Back to Journals » Cancer Management and Research » Volume 14
Outcomes of Patients with EGFR-Mutant Advanced NSCLC in a Developing Country in Southeast Asia
Authors How SH, Liam CK , Zainal Abidin MA, Hasbullah HH, Tho LM, Ho GF , Muhamad Nor I, Pang YK , Ho KF, Thiagarajan M, Ariffin R, Samsudin A, Omar A, Tan SN, Ong CK, Soon SY, Poh ME
Received 11 March 2022
Accepted for publication 27 May 2022
Published 16 June 2022 Volume 2022:14 Pages 1995—2005
DOI https://doi.org/10.2147/CMAR.S364713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Soon Hin How,1,2 Chong Kin Liam,3 Muhammad Adil Zainal Abidin,1 Harissa H Hasbullah,4,5 Lye Mun Tho,6 Gwo Fuang Ho,7 Ibtisam Muhamad Nor,5 Yong Kek Pang,3 Kean Fatt Ho,8 Muthukkumaran Thiagarajan,5 Roziana Ariffin,9 Azlina Samsudin,10 Azza Omar,11 Sin Nee Tan,2 Choo Khoon Ong,12 Sing Yang Soon,13 Mau Ern Poh3
1Kulliyyah of Medicine, International Islamic University Malaysia, Kuantan, Pahang, Malaysia; 2Hospital Tengku Ampuan Afzan, Kuantan, Pahang, Malaysia; 3Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia; 4Faculty of Medicine, Universiti Teknologi Mara, Sungai Buloh, Selangor, Malaysia; 5Oncology and Radiotherapy Department, General Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; 6Department of Clinical Oncology, Beacon Hospital, Petaling Jaya, Selangor, Malaysia; 7Clinical Oncology Unit, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia; 8Mount Miriam Cancer Hospital, Penang, Malaysia; 9Hospital Tunku Azizah, Kuala Lumpur, Malaysia; 10Hospital Sultanah Nur Zahirah, Kuala Terengganu, Terengganu, Malaysia; 11Respiratory Unit, Medical Department, Hospital Raja Perempuan Zainab II, Kota Bharu, Kelantan, Malaysia; 12Gleneagles Hospital, Penang, Malaysia; 13Sarawak Heart Centre, Kuching, Sarawak, Malaysia
Correspondence: Mau Ern Poh, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia, Tel +60 3 7949 4422, Email [email protected]
Background: Although first- and second-generation EGFR TKIs are considered first-line treatment in EGFRm+ NSCLC, most patients develop resistance and progress, commonly, EGFR T790M mutation. The third-generation EGFR-TKI has demonstrated efficacy in patients with progressive disease harboring the T790M mutation and in the first-line setting, bypassing this mode of resistance. The primary objectives of this study are to describe the proportion of EGFRm+ NSCLC patients treated with first-, second- and third-generation EGFR TKIs, and cytotoxic chemotherapy in the first-line setting, and the time on treatment for each category. Secondary objectives are to determine the dropout rate, the rates for T790M mutation testing at disease progression and the type of subsequent treatment.
Methods: This multicenter retrospective study utilized data from the Malaysian Lung Cancer Registry that actively registers all lung cancer patients ≥ 18 years, with primary lung cancer confirmed histologically or cytologically. All patients diagnosed with advanced stages (ie stages IIIB, IIIC and IV) EGFRm+ NSCLC from 1st of January 2015 to 31st December 2019 were included.
Results: Of 406 patients with EGFRm+ NCSLC, 351 were treated. Types of first-line treatment were as follows: EGFR-TKIs (first generation – 54.1%, second generation – 25.6% and third-generation – 12.5%) and chemotherapy (7.7%). The median time of treatment for each generation of EGFR-TKI was 12 months, 12 months and 24 months, and 2 months for chemotherapy. The dropout rate was 28.7% (n = 101). Nearly half (49.4%) of patients who were on first- or second-generation EGFR-TKI had further genetic testing via liquid or tissue biopsies upon disease progression. About 24.9% of those who developed disease progression after first- or second-generation EGFR TKI were started on a third-generation EGFR TKI.
Conclusion: In the real-world, the management of EGFRm+ advanced NSCLC patients in an Asian cost-restrictive setting may adversely affect the choice of first-line therapy, time on each line of treatment and subsequently the overall survival of patients.
Keywords: tyrosine kinase inhibitors, lung cancer, time on treatment, overall survival
Introduction
In 2018, the World Health Organization estimated that globally, lung cancer was the most common cancer (2.09 million cases) and the leading cause of cancer death (1.76 million deaths). The Malaysian National Cancer Registry published in 2016 showed that lung cancer is the third most common cancer (9.8%) in Malaysia with an incidence of 13.2 per 100,000 in males and 6.9 per 100,000 in females.1 The Malaysian Study on Cancer Survival (2018) determined that the 1-year and 5-year overall survival rates in patients with lung cancer were 35.5% and 11.0%, respectively.2
A review of lung cancer research in Malaysia indicated that up to ~80.0% of lung cancer patients have non-small cell lung cancer (NSCLC).3 The estimated rate of epidermal growth factor receptor (EGFR) mutation (EGFRm+) in Malaysian lung cancer patients was 45.7% (n = 484).4 This finding was similar to an updated global analysis based on original studies from various countries, which determined that the prevalence of EGFRm+ lung cancer was higher in parts of Asia (42.7%, p < 0.001).5 However, in a smaller prospective study of 151 multi-ethnic Malaysian patients,6 the EGFRm+ rate was 36.4%, which is in line with the global median overall prevalence of 33.1%.5 There was also no significant difference among the different ethnic populations (p = 0.08).6 EGFRm+ was found mainly in the adenocarcinoma subtype,4 which matches the global epidemiological data.5
With the advent of targeted therapy, the use of EGFR tyrosine kinase inhibitors (TKIs) is currently the recommended first-line treatment for EGFRm+ NSCLC.7 First- and second-generation EGFR-TKIs have shown significant efficacy in this group of patients.8–11 However, most patients develop progressive disease (PD) within 9.0–14.7 months.12–15 The most common reason for PD is the development of EGFR TKI resistance, of up to 69.0% due to an acquired second mutation, ie pThr790Met point mutation (T790M+)13,15–17 In the Phase III randomized-controlled AURA 3 study,18 of 1036 patients with advanced or metastatic NSCLC and PD after first-line EGFR TKI therapy, more than half (52.0%) were T790M+.
A challenge of novel, molecular-targeted therapies is the access to the specific genetic testing required before initiating treatment, and the cost of the treatment itself.19 First- and second-generation EGFR TKIs being more accessible, are usually offered upfront instead of third-generation TKIs, even though the third-generation TKI osimertinib has been approved for use as a second-line treatment in Malaysia since May 2017 and as a first-line treatment since November 2018. For patients who progress on first- and second-generation EGFR TKI, many might not have access to third-generation EGFR TKI resulting in the utilization of chemotherapy or other alternatives. This lack of accessibility to third-generation EGFR TKI will impact their time on treatment (TOT) and the overall survival.
The primary objectives of this multicenter, retrospective study are to describe the proportion of Malaysian NSCLC patients with EGFRm+ according to the types of mutation (eg exon 19 deletion [Del19] and exon 20 L858R insertion) and describe the proportion of EGFRm+ NSCLC patients treated with first-, second- and third-generation EGFR TKIs, and cytotoxic chemotherapy in the first-line setting, and the TOT in each treatment category. Secondary objectives are to determine the dropout rate, rates for T790M+ testing at PD and subsequent treatment based on the presence or absence of T790M+ as an acquired resistance. We aim to use data from a large, multicenter registry to highlight real-world prevalence and therapy outcomes in the setting of a resource challenged, developing country like Malaysia.
Materials and Methods
Study Design and Data Source
The data for this multicenter retrospective study were taken from the Malaysian Lung Cancer Registry. Eighteen major hospitals from the public, university and private sectors (75.0% contribution by public and university hospitals, and 25.0% from private hospitals) across Malaysia actively register all lung cancer patients 18 years and above, with a prior or current diagnosis of primary lung cancer confirmed histologically or cytologically since 1st January 2015. Electronic case report forms were used to collect data for the registry. The data collected include patients’ baseline demographics, clinical characteristics such as smoking and Eastern Cooperative Oncology Group [ECOG] performance status, radiological and pathological findings, and treatment regimens of patients with EGFRm+ NSCLC. For this study, informed consent was waived with approval by the University of Malaya Medical Centre Medical Research Ethics Committee as the individual patient medical information in the database was considered confidential and this was ensured using patient identification code numbers.
Study Population
All patients diagnosed with advanced stage (ie stages IIIB, IIIC and IV) EGFRm+ NSCLC patients from 1st of January 2015 to 31st December 2019 were included in this study. Clinical staging was based on the 8th edition of the International Association for the Study of Lung Cancer (IASLC) tumor, lymph node and metastasis (TNM) classification.20 Patients with incomplete staging or treatment information were excluded from the analysis. Patients’ EGFRm+ status was classified into Del19 (including patients with mixed exon 19 deletion and other co-mutations), L858R (including patients with mixed exon 21 L858R and other co-mutations) and rare mutations.
Data Collection
The electronic case report forms (eCRFs) retrieved from the Malaysian Lung Cancer registry were screened for any missing data. eCRFs of patients with missing data that could not be resolved after checking with the site investigator were excluded from analysis. Patients lost to follow-up and not contactable by phone at the time of analysis were excluded from OS analysis.
The demographic and the clinical characteristics of the patients, the type of treatment (chemotherapy or targeted therapy), time on treatment (TOT) on first-line treatment, PD, and median overall survival (OS) were documented. Type of treatment was categorized as first- and second-generation EGFR TKI with or without chemotherapy, third-generation EGFR TKI with or without chemotherapy, chemotherapy alone, and others which included any treatment that did not fall into the previous categories such as immune checkpoint inhibitor therapy with or without chemotherapy, bevacizumab with chemotherapy, and so on. TOT was defined as the duration from the first day of therapy to the day of the last dose of therapy. PD was evaluated clinically and radiologically every six weeks to three months depending on the individual center management protocols, and was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). OS was defined as the time from initiation of therapy until death from any cause.
Statistical Analysis
The analysis was then performed on complete eCRFs using IBM SPSS Statistics version 23. Descriptive analysis was performed for categorical variables and presented as percentages (%). Continuous variables including age and TOT were described using medians. Significance level was taken at alpha of 0.05, and all p values were reported to be 2-sided.
The Kaplan–Meier method was used to estimate TOT and OS, and Log rank test was used to test the TOT and survival differences between groups. All the assumptions were checked prior to analysis. Outcome was defined as when the patient changed treatment regime whilst censored was defined by the end of the study or when the patient was lost to follow up. Duration was expressed in months.
Results
A total of 406 patients with EGFRm+ NSCLC were registered in the Malaysian Lung Cancer Registry. Of these, 22 (5.4%) patients were excluded from analysis because of incomplete staging or treatment details (Figure 1). A total of 33 (8.6%) patients did not receive treatment after diagnosis, and the two most common reasons for non-treatment were death before treatment initiation (14 patients, 42.4%) and poor ECOG performance status at presentation (10 patients, 30.3%).
The baseline characteristics of the 351 patients included in the treatment and survival analyses are shown in Table 1. The majority (80.6%) of patients were non-smokers and female (61.5%). Del19 was the most common mutation in 195 (55.6%) patients followed by L8585R mutation in 113 (32.2%) patients. Rare mutations were detected in 43 (12.3%) patients (G719X = 12, 20 insertion = 8, L861Q = 8, S768i = 4, T790M = 4, and others).
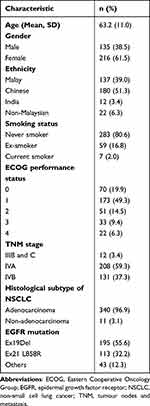 |
Table 1 Baseline Characteristics of Patients in the Analysis Set (N = 351) |
Of the 351 patients who were treated, 92.3% were treated with first-line targeted therapy, including 10 patients who were treated first-line with a combination of chemotherapy and EGFR TKI (Figure 1). First-generation EGFR TKIs were the most frequently used (54.1%) followed by the second-generation EGFR TKI (25.6%). Only 12.5% patients were on first-line third-generation EGFR-TKI (osimertinib) and 7.7% were treated with chemotherapy alone. The median follow-up time for those on first- and second-generation EGFR TKIs was 24 months (95% CI 21.4, 26.6), on osimertinib was 23 months (95% CI 17.5, 28.5), and for those on chemotherapy was 21 months (95% CI 13.9, 28.1).
At the time of analysis, 279 (79.5%) patients had PD. For the 24 patients treated with first-line osimertinib, only three (12.4%) patients had genetic testing performed at PD. Eighty-three (36.2%) of the 229 patients treated with first-line first- or second-generation EGFR TKIs had genetic testing upon progression (Figure 1). The most common genetic testing method was a blood liquid biopsy (n = 65; 78.3%) followed by a core tissue biopsy (n = 18; 21.7%) and cytological specimens were utilized for the remaining three patients. Tissue-based biopsies were performed using the cobas® version 2 (Roche Diagnostics) platform whilst liquid biopsies were performed using droplet digital polymerase chain reaction. Overall, EGFR T790M+ was detected in 54 of 83 (65.1%) patients who failed first- or second-generation EGFR TKI. EGFR T790M+ was detected in 41 of 65 (63.1%) using liquid biopsies and in 10 of 18 (55.6%) patients using core tissue biopsies (including one patient who had positive EGFR T790M from both liquid and tissue biopsy). All three patients who were tested using cytological specimens were EGFR T790M positive.
The median TOT for first-, second- and third-generation EGFR TKIs were 12.0, 12.0 and 24.0 months in the first-line setting, respectively. Since the median TOT for first- and second-generation EGFR-TKI in the first-line setting were the same (12.0 months), we combined their results when performing further analysis. Patients treated with first-line chemotherapy had significantly shorter TOT (2.0 months) compared to patients who were on first-line first- or second-generation (12.0 months), and third-generation (24.0 months) EGFR TKIs (p < 0.001, Figure 2). A hundred and one (28.8%) patients were lost to follow up at the time of analysis despite all efforts by their treating physicians to contact them. The median OS for the whole cohort (n = 250) was 30.0 months (95% CI, 25.4–34.6) (Figure 3). By category of treatment, patients on first-line chemotherapy and osimertinib had significantly longer median OS than those on first-line first- or second-generation EGFR TKI (median OS not reached vs 28.0 (95% CI, 22.1–34.0) months) (Figure 3). Among those who received first-line first- and second-generation EGFR TKIs, initiating third-generation EGFR TKI as second-line treatment on PD doubled the median OS (60.0 months) compared to those who did not receive osimertinib as second-line treatment (Figure 4).
 |
Figure 2 Time on treatment (TOT) according to first-line treatment excluding patients lost to follow-up (N=279). |
 |
Figure 3 Overall survival (OS) according to first-line treatment excluding patients lost to follow-up (N=250). |
 |
Figure 4 Overall survival (OS) after first-line first- and second-generation EGFR TKIs with and without third-generation EGFR-TKI. |
Upon PD on first-line treatment, 174 of 351 patients were started on second-line treatment at the time of analysis with 116 patients (66.7%) receiving second-line targeted therapy with or without chemotherapy and 53 patients (30.5%) treated with second-line chemotherapy alone (Table 2). Only two patients (1.1%) were started on immune checkpoint inhibitor with chemotherapy, and the remaining were given chemotherapy with bevacizumab or others. Of the 229 patients who developed PD on first- or second-generation EGFR TKI, 83 patients had genetic testing. Nearly half of these patients [41 of 83 (49.4%)] were started on the third-generation EGFR-TKI, osimertinib, while only 16 of 146 patients (11.0%) who did not have genetic testing received osimertinib. Of the 26 patients who had PD on first-line chemotherapy, 22 received second-line treatment with the majority [20 of 22 (90.9%)] receiving second-line EGFR TKI. Thirty-four of 138 (24.6%) patients who failed first-line first- or second-generation EGFR TKI received a different first- or second-generation EGFR TKI as second-line treatment.
 |
Table 2 Second-Line Treatment in Patients Who Progressed After First-Line Treatment |
Discussion
This multicenter registry-based, retrospective study aimed to highlight the treatment patterns of EGFRm+ advanced stage NSCLC patients in a real-world, resource-limited setting. Like other local and international studies,4–6 we found a higher proportion of female patients and those who never smoked with EGFRm+ NSCLC.
Though Malaysia is categorized as a developing country, most of our patients (92.3%) were treated with EGFR TKI compared to chemotherapy as the standard of care in the first-line setting.15,21 This is comparable to Singapore where 90.6% of EGFRm+ NSCLC patients received EGFR TKI monotherapy (88.6%) or in combination with chemotherapy (3.6%),22 and Taiwan where 95.7% of patients received EGFR TKI monotherapy.23 Even though the third-generation EGFR TKI, osimertinib has shown superior treatment outcomes compared to first-generation EGFR TKI,24 the proportion of our patients on first-line osimertinib was only 12.5%. First- and second-generation EGFR TKIs are reimbursed by the government, whereas the third-generation is self-funded. This is the most likely explanation for the preference of first- and second-generation EGFR TKIs in the first-line treatment setting and also explains the small uptake of patients treated with first-line third-generation TKI, namely osimertinib.
The median TOT for our patients treated with first-line first- or second-generation EGFR TKIs was comparable with that of other real-world studies showing a median TOT ranging from 12.1 to 17.9 months but falling at the lower end of this range.14,25,26 This could partly be due to 15.7% of our patient having poor ECOG performance status of 3 or 4. Interestingly, the median TOT in patients with poor ECOG performance status who were included in the study was 11.0 months. This suggests that patients with advanced EGFRm+ NSCLC despite having poor ECOG performance status, do derive OS benefits when treated with EGFR TKIs as previously reported.27,28
Only about 30.0% of our patients treated with first-line EGFR TKI who had PD underwent further genetic testing for acquired T790M mutation. One possible explanation for this low percentage is that while this registry was started in 2015, second-line osimertinib was only approved in Malaysia in May 2017. Although there was an expanded early access program for second-line osimertinib from 2016 to early 2017, the scope for access was not universal. The second reason is the high cost of second-line osimertinib treatment, which has to be self-funded for most patients. Nevertheless, our data revealed that those who had genetic testing to detect acquired T790M mutation were more likely to receive second-line osimertinib. The type of testing performed was mostly liquid biopsy. Although liquid biopsy is less sensitive and cannot detect small cell transformation, and there is a strong recommendation for tissue biopsy,29 patients and treating physicians seemed to prefer liquid biopsy. Physicians prefer liquid biopsy as it is less invasive, costs less and is easier to organize especially in a busy hospital setting. In the real-world FLATIRON study conducted in the US, the frequency of testing for T790M+ was only 39.4% after failure of first-line EGFR TKI therapy.30 A real-world study looking at T790M+ testing in advanced NSCLC patients in Japan29 revealed a much higher rate of 86.9% (of 236 patients) with the majority (58.1%) tested using liquid biopsy. Regardless, the positive rate for T790M+ in the patients treated with first- and second-generation EGFR TKI in our study is similar to that reported by other studies.13,15–17 Patients who fail first- and second-line osimertinib should ideally undergo tissue rebiopsy to detect targetable resistance molecular alterations such as MET amplification13,15,16 but only 12.5% of our patients who failed first-line osimertinib underwent a tissue rebiopsy. This can be attributed to the non-availability of next-generation sequencing testing during the study period, as well as its high cost (~USD 2500 per test), and the lack of molecular targeted treatment either commercially or in clinical trials post-osimertinib.
Less than 50.0% of our patients received osimertinib as second-line treatment. This percentage is low compared to other countries where the rate is 73.0–95.7%.29–31 The reason for the low uptake is mainly the cost of osimertinib in Malaysia. Approximately 20.0% of our patients who progressed on first-line first- or second-generation EGFR TKI were switched to a different first- or second-generation EGFR TKI with or without chemotherapy. This was a common practice before the availability of osimertinib in Malaysia and still is despite its availability because of its high cost. In clinical practice, first- and second-generation EGFR TKIs are often used beyond PD in patients who have asymptomatic progression and have been shown to prolong OS compared to switching to chemotherapy.15 The National Comprehensive Cancer Network also recommends continuing first- and second-generation EGFR TKIs in combination with local therapy in patients with asymptomatic or minimally symptomatic progression.32
The median OS in our cohort of patients was 30.0 months, which is comparable to the Japanese real-world study (median OS 29.7 months, 95% CI 28.1–31.4).26 The median OS for patients treated with both first-line third-generation EGFR TKI and first-line chemotherapy had not been reached at the time of analysis. The OS benefit among the patients who received first-line chemotherapy who did as well as osimertinib was interesting. However, this apparent good outcome of those on first-line chemotherapy is most likely because more than 90.0% of these patients were switched to second-line EGFR TKI therapy upon progression of disease. Additionally, the proportion of patients on first-line chemotherapy was proportionally smaller than those who received first- or second-line EGFR TKI (27 vs 280). The shorter median OS of patients on first-line first- or second-generation EGFR TKI is most likely due to only 60.2% of them going on to receive second-line treatment. Hence, we cannot conclude that chemotherapy as first-line therapy is better than the EGFR TKIs. It also suggests that subsequent lines of therapy, whether it is with targeted therapies, chemotherapy or immune checkpoint inhibitor combinations, are vital for prolonging OS in these patients.
Two separate phase III randomized trials of the same design conducted in Japan33 and India34 illustrate the importance of access to a subsequent line of treatment in prolonging the OS. The objective of the Japanese NEJ009 study and the Indian study was to compare the efficacies of gefitinib versus gefitinib plus chemotherapy in patients with EGFRm+ advanced NSCLC. The median OS in both studies were drastically different. The median OS of the gefitinib arm was 38.8 months in the Japanese study compared to only 17.0 months in the Indian study. This is because more patients (23.3%) in the Japanese study received osimertinib on PD compared to only 11.0% in the Indian study. We postulate that the difference in the OS between the Japanese and Indian studies was likely due to a difference in access to second-line osimertinib determined by its cost and reimbursement in the two countries.
The limitation of our study is that it is retrospective, which is true for most real-world studies. Although the Malaysian Lung Cancer Registry has an active participation from many centers across Malaysia, it is limited by incomplete eCRFs and a high loss to follow-up rate. These deficiencies could be attributed to the time constraints of the busy participating clinicians and the lack of dedicated human resources to monitor the patients’ follow-up appointment.
Conclusion
Overall accessibility to first-line EGFR TKI is high but limited to first- or second-generation TKI. Patients who received chemotherapy as first-line therapy may have a good OS if they receive targeted therapy subsequently. The percentage of patients undergoing genetic testing following progression on first-line EGFR TKI remains modest. Liquid biopsy is a preferred option among treating physicians. The lack of accessibility to effective but costly newer treatments in the first- and second-line settings can adversely affect survival.
Our study suggests that in the real-world, the management of EGFRm+ advanced NSCLC patients in an Asian cost-restrictive setting can adversely affect the choice of first-line therapy, time on each line of treatment and subsequently overall survival of the patient.
Acknowledgments
The authors wish to thank the Malaysian Thoracic Society for the research grant, Malaysian Association for Thoracic & Cardiovascular Surgery for maintaining the lung cancer database, and the study coordinators at each of the participating centres. We would also like to thank MediConnexions Sdn Bhd for their editorial support.
Funding
This work was supported by the Malaysian Thoracic Society and Astra Zeneca (Malaysia).
Disclosure
CKL has received research grants and honoraria from Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, Pfizer, Roche and Zuellig Pharma. HHH has received research grants and honoraria from Astra Zeneca, EISAI, MSD, Novartis and Tessa Therapeutics; AB science, AdipoLab, Arcus Bioscience. GFH has received research grants and honoraria from AB Science, Arcus Biosciences, Ipsen, Astellas, Astra Zeneca, Eli Lily, Boehringer Ingelheim, BMS, MSD, MIRARI Therapeutics, Novartis, Pfizer, Regeneron, Roche and Tessa Therapeutics, respectively. LMT has received honoraria from Astra Zeneca, Boehringer Ingelheim, Roche, MSD and Pfizer. The other authors have no conflicts of interest to declare in this work.
References
1. Ministry of Health, Malaysia. Malaysia National Cancer Registry report 2012–2016; 2019.
2. Ministry of Health, Malaysia. Malaysian study on cancer survival (MySCan); 2018.
3. Kan CS, Chan KM. A review of lung cancer research in Malaysia. Med J Malaysia. 2016;71(Suppl 1):70–78.
4. Shi Yeen TN, Pathmanathan R, Shiran MS, Ahmad Zaid FA, Cheah YK. Detection of epidermal growth factor receptor mutations in formalin fixed paraffin embedded biopsies in Malaysian non-small cell lung cancer patients. J Biomed Sci. 2013;20(1):22.
5. Werutsky G, Debiasi M, Sampaio FH, et al. P1.08: updated analysis of global epidemiology of EGFR Mutation in advanced non-small cell lung cancer: track: prevention, early detection, epidemiology and tobacco control. J Thorac Oncol. 2016;11(10):S184–S185. doi:10.1016/j.jtho.2016.08.030
6. Liam CK, Leow HR, How SH, et al. Epidermal growth factor receptor mutations in non- small cell lung cancers in a multiethnic Malaysian patient population. Asian Pac J Cancer Prev. 2014;15(1):321–326. doi:10.7314/APJCP.2014.15.1.321
7. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up††footnotes approved by the ESMO Guidelines Committee: February 2002, last update September 2018. Ann Oncol. 2016;27(Suppl 5):v1–v27.
8. Ho G-F, Chai C-S, Alip A, et al. Real-world experience of first-line Afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer. 2019;19(1):896. doi:10.1186/s12885-019-6107-1
9. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
10. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
11. Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X
12. Lavacchi D, Mazzoni F, Giaccone G. Clinical evaluation of dacomitinib for the treatment of metastatic non-small cell lung cancer (NSCLC): current perspectives. Drug Des Devel Ther. 2019;13:3187–3198. doi:10.2147/DDDT.S194231
13. Ma C, Wei S, Song Y. T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis. 2011;3(1):10–18. doi:10.3978/j.issn.2072-1439.2010.12.02
14. Wu S-G, Chiang C-L, Liu C-Y, et al. An observational study of acquired EGFR T790M-dependent resistance to EGFR-TKI treatment in lung adenocarcinoma patients in Taiwan. Front Oncol. 2020;10:1481. doi:10.3389/fonc.2020.01481
15. Wu S-G, Shih J-Y. Management of acquired resistance to EGFR TKI–targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38. doi:10.1186/s12943-018-0777-1
16. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi:10.1200/JCO.2016.70.3223
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.CCR-12-2246
18. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
19. Carbonnaux M, Souquet P-J, Meert A-P, Scherpereel A, Peters M, Couraud S. Inequalities in lung cancer: a world of EGFR. Eur Respir J. 2016;47(5):1502–1509. doi:10.1183/13993003.01157-2015
20. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–577. doi:10.1097/JTO.0b013e3181a0d82e
21. Greenhalgh J, Dwan K, Boland A, et al.First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;(5):Cd010383. doi:10.1002/14651858.CD010383.pub2
22. Chua B, Tan EH, Lim DWT, et al. Real world data on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) use in advanced/metastatic non-small cell lung cancer (NSCLC) with EGFR mutations in Singapore. Ann Oncol. 2018;29:ix161. doi:10.1093/annonc/mdy425.033
23. Tu C, Chen C, Hsia T, Liao W, Chen W, Hsu W. P1.03-053 Taiwan real word efficacy of 1st line EGFR TKIs treatment in EGFR mutation positive advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(11):S1971. doi:10.1016/j.jtho.2017.09.858
24. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2017;378(2):113–125. doi:10.1056/NEJMoa1713137
25. Hochmair M, Schwab S, Burghuber O, et al. P2.03-025 prevalence of EGFR T790M mutation in NSCLC patients after afatinib failure, and subsequent response to osimertinib. J Thorac Oncol. 2017;12(11):S2137. doi:10.1016/j.jtho.2017.09.1276
26. Okamoto I, Morita S, Tashiro N, et al. Real world treatment and outcomes in EGFR mutation-positive non-small cell lung cancer: long-term follow-up of a large patient cohort. Lung Cancer. 2018;117:14–19. doi:10.1016/j.lungcan.2018.01.005
27. Jovanovic D, Stevic R, Velinovic M, et al. Durable complete remission of poor performance status metastatic lung adenocarcinoma patient treated with second-line erlotinib: a case report. Onco Targets Ther. 2017;10:4347–4354. doi:10.2147/OTT.S131756
28. Nishii Y, Hataji O, Ito K, et al. Efficacy of osimertinib in a patient with non-small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status. Mol Clin Oncol. 2018;8(2):246–249. doi:10.3892/mco.2017.1522
29. Seto T, Nogami N, Yamamoto N, et al. Real-world EGFR T790M testing in advanced non-small-cell lung cancer: a prospective observational study in Japan. Oncol Ther. 2018;6(2):203–215. doi:10.1007/s40487-018-0064-8
30. Chiang AC, Fernandes AW, Pavilack M, et al. EGFR mutation testing and treatment decisions in patients progressing on first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer. 2020;20(1):356. doi:10.1186/s12885-020-06826-0
31. So YJ, Fraser A, Rivalland G, McKeage M, Sullivan R, Cameron L. Osimertinib in NSCLC: real-world data from New Zealand. JTO Clin Res Rep. 2020;1(2):100022. doi:10.1016/j.jtocrr.2020.100022
32. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. doi:10.6004/jnccn.2017.0050
33. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J Clin Oncol. 2020;38(2):115–123. doi:10.1200/JCO.19.01488
34. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR -mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi:10.1200/JCO.19.01154
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

