Back to Journals » Journal of Inflammation Research » Volume 15
Variant Angina is Associated with Myocarditis
Authors Xu X, Wang JJ, Zhao H, Miao K, Cui G, Zhang Y, Yang X , Wang L, Wu J, Wang DW
Received 17 June 2022
Accepted for publication 20 August 2022
Published 29 August 2022 Volume 2022:15 Pages 4939—4949
DOI https://doi.org/10.2147/JIR.S378152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xin Xu,1,2,* James Jiqi Wang,1,2,* Hu Zhao,1,2 Kun Miao,1,2 Guanglin Cui,1,2 Yuxuan Zhang,1,2 Xiaoyun Yang,1,2 Luyun Wang,1,2 Junfang Wu,2 Dao Wen Wang1,2
1Division of Cardiology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Hubei Key Laboratory of Genetics and Molecular Mechanism of Cardiological Disorders, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dao Wen Wang; Junfang Wu, Division of Cardiology, Department of Internal Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095# Jiefang Ave, Wuhan, 430030, People’s Republic of China, Tel/Fax +86-278366-3280, Email [email protected]; [email protected]
Background: Vasospastic angina (VSA) is caused by severe diffuse or segmental coronary artery spasms. Patients with variant angina have poor clinical outcomes, although nitrates and calcium blockers help improve patient symptoms because there is no understanding of the etiology and causal treatment. The present study investigated whether VSA is associated with inflammation of the heart.
Patients and Methods: A total of 109 patients with VSA diagnosed by the presence of recurrent angina pectoris, typical electrocardiography, and coronary angiography were recruited, and 61 normal participants and 61 patients with acute myocardial infarction (AMI) and coronary artery stenosis were recruited as controls. The plasma levels of 24 cytokines were measured using a magnetic Luminex assay, and endothelin-1 and histamine levels tested using enzyme-linked immunosorbent assay and mass-spectrometry, respectively, for all participants. Furthermore, four patients with VSA underwent 18-fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT).
Results: The plasma levels of interleukin (IL)-12p70, IL-13, PDL-1, IL-10, IL-6, IL-15, macrophage inflammatory protein (MIP)-1α, and MIP-1β in patients with VSA were significantly higher than those in both normal controls and patients with AMI (p< 0.001) but did not differ between normal controls and patients with AMI. 18F-FDG PET/CT showed that the left ventricle, coronary perivascular tissue volume, and coronary perivascular FDG uptake were significantly increased in all four patients.
Conclusion: Our findings demonstrate that VSA patients have significantly elevated plasma cytokine levels and myocardial and pericoronary inflammation, suggesting that VSA is associated with myocarditis. This study provides novel insights into the etiology and treatment of VSA.
Keywords: variant angina, myocarditis, cytokines, inflammation
Introduction
Vasospastic angina (VSA), formerly known as variant angina or Prinzmetal angina, is mainly caused by transient narrowing due to vasoconstriction of the coronary artery, leading to clinical symptoms that present as chest pain and are usually accompanied by ST-segment elevation or ST-segment depression on the ECG.1 It is classified as a focal or diffuse coronary artery spasm (CAS). Clinically, severe vasospastic angina causing total coronary occlusion can induce a wide range of myocardial infarctions and is life-threatening.2 It was reported that the prevalence rate of VSA ranged from 33.4% to 57.6% in western countries and 40% to 79% in Asian countries. Importantly, patients with VSA have relatively poor clinical outcomes, even if current therapies with calcium channel blockers and nitrates can effectively relax spastic coronary arteries and improve angina symptoms in these patients.3
The exact etiology of VSA remains unknown, and spastic coronary artery segments have no stenosis in most patients. Therefore, VSA may differ from atherosclerotic lesions. Furthermore, statins have been used worldwide to reduce adverse events in patients with coronary artery disease (CAD), and antiplatelet therapy using aspirin or aspirin plus clopidogrel is used for the primary and secondary prevention of CAD. However, the application of these treatments has not reduced adverse events as expected in patients with VSA.4,5 These studies suggest that hypercholesterolemia and platelet activation are not pathogenic factors of VSA.
An early study found that platelet-activating factor (PAF) can induce coronary spasm, inflammatory cell-derived interleukin 1 (IL-1) is a potent inducer of PAF,6,7 and mast- -and eosinophil-released histamine (HA) induces coronary artery contraction, which suggests that inflammation and inflammatory cytokines may be an important cause of VSA. One example is that allergic reactions (usually severe reactions) can cause VSA by releasing HA and other inflammatory mediators (Kounis syndrome).8
It is well known that inflammatory processes are involved in various vascular diseases. Previous observations have shown that coronary inflammatory changes may be more important in the pathogenesis of CAS than coronary atherosclerotic changes.9 Shimokawa et al found that chronic treatment with IL-1β, a pro-inflammatory cytokine, induced intimal thickening and CAS in vivo in animal experiments.9 An interesting study in 15 patients with VSA found that coronary adventitial and perivascular adipose tissue inflammation are associated with CAS using 18-fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT).10 This primary exploration suggests that VSA is associated with inflammation. However, more evidence is needed to establish the direct association between inflammation, especially in the heart, and VSA.
In the present observational study, we investigated whether the increased plasma inflammatory cytokines levels are associated with recurrent CAS using multivariate logistic regression to establish a predictive model to evaluate whether the levels of cytokines serve as important biomarkers to identify VSA. We further evaluated whether myocarditis and coronary adventitial and perivascular adipose tissue inflammation exist in the hearts of patients with VSA.
Methods
Study Participants and Blood Sample Collection
Patients who visited the Department of Cardiovascular Medicine, Tongji Hospital (Wuhan, China) donated blood samples for this study. Informed consent was obtained from the participants. This study was approved by the Ethics Committee of Tongji Hospital and the trial conformed to the principles outlined in the Declaration of Helsinki. The process of patient screening and selection is documented in detail in a flowchart (Supplementary Figure 1). Between March 2012 and January 2021, 137 patients were clinically diagnosed with VSA at Tongji Hospital (Wuhan, China). Electrocardiography (ECG), Holter ECG, and coronary angiography were performed. Six patients with suspected VSA who did not meet the criteria were excluded according to the latest diagnostic criteria for VSA. We excluded 22 patients with VSA because blood samples were not obtained from these patients during hospitalization. The exclusion criteria included non-ST-elevation myocardial infarction, stable angina, and unstable angina. Finally, 109 patients with VSA who met the diagnostic criteria, from whom blood samples were obtained, were successfully recruited for the study. We enrolled 61 normal controls (CON) and 61 patients with ST-segment elevation myocardial infarction (ST) determined by the latest diagnostic criteria as negative and positive controls to reflect the clinical situation more accurately. Reports of this research adhered to the STROBE checklist for cohort studies (Supplementary material).
Peripheral blood samples were collected in ethylenediamine tetra-acetic acid-coated tubes and centrifuged at 2800 rpm for 8 min, followed by the collection of plasma into cryovials. Plasma was stored at −80°C until analysis. In addition, plasma samples needed to be thawed overnight at 4°C before testing.
Cytokine Measurement of Plasma
Cryopreserved plasma cytokine concentrations were quantified using the Human Immunotherapy Magnetic Luminex Performance Assay 24-plex panel (R&D Systems, USA) according to the manufacturer’s instructions: granulocyte-macrophage colony-stimulating factor, granzyme B, interferon-α (IFN-α), IFN-γ, IL-1α, IL1-β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-33, IFN inducible protein 10, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, macrophage chemoattractant protein-1 (MCP-1), programmed death ligand 1 (PD-L1), tumor necrosis factor-α (TNF-α), and CD40L. A two-fold dilution with a calibrator was used for all samples, based on pre-experimental results. The plate was read using a Luminex magnetic analyzer (R&D Systems, USA).
Enzyme-Linked Immunosorbent Assay (ELISA)
The protein expression of endothelin-1 (ET-1) was detected using human ET-1 ELISA kits (Immunoway, USA) following the manufacturer’s instructions. The absorbance at 450 nm was measured using a microplate reader.
Metabolomic Analysis
The native standards, acetylcholine (ACh), HA, and its deuterated internal (d9-ACh and d4-HA), were procured from Sigma-Aldrich (St. Louis, MO, USA). Methanol and acetonitrile were procured from Merck Millipore (Billerica, MA, USA). Ammonium hydroxide (25%, w/v) solution and formic acid were procured from Fisher Scientific. All the solvents and chemicals used were of high-performance liquid chromatography grade or higher.
Ach and HA were quantified using previously optimized methods.11 Briefly, plasma samples (20 µL) from each patient, 10 µL of IS solution (d9-Ach concentration 50 ng/mL, d4-HA concentration 2 ng/mL), and 170 µL of methanol were vortex-mixed for 2 min, followed by centrifugation at 12,000 × g at 4°C for 15 min. The supernatant (100 µL) was then transferred to a chromatographic vial for further measurement. Five microliters of plasma from each patient were prepared in a pooled sample to define the quality control.
Metabolites were obtained using a UPLC system interfaced with a mass spectrometer (Waters Xevo™ TQXS system). The injection volumes of 5 µL were employed in a column (ACQUITY UPLC BEH Amide, 100 mm × 2.1 mm, 1.7 μm, Waters, MA, USA) and maintained at 40°C at a flow rate of 0.5 mL/min. The mobile phases comprised (A) 0.05% ammonium hydroxide, 20 mM ammonium formate in water and (B) 0.05% ammonium hydroxide, 20 mM ammonium formate in 90% acetonitrile. A gradient elution was optimized as following: 0–3.0 min, 95–45% B; 3.0–6 min, 50% B; 6–6.1 min, 95% B; 6.1–9 min, 95% B. The optimized parameters for mass spectrometry were: capillary voltage, 4.5 kV; cone voltage, 50 V; source temperature, 150°C; desolvation temperature, 500°C; cone gas flow, 150 L/h; desolvation gas flow, 800 L/h; collision gas flow, 0.5 mL/min. Measurements were performed using multiple reaction monitoring in positive mode. A quality control sample was acquired for every 10 samples to assess the stability of the experiment. Quantification was performed against the area of the internal standards using Masslynx (version 4.2; Waters, USA). The concentration is presented in ng/mL for each compound. (ClinicalTrials.gov Identifier: NCT05282511. Registered 16 March 2022, retrospective, https://www.clinicaltrials.gov/ct2/show/NCT05282511)
Statistical Analysis
Cytokines were transformed using log2 to prevent the skewing of results by outliers. The data are presented as scatter plots with each circle representing a single individual. All data were analyzed using GraphPad Prism 9 (version 9.0), R software (version 4.1.1), and R Studio (version 1.4.1717). Continuous variables are expressed as mean ± standard deviation or median (interquartile range), according to their distribution, and categorical variables as frequencies and percentages. The difference in cytokine levels in the plasma samples was assessed using the two-tailed Mann–Whitney U and Kruskal–Wallis tests. The three groups were compared using Dunn’s post hoc test to correct for multiple comparisons. Generalized linear modeling-logistic regression (GLM-LR) was used to assess the ability of cytokines with significant differences to classify VSA, and stepwise regression was used to screen the optimal model. Model performance was evaluated based on the area under the receiver operating characteristic curve using pROC (package version 1.18.0). The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of the model were determined using standard definitions. Statistical significance was set at p<0.05.
Results
Baseline Characteristic of the Study Population
Plasma samples from 224 participants, comprising 60 normal participants, 56 patients with ST, and 108 patients with VSA, were included in this study. The clinical characteristics are summarized in Table 1. There was no difference in the prevalence of coronary risk factors (age, sex, current smoking status, alcohol consumption, and blood pressure) among the three groups, except for diabetes mellitus (p=0.008) and dyslipidemia (p=0.020). Similarly, no statistically significant difference was observed in the history of asthma (p=0.646) or allergy (p=0.596) among the participants in the various groups. Among the cardiac markers, erythrocyte sedimentation rate levels were comparable among the three groups (p=0.164), whereas the levels of high-sensitivity C-reactive protein (hs-CRP) increased significantly in the VSA and ST groups compared with the CON group (p=0.002). Regarding medical treatment, there was no difference in nitrate (0.078) and corticosteroids (0.137) among the various groups, whereas the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers and calcium channel blockers increased significantly in the VSA and ST groups compared with the CON group (p<0.001 and p<0.001, respectively). Similarly, the use of statins and beta-blockers was significantly higher in the CON and ST groups than that in the VSA group (p<0.001 and p<0.001, respectively).
 |
Table 1 Baseline Clinical Characteristics and Treatments of the CON Group, ST Group and VSA Group |
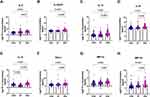
Plasma Levels of Cytokines and Chemokines were Elevated in the VSA Group Compared with the both CON and ST Groups
Plasma levels of all 24 cytokine were measured properly and 17 of them had significantly different between VSA, ST and normal control (Table 2). Interestingly, plasma levels of the pro-inflammatory cytokines IL-6, IL-12p70, and IL-15 (Figure 1A, B and E) and the anti-inflammatory cytokines IL-13 and IL-10 (Figure 1C and D) were elevated in the VSA group compared with the CON and ST groups in this study. In addition, plasma chemokine levels of PD-L1, MIP-1α and MIP-1β (Figure 1F–H) were higher in the VSA group than in the CON and ST groups. In addition, the levels of these cytokines were not significantly different between the CON and ST groups, except for IL-15.
 |
Table 2 Cytokines with Significant Difference in CON Group, ST Group and VSA Group |
Plasma Levels of Cytokines Were Elevated in the VSA Group Compared with the ST Group
It is noteworthy that the levels of the pro-inflammatory cytokines IL-4, IFN-α, IL1-α, IL-2 and chemokine MCP-1 (Figure 2A–E) were significantly higher in the VSA group than in the ST group. This indicates that these cytokines can distinguish between the VSA and ST groups.
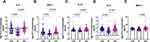 |
Figure 2 (A–E) Plasma levels of cytokines are elevated in VSA group compared to ST group. |
Plasma Levels of IL-8, IFN-γ, and Anti-Inflammatory Cytokine IL-1ra are Elevated in Patients with VSA Compared with the Normal Participants
The levels of the pro-inflammatory cytokines IL-8 and IFN-γ (Figure 3A, C) and anti-inflammatory cytokine IL-1ra (Figure 3B) in the VSA group increased compared with the CON group. This also shows that these three cytokines could distinguish between the VSA and CON groups. In addition, the plasma levels of IFN-γ and IL-1ra in patients with ST were higher than those in patients with VSA. Other significantly different cytokines are shown in the Supplementary Material.
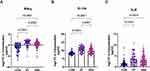 |
Figure 3 (A–C) Plasma levels of cytokines are elevated in VSA group compared to CON group. |
Plasma Vasoconstrictor Metabolites Increased in the VSA and ST Groups
We found that the levels of plasma vasoconstrictor metabolites, HA and ET-1 (Figure 4A and B) were significantly higher in the VSA and ST groups compared with those in the CON group. These vasoconstrictor metabolites showed a higher trend in the VSA group than in the ST group, although the difference was not significant.
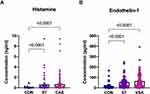 |
Figure 4 (A and B) Plasma vasoconstrictor metabolites are increased in both VSA and ST group. |
Plasma Levels of Cytokines Serve as Biomarkers of VSA to Distinguish Between the VSA and ST and CON groups
The GLM-LR model was used to evaluate the predictive power of cytokines with significant differences. The area under the curve (AUC) was 0.84 (95% confidence interval [CI] 0.78–0.90) between the VSA and CON groups, and the sensitivity and specificity of developing VSA were 71.56% and 86.88%, respectively (Figure 5A). The AUC was 0.94 (95% CI 0.91–0.98) between the VSA and ST groups, and the sensitivity and specificity of developing VSA were 87.16% and 88.53%, respectively (Figure 5C). We also assessed the predictive power of other clinical markers of inflammation, such as hs-CRP and common inflammatory cytokines (including IL-1β, IL-6, IL-8, IL-10, TNF-α.). The AUC of the two predictors were 0.55 and 0.78 between the VSA and CON groups (Figure 5B). The AUC of the two predictors were 0.76 and 0.59 between the VSA and ST groups (Figure 5D). This showed that the predictive power of these cytokines was better than that of hs-CRP and inflammatory cytokines.
Case Presentation
We performed 18F-FDG PET/CT in four patients and found that all patients had a significantly increased 18F-FDG uptake in the left ventricle and also showed a marked increase in the 18F-FDG uptake in the proximal segment of the right coronary artery. We also tried to treat these four patients with VSA using glucocorticoids, and long-term remission was achieved during the follow-up period of 3 and 6 months. Below are the reports of two typical cases.
Patient 1:
A 51-year-old man had a history of recurrent allergic asthma. The patient was admitted for recurrent chest pain for 7 years, accompanied by chest pain-related syncope five times in 3 months. The chest pain was not related to effort but to asthma occurrence, aggravation, or sleep, and lasted for 5–30 min. Three months ago, he experienced chest pain again after getting a cold, and when it was severe, he lost consciousness for approximately 2 min. He previously underwent ECG and Holter ECG showing recurrent ST-segment elevation in leads II and III, as well as augmented vector foot and chest leads and intermittent cardioplegia for up to 9 s. On admission, ECG demonstrated ST-segment elevation and Q-wave myocardial infarction and grade III atrioventricular block (Supplementary Figure 2). Coronary angiography revealed severe local stenosis of the right coronary artery, which resolved after nitroglycerine injection (Supplementary Figure 3). Additionally, the left anterior descending artery and left circumflex artery narrowed down by approximately 20%, and the right coronary artery narrowed down by approximately 30% locally. Serum cardiac troponin was significantly elevated (3000.0 pg/mL, reference <34 pg/mL), and N-terminal pro-brain natriuretic peptide was mildly elevated (300 pg/mL). It was interesting that the blood test revealed elevated white blood cell and eosinophil levels of 15.1 × 109/L and 0.84 × 109/L while he was symptomatic. Plasma cytokine assays showed that concentrations of IL-8, TNF-α, and soluble suppression of tumorigenesis-2 (sST2) were significantly elevated (0.15 ng/mL, 0.03 ng/mL, and 80 ng/mL, respectively). 18F-FDG PET/CT revealed a diffuse FDG uptake increase in the coronary artery and the left ventricle (Supplementary Figure 4). Based on clinical symptoms and angiography and ECG results, the patient was diagnosed with VSA caused by the inflammatory response of the coronary artery. He subsequently underwent immunomodulatory treatments with intravenous injection of methylprednisolone (80 mg/day) and immunoglobulin (10 g/day), as well as oral sustained-release isosorbitol dinitrate and diltiazem (90 mg/day). After treatment for 1 week, he noted significant improvement in his symptoms, and IL-8, TNF-α, and sST2 levels returned to normal. During the 6-month follow-up period, there was no onset of chest pain.
Patient 2:
A 64-year-old woman was hospitalized for appendicitis and experienced sudden chest pain 2 days after admission. The ECG revealed inferior T-wave inversion and ST-segment flat, but T wave disappear in all leads when she has mild chest pain, which is different from normal condition (no chest pain) during Holter ECG monitoring (Supplementary Figure 5). Her troponin I level was 11.02 ng/mL. Moreover, her blood tests revealed an increased neutrophil ratio and elevated hs-CRP (76.3% and 7.8 mg/L, respectively). On day 4 of hospitalization, she had ventricular fibrillation during coronary angiography of the right coronary artery which showed diffuse spasm (narrowed >85%), and the left coronary artery also showed mild diffuse spasm. After defibrillation and vasodilator therapy (intracoronary nitroglycerine injection, 100 µg), the coronary spasm improved. Spontaneous circulation returned (Supplementary Figure 6). On day 5 of hospitalization, the blood test revealed an elevated neutrophil count (89.7%), and the 18F-FDG PET/CT revealed that FDG uptake in the right coronary artery was elevated and diffusely increased in the left ventricle (Supplementary Figure 7). Therefore, we believe that pericoronary inflammation causes coronary spasms. She was treated with an intravenous injection of methylprednisolone (40 mg/day) and immunoglobulin (5 g/day) to inhibit the inflammatory response. Eventually, her plasma inflammatory cytokine levels returned to normal, and she was asymptomatic. After 2 months of follow-up, the patient had no chest pain.
Discussion
This study is the first to investigate the relationship between the levels of multiple plasma inflammatory cytokines and VSA in a Chinese cohort and included patients with ST and normal participants as controls. We found that (1) of the tested 24 inflammatory factors, eight were significantly elevated in patients with VSA than in patients with ST and normal controls (p<0.001). IL-4, IFN-α, IL1-α, IL-2, and MCP-1 were lower in patients with ST than in patients with VSA. Plasma levels of IL-8, IFN-γ, and IL-1ra were significantly elevated in patients with VSA and ST compared with the controls, which suggests that VSA is an inflammation-related disease and it is different from acute myocardial infarction (AMI); (2) substances that caused VSA, namely HA and ET-112,13 were also elevated; (3) further, we observed that patients with VSA had pericoronary inflammation as well as myocarditis, as shown by 18F-FDG PET/CT. Our findings reveal that VSA is associated with inflammation, particularly myocarditis. In addition, we found that a panel of inflammatory cytokines might help diagnose VSA.
Previous case reports14–17have indicated that CAS is associated with inflammation and myocarditis, as confirmed by endomyocardial biopsy. 18F-FDG uptake can effectively reflect tissue metabolism; therefore, 18F-FDG PET/CT can assess tissues, including the cardiac inflammatory status, by 18F-FDG uptake.18 A small cohort study using 18F-FDG PET/CT found that coronary perivascular FDG uptake significantly increased in the VSA group but not in controls, suggesting coronary adventitial and perivascular adipose tissue inflammation in patients with VSA.10 The present study demonstrates that the plasma levels of multiple inflammatory cytokines in patients with VSA are markedly elevated, which is different from AMI. These findings reveal that VSA is an inflammatory-associated disease.
The mechanisms of coronary spasms are important to understand this special recurrent angina pectoris. Clinically, coronary spasms can be induced by ACh or ergoline. Nihei et al found that Rho-kinase activity in circulating leukocytes in patients with VSA increased, and during the 3-year follow-up period, cardiac events occurred in 10 patients (5.7%) with VSA but in none of the patients without VSA, while VSA patients with higher Rho-kinase activity had a significantly worse prognosis than other comparable patients.19 Increased Rho-kinase activity represents an inflammatory condition and can activate the mitogen-activated protein kinase and nuclear factor kappa B signaling pathways, as well as the 20-kDa regulatory myosin light chain, thus inducing vessel contraction.20 Increased cytokine levels from neutrophils and macrophages, such as IL-6, can enhance coronary contractions by activating Rho-associated kinase.21,22 In addition, IL-1 induces the synthesis of inflammatory mediators, prostacyclin, and platelet-activating factor, which directly induce CAS.6,23
Hypersensitivity has been recognized to be related to VSA, which is referred to as Kounis syndrome.8 HA, a key factor in inflammation, especially mast cells, is an important mediator of inflammation and vasospasm. Therefore, Kounis syndrome represents a subset of patients with VSA due to inflammation. Shimokawa et al found that the local coronary segment exposed to inflammatory stimuli (IL-1β) was linked to vascular smooth muscle cell hyperreactivity in a porcine model with significantly elevated levels of serotonin, HA, and prostaglandin F2.9 Our observation found that plasma HA and ET-1 levels in patients with VSA increased, as proven by Figeuras et al.24 Two of our VSA patients with refractory angina pectoris achieved long-term remission after receiving treatment with glucocorticoids and immunoglobulin. The detection of inflammation may provide a new approach for diagnosing recurrent coronary spasms. Furthermore, our case report not only contributes to the diagnosis of CAS but also paves the way for alternative therapy in patients with VSA. This is also our first attempt to use glucocorticoids combined with immunoglobulin to treat patients with coronary spasms caused by inflammation. Although we have only observed a small number of clinical cases, the therapy has proven to be effective in practice.
In summary, the levels of multiple inflammatory cytokines in patients with VSA were generally increased, different from that in patients with AMI. Therefore, we were able to set a multiple inflammatory cytokine biomarker to help differentiate VSA from AMI or regular angina and provide some insight into mechanical therapy. A previous study reported that plasma levels of hs-CRP are higher in patients with coronary spasms than in those without, and elevated CRP is independently and significantly associated with coronary spasm.25 To further explore the relationship between inflammatory cytokines and other inflammatory markers, our multivariate logistic regression showed that the predictive power of these cytokines was significantly better than that of hs-CRP and commonly inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-10, and TNF-α.
The next question concerns the origin of the inflammatory cytokines. Combining the literature and this study, we suggest that VSA is a complication of myocarditis in most patients. Thus, for patients in the acute phase of VSA or recurrent attacks of VSA, anti-inflammatory therapy such as using glucocorticoids is reasonable.
A limitation of this study is its relatively small sample size. In addition, for most patients in this cohort, we only provided plasma levels of multiple inflammatory cytokines and baseline data; however, 18F-FDG PET/CT and positive responses to glucocorticoid therapy were available for only a few patients. Finally, regarding the predictive efficacy of inflammatory factors, we still lack a validation population for further confirmation. Therefore, further studies, including therapeutic clinical trials and multivariate cytokine panel validation, are required.
The present study demonstrates that various inflammatory cytokines in the plasma are increased and that elevated inflammatory cytokines may be useful for diagnosis and serve as a guide for anti-inflammatory treatment in patients with VSA.
Data Sharing Statement
The datasets generated and analyzed in this study will be available by the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Tongji Hospital and the trial conformed to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Acknowledgments
The authors acknowledge the participants and support for this study. Additionally, we thank all our colleagues from the Department of Internal Medicine, Tongji Hospital for their tremendous efforts.
Author Contributions
All authors significantly contributed to the reported work related to the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the manuscript; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by project and grant of National Key Research and Development Program of China (2021YFC2500604) and National Nature Science foundation of China (No. 81790624, 31971358 and 81900341), Major Projects of Technological Innovation of Hubei province (No. 2017ACA170) and Tongji Hospital Clinical Study of Fulminant myocarditis (No. 2019YBKY019).
Disclosure
The authors declare that they have no competing interests.
References
1. Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris I. A variant form of angina pectoris. Am J Med. 1959;27(3):375–388. doi:10.1016/0002-9343(59)90003-8
2. Pol D, Sethwala A, Wynn G. Severe vasospastic angina causing total coronary occlusion. Eur Heart J. 2020;41(37):3590. doi:10.1093/eurheartj/ehaa368
3. Bil J, MoZeNska O, Segiet-SwiEcicka A, Gil RJ. Revisiting the use of the provocative acetylcholine test in patients with chest pain and nonobstructive coronary arteries: a five-year follow-up of the AChPOL registry, with special focus on patients with MINOCA. Transl Res. 2021;231:64–75. doi:10.1016/j.trsl.2020.11.009
4. Cho -S-S, Jo S-H, Han SH, et al. Clopidogrel plus aspirin use is associated with worse long-term outcomes, but aspirin use alone is safe in patients with vasospastic angina: results from the VA-Korea Registry, A prospective multi-center cohort. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-54390-w.
5. Oh MS, Yang JH, Lee DH, et al. Impact of statin therapy on long-term clinical outcomes of vasospastic angina without significant stenosis: a propensity-score matched analysis. Int J Cardiol. 2016;223:791–796. doi:10.1016/j.ijcard.2016.08.229
6. Bussolino F, Breviario F, Tetta C, Aglietta M, Mantovani A, Dejana E. Interleukin 1 stimulates platelet-activating factor production in cultured human endothelial cells. J Clin Invest. 1986;77(6):2027–2033. doi:10.1172/JCI112532
7. Koltai M, Hosford D, Guinot P, Esanu A, Braquet P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part I). Drugs. 1991;42(1):9–29. doi:10.2165/00003495-199142010-00002
8. Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol. 2006;110(1):7–14. doi:10.1016/j.ijcard.2005.08.007
9. Shimokawa H, Ito A, Fukumoto Y, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. J Clin Invest. 1996;97(3):769–776. doi:10.1172/JCI118476
10. Ohyama K, Matsumoto Y, Takanami K, et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71(4):414–425. doi:10.1016/j.jacc.2017.11.046
11. Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7(5):872–881. doi:10.1038/nprot.2012.024
12. Toyo-Oka T, Aizawa T, Suzuki N, et al. Increased plasma level of endothelin-1 and coronary spasm induction in patients with vasospastic angina pectoris. Circulation. 1991;83(2):476–483. doi:10.1161/01.CIR.83.2.476
13. Shimokawa H, Tomoike H, Nabeyama S, Yamamoto H, Nakamura M. Histamine-induced spasm not significantly modulated by prostanoids in a swine model of coronary artery spasm. J Am Coll Cardiol. 1985;6(2):321–327. doi:10.1016/S0735-1097(85)80167-4
14. Hirota Y, Nakamori S, Hiramatsu D, Moriwaki K, Imanaka-Yoshida K, Dohi K. Pathological Q-waves with coronary artery spasm. JACC Case Rep. 2021;3(4):555–560. doi:10.1016/j.jaccas.2020.11.033
15. Sheibani H, Javedani Masroor M. Pericarditis as a trigger for prinzmetal angina - a case report. J Med Life. 2021;14(6):853–861. doi:10.25122/jml-2021-0061
16. Forman MB, Oates JA, Robertson D, Robertson RM, Roberts LJ 2nd, Virmani R. Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. 1985;313(18):1138–1141. doi:10.1056/NEJM198510313131807
17. Klein RM, Schwartzkopff B, Strauer BE. Evidence of endothelial dysfunction of epicardial coronary arteries in patients with immunohistochemically proven myocarditis. Am Heart J. 1998;136(3):389–397. doi:10.1016/S0002-8703(98)70211-X
18. Schonau V, Vogel K, Englbrecht M, et al. The value of (18) F-FDG-PET/CTin identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): data from a prospective study. Ann Rheum Dis. 2018;77:70–77. doi:10.1136/annrheumdis-2017-211687
19. Nihei T, Takahashi J, Hao K, et al. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J. 2018;39(11):952–959. doi:10.1093/eurheartj/ehx657
20. Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104(2):109–115. doi:10.1254/jphs.CP0070027
21. Lin YK, Yeh CT, Kuo KT, et al. Apolipoprotein (a)/Lipoprotein(a)-Induced oxidative-inflammatory alpha7-nAChR/p38 MAPK/IL-6/RhoA-GTP signaling axis and M1 macrophage polarization modulate inflammation-associated development of coronary artery spasm. Oxid Med Cell Longev. 2022;2022:9964689. doi:10.1155/2022/9964689
22. Hung MY, Wu YH, Bamodu OA, et al. Activation of the monocytic alpha7 nicotinic acetylcholine receptor modulates oxidative stress and inflammation-associated development of coronary artery spasm via a p38 MAP-kinase signaling-dependent pathway. Free Radic Biol Med. 2018;120:266–276. doi:10.1016/j.freeradbiomed.2018.03.050
23. Dejana E, Ji-Ming W, Mantovani A. The recruitment of leukocytes and their interaction with the vessel wall: the role of interleukin-1 and tumor necrosis factor. Scand J Rheumatol Suppl. 1987;66:19–25. doi:10.3109/03009748709102518
24. Figueras J, Garcia-Dorado D, Agullo L, et al. Activation of polymorphonuclear leukocytes and increased plasma vasoconstrictors in vasospastic and nonvasospastic angina. Can J Cardiol. 2011;27(5):601–605. doi:10.1016/j.cjca.2011.01.004
25. Park JY, Rha SW, Li YJ, et al. The impact of high sensitivity C-reactive protein level on coronary artery spasm as assessed by intracoronary acetylcholine provocation test. Yonsei Med J. 2013;54(6):1299–1304. doi:10.3349/ymj.2013.54.6.1299
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.