Shen J, et al. TGF-β1 induces erlotinib resistance in non-small cell lung cancer by down-regulating PTEN. Biomed Pharmacother. 2016;77:1–6.
41. Nishino M, Cardarella S, Jackman DM, et al. RECIST 1.1 in NSCLC Patients With EGFR Mutations Treated With EGFR Tyrosine Kinase Inhibitors: comparison With RECIST 1.0. Am J Roentgenol. 2013;201(1):W64–W71.
42. Zhan P, Xi G, Zhang B, et al. NCAPG2 promotes tumour proliferation by regulating G2/M phase and associates with poor prognosis in lung adenocarcinoma. J Cell Mol Med. 2017;21(4):665–676.
43. Feng Z, Zhang J, Zheng Y, et al. Elevated expression of ASF1B correlates with poor prognosis in human lung adenocarcinoma. Pers Med. 2021;18(2):115–127.
44. Zhang Y, Zhang L, Xu Y, et al. Immune‐related long noncoding RNA signature for predicting survival and immune checkpoint blockade in hepatocellular carcinoma. J Cell Physiol. 2020;12(235):9304–9316.
45. Wang W, Zhao Z, Yang F, et al. An immune-related lncRNA signature for patients with anaplastic gliomas. J Neuro-Oncol. 2018;136(2):263–271.
46. Lu X, Jiang L, Zhang L, et al. Immune Signature-Based Subtypes of Cervical Squamous Cell Carcinoma Tightly Associated with Human Papillomavirus Type 16 Expression, Molecular Features, and Clinical Outcome. Neoplasia. 2019;21(6):591–601.
47. Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2012;41(D1):D955–D961.
48. Reimand J, Isserlin R, Voisin V, et al. Pathway enrichment analysis and visualization of omics data using g: profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019;14(2):482–517.
49. Zengin T, Önal-Süzek T. Comprehensive profiling of genomic and transcriptomic differences between risk groups of lung adenocarcinoma and lung squamous cell carcinoma. J Personalized Med. 2021;11(2):154.
50. Jin R, Peng L, Shou J, et al. EGFR-mutated squamous cell lung cancer and its association with outcomes. Front Oncol. 2021;11:48.
51. Zhao Y, Du T, Du L, et al. Long noncoding RNA LINC02418 regulates MELK expression by acting as a ceRNA and may serve as a diagnostic marker for colorectal cancer. Cell Death Dis. 2019;10(8):568.
52. Zhou R, Zhang E, Sun Q, et al. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. Bmc Cancer. 2019;19(1):799.
53. Jiang Q, Xue D, Shi F, Qiu J. Prognostic significance of an autophagy-related long non-coding RNA signature in patients with oral and oropharyngeal squamous cell carcinoma. Oncol Lett. 2021;21(1):29.
54. Wu H, Liu T, Qi J, Qin C, Zhu Q. Four Autophagy-Related lncRNAs Predict the Prognosis of HCC through Coexpression and ceRNA Mechanism. Biomed Res Int. 2020;1–19.
55. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–i19.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.