Back to Journals » Journal of Inflammation Research » Volume 15
May the Nitrosative and Carbonyl Stress Promote Inflammation in Patients with Colorectal Cancer?
Authors Dorf J, Zaręba K , Matowicka-Karna J , Pryczynicz A , Guzińska-Ustymowicz K, Zalewska A , Maciejczyk M
Received 2 June 2022
Accepted for publication 22 July 2022
Published 11 August 2022 Volume 2022:15 Pages 4585—4600
DOI https://doi.org/10.2147/JIR.S374387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Justyna Dorf,1 Konrad Zaręba,2 Joanna Matowicka-Karna,1 Anna Pryczynicz,3 Katarzyna Guzińska-Ustymowicz,3 Anna Zalewska,4 Mateusz Maciejczyk5
1Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Bialystok, Poland; 2 2nd Clinical Department of General and Gastroenterological Surgery, Medical University of Bialystok, Bialystok, Poland; 3Department of General Pathomorphology, Medical University of Bialystok, Bialystok, Poland; 4Independent Laboratory of Experimental Dentistry, Medical University of Bialystok, Bialystok, Poland; 5Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Bialystok, Poland
Correspondence: Justyna Dorf, Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Waszyngtona 15a St, Białystok, 15-269, Poland, Tel +48 85 8 31 87 16, Email [email protected]
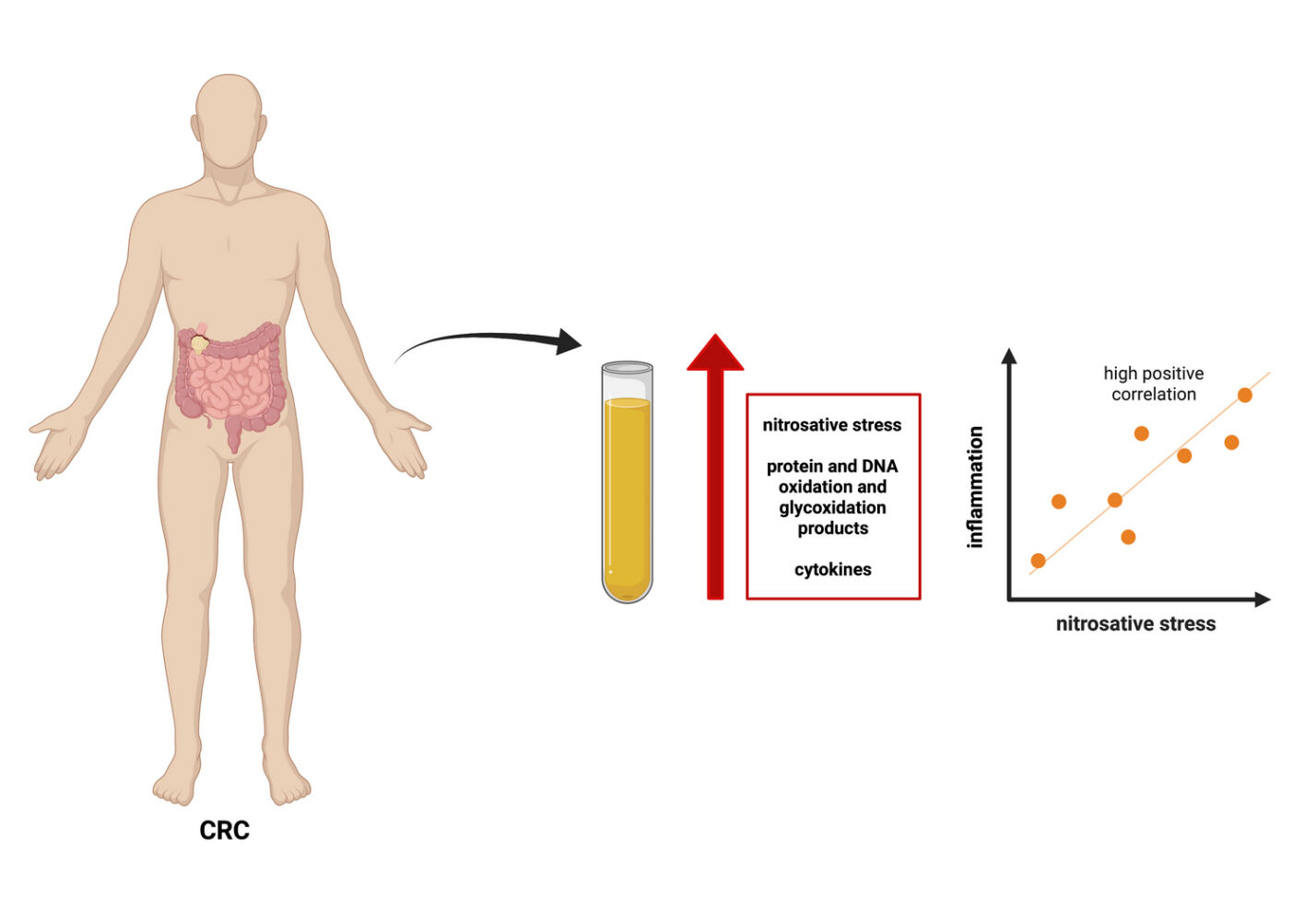
Purpose: Overproduction of reactive nitrogen species (RNS) causes the nitrosative stress, which plays a vital role in the development of metabolic, inflammatory, and cancerous diseases. However, the role of nitrosative and carbonyl stress in the biology of colorectal cancer (CRC) is still not well understood. Therefore, this study evaluated nitrosative stress, protein and DNA oxidation/glycoxidation, and pro- and anti-inflammatory cytokines in CRC patients compared with healthy controls.
Patients and Methods: Fifty-five CRC patients (21 women, 34 men) and 55 healthy controls matched for sex and age were included in the experiment. Nitrosative stress parameters (nitric oxide (NO), peroxynitrite, S-nitrosothiols, and nitrotyrosine), protein oxidation (total thiols) and glycoxidation products (kynurenine N-formylkynurenine, dityrosine, Amadori products, and amyloid), and DNA damage markers (8-hydroxydeoxyguanosine (8-OHdG)), as well as levels of pro- and anti-inflammatory cytokines, were measured in serum or plasma samples.
Results: The levels of NO, peroxynitrite, S-nitrosothiols, nitrotyrosine, total thiols, kynurenine, N-formylkynurenine, dityrosine, Amadori product, amyloid, and 8-OHdG, as well as IL1α, IL1β, IL6, IL10, and TNF-α, were significantly higher in CRC patients than in controls. Oxidation and glycoxidation products were positively correlated with pro-inflammatory (IL1α, IL1β, IL6, TNFα) and anti-inflammatory cytokines (IL10), indicating that redox damages may promote inflammation in CRC patients. Many redox biomarkers differentiate patients with CRC from healthy individuals with high sensitivity and specificity.
Conclusion: Correlations of chosen oxidative products with pro-inflammatory (IL1α, IL1β, IL6, TNFα) and anti-inflammatory cytokines (IL10) suggest that redox damages may promote inflammation in CRC patients. Thus, our research is the first point for further clinical trials focusing on the evaluation of the diagnostic utility of nitrosative stress biomarkers in a larger group of CRC patients.
Keywords: nitrosative stress, cytokines, inflammation, colorectal cancer
Graphical Abstract:
Introduction
Although screening tests such as stool examinations and colonoscopy are widely available, colorectal cancer (CRC) remains one of the cancers with the worst prognosis.1 According to the database published by Global Cancer Observatory (GLOBOCAN), the total number of deaths due to colorectal cancer in 2020 was 579,858, whereas the number of new cases was 1,148,515. This classified CRC in third place of the most common cancers globally.1 Simultaneously, GLOBOCAN predicts that the number of new cases will increase by 58.8% to 1.92 million within twenty years, and the number of deaths will increase by 71.9% to 991,332.1 Over 20% of CRC patients have an IV stage with metastasis at diagnosis, which precludes adequate surgical resection. In patients with metastasis, only 70–75% survive one year, 30–35% over three years, and about 20% of CRC patients survive beyond five years from the time of diagnosis.2 Therefore, to improve these unfavorable statistics, new diagnostic methods would be helpful in the diagnosis of colorectal cancer. There is still a great need for additional trials on identifying new non-invasive biomarkers associated with the early events of colorectal carcinogenesis and the development of new, more effective therapies in CRC treatment. The basis for further studies is knowledge increasing CRC biology and finding a connection between biochemical alterations in cancerous tissues and blood.
One of the factors associated with carcinogenesis (also in the colon) is oxidative and nitrosative stress reflecting the lack of balance between the production of reactive oxygen (ROS) and nitrogen (RNS) species and the ability to detoxify/remove free radicals by antioxidants both enzymatic and non-enzymatic.3 It is well known that increased production of ROS and RNS leads to proteins, lipids, and DNA damages. Overproduction of free radicals is also responsible for chronic inflammation.4,5 The effect of inflammation is the induction of immune cells to the secretion of cytokines and chemokines.6 Exposure of colon cells to cytokines may induce inflammatory pathways such as JAK-STAT, NF-kB, or MAPKs cascades, increase the expression of pro-inflammatory enzymes, and induce the production of pro-inflammatory mediators (such as interleukin 1β or tumour necrosis factor α).7 It has been observed that TNF-α derived by activated macrophages and T-cells may promote inflammation and initiate carcinogenesis by inducing DNA damage.8
It is well known that inflammation may lead to carcinogenesis and then modulate cancer cells by stimulating or inhibiting their growth. The activity of cells associated with inflammation and inflammation-regulating factors may modulate the balance between their pro- and antitumour events.9 Acute inflammation in the early stage of cancer may show anticancer action, and in some types of cancers, the ability to induction of controlled acute inflammatory process may be used as an element of therapy. Nevertheless, in chronic inflammation, inflammatory cells promote the cancer cells growth and stimulate proliferation and survival.10 Understanding the mechanisms linking inflammation with carcinogenesis may provide significant benefits both in the clinical and scientific aspects linked to developing new diagnostic and therapeutic methods.
Therefore, our study’s main aim was to assess the relationship between nitrosative stress and pro-inflammatory mediators in colorectal cancer patients in comparison with healthy controls. We also assessed the diagnostic utility of nitrosative stress and pro-inflammatory mediators using the receiver operating characteristic (ROC) analysis.
Materials and Methods
Patients
The study was approved by the Bioethics Committee of the Medical University of Bialystok, Poland (permission number APK.002.99.2021). After a detailed description of the aim of the research and the possible risk, all patients qualified to the study agreed in writing for participation in the experiment. The study was performed in accordance with the World Medical Association Declaration of Helsinki for ethical principles for medical research involving human subjects.
The study included 55 patients (21 women and 34 men) treated surgically due to colorectal cancer in the 2nd Clinical Department of General and Gastroenterological Surgery at the Medical University of Bialystok Clinical Hospital in 2017–2021. Patients were qualified based on the following criteria: men and women without coexistence of other systemic diseases who did not receive radio- or chemotherapy before the surgery. Exclusion criteria for patients with colorectal cancer were as follows: acute inflammatory diseases, infectious diseases (HIV/AIDS, hepatitis A, B, or C), autoimmune diseases (Crohn’s and Hashimoto’s disease, ulcerative colitis, rheumatoid arthritis, and psoriasis), cardiovascular diseases, metabolic diseases, such as osteoporosis, type 1 diabetes, mucopolysaccharidosis and gout, digestive, respiratory or genitourinary systems diseases. Additionally, smokers and patients taking drugs (antibiotics, non-steroidal anti-inflammatory drugs, glucocorticosteroids, vitamins, and dietary supplements) for the three months before the surgery were excluded from the study.
The time from cancer diagnosis to the surgical resection ranged from a minimum of two days to a maximum of four weeks. The study material was taken from all patients before surgery.
The control group included 55 healthy patients (selected by sex and age to match the study group) attending follow-up visits at the Department of Restorative Dentistry at the Medical University of Bialystok from January 2018 to January 2020. To the control group were qualified patients with normal results of complete blood count (CBC) and biochemical blood tests (Na+, K+, creatinine, AST, ALT).
The number of patients in the study and in the control group was set based on a previously conducted pilot study. The power of the study was set at 0.8.
Histopathological Analysis
Histopathological diagnosis was determined using hematoxylin and eosin (H+E) staining and included the following parameters: histological type, the grade of histological malignancy according to the World Health Organization (WHO) guidelines11 and tumour stage according to the TNM classification standard of the Union for International Cancer Control.12
Each tumour was cut along a line that was parallel to the longest tumour axis. In this way, 4 to 8 slices contained cancer and adjacent macroscopically unchanged tissues of 1–1.5 cm in size were taken. Tissues were fixed in 10% buffered formalin but no longer than 24 hours. The specimens were embedded in paraffin at a temperature of 56 °C. Paraffin blocks were cut into 4 μm thick sections on a microtome Microm H340. The obtained sections were stained with hematoxylin-eosin and reviewed by two independent pathologists on a microscope Olympus CX22 under 200× and 400× magnification.
Blood Collection
Fasting venous blood (10 mL) was taken from all patients on an empty stomach, upon overnight rest, using the S-Monovette® K3 EDTA and S-Monovette® serum collection system (Sarstedt, Germany). Immediately after collection, blood was centrifuged at 1500 x g for 10 min at +4°C (MPW 351, MPW Med. Instruments, Warsaw, Poland) to separate plasma or serum from erythrocytes. The top layer (plasma or serum) was then taken. About 0.5 M butylated hydroxytoluene (20 μL/2 mL plasma or serum) was added to prevent sample oxidation. Until redox determinations, all samples were stored at −80°C.
Determination of Redox Markers
All the determinations were conducted in duplicate in the plasma or serum samples. The Infinite M200 PRO Multimode Microplate Reader (Tecan) was using for measurement of absorbance/fluorescence. The results were standardized to 100 mg of total protein. The total protein content was estimated colorimetrically at 562 nm wavelength with the bicinchoninic acid (BCA) method. A commercial kit was used according to the manufacturer’s instructions (Thermo Scientific PIERCE BCA Protein Assay; Rockford, IL, USA).
Nitrosative Stress
Nitric oxide (NO) concentration was determined indirectly by measuring its stable decomposition products NO2- and NO3- using the Griess reaction. For this purpose, sulfanilamide and NEDA·2 HCl (N-(1-naphthyl)-ethylenediamine dihydrochloride) were used.32,33 The absorbance was analyzed at 490 nm.13,14,15
The peroxynitrite concentration was determined spectrophotometrically based on peroxynitrite-mediated nitration resulting in the nitrophenol formation.16 The absorbance was assessed at 320 nm.
The concentration of S-nitrosothiols was analyzed spectrophotometrically using Griess’s assay based on the reaction with Cu2+ ions.17 The absorbance was assessed at 490 nm.
The concentration of nitrotyrosine was determined spectrophotometrically using an ELISA kit (Immundiagnostik AG; Bensheim, Germany) according to the manufacturer’s instructions.
Protein Glycoxidation
A fluorescence evaluation of oxidative protein products was carried out. Dityrosine, kynurenine, and N-formylkynurenine levels were determined fluorimetrically. Immediately before measurement, serum samples were diluted in 0.1 M H2SO4 (1:5, v/v).18 The characteristic fluorescence at 330/415, 365/480, 325/434, and 295/340 nm, respectively, was measured in 96-well black-bottom microplates.19 The results were expressed in arbitrary fluorescence units (AFU)/mg protein.
The formation of amyloid cross-βstructure was measured colorimetrically using thioflavin T.20 The characteristic fluorescence was measured at 435/485 nm.
The formation of the Amadori product was analyzed colorimetrically using nitro blue tetrazolium (NBT) assay.21 The absorbance was measured at 525 nm, and an extinction coefficient of 12,640 cm−1mol−1 L for monoformazan was used.
Oxidative Damage Products
The concentration of total thiols was measured colorimetrically at 420 nm using Ellman’s reagent.22 The concentration of thiol groups was calculated from the calibration curve using reduced glutathione (GSH) as a standard.
The 8-hydroxydeoxyguanosine (8-OHdG) level was determined colorimetrically with commercial ELISA kits (Cayman Chemical, Miami, USA; USCN Life Science, Wuhan, China, respectively), according to the manufacturer’s instructions.
Inflammatory Cytokines
The concentrations of interleukin 1 alpha (IL1α), interleukin 1 beta (IL1β), interleukin 6 (IL6), interleukin 10 (IL10), and tumour necrosis factor alpha (TNF-α) were analysed using commercial ELISA kits from EIAab, Wuhan, China, according the instructions provided by the manufacturer.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.4.3 (GraphPad Software, La Jolla, USA). The Shapiro–Wilk test was used to determine the normality of distribution. For a normal distribution, the Student’s t-test was used. In the case of the lack of normal distribution, the Mann–Whitney U-test was used. The results were presented as median (minimum-maximum), and the value of p < 0.05 was considered statistically significant. Spearman correlation coefficient was used to evaluate the relationships between redox and clinicopathological parameters describing the study group. In order to identify factors that determine the levels of redox biomarkers, we performed multiple regression analyses. Tumour’s location, tumour’s size, histological type, pT, pN, and pM were included as independent variables; 95% confidence intervals (CI) were noted along with regression parameters. Receiver Operating Characteristic (ROC) analysis was used to assess the diagnostic utility of the redox biomarkers. AUC (area under the curve) and optimal cutoff values were determined for each parameter, ensuring high sensitivity and specificity.
Results
Clinical Findings
The study group consisted of 55 patients with colorectal cancer, and the control group included 50 healthy people matched by age and sex with the study group. The tumour differentiation grade in all patients with CRC was G2 (moderately differentiated). Tumour location was divided into two groups: left-side tumours (descending colon, sigmoid colon, and rectum) and right-side tumours (caecum, ascending colon, transverse colon).23 Right-side colorectal cancer (RCRC) occurred in 40% of patients, whereas left-side (LCRC) in 60% of patients. Forty-four patients had adenocarcinoma, whereas 11 patients had mucinous adenocarcinoma. About 24% of patients had a pT1, 27% - pT2, 36% - pT3 and 13% had a pT4 stage of tumour. About 43% of patients have lymph nodes (N1+N2), and 15% have distant metastasis (M1). Most of the patients had a normal level of tumour markers – CEA, CA19-9, and CA72-4. Detailed patient characteristics are shown in Table 1.
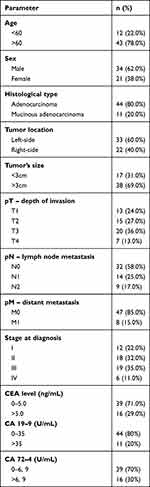 |
Table 1 Characteristics of Study Group |
Nitrosative Stress
The levels of NO, peroxynitrite, S-nitrosothiols, and nitrotyrosine were significantly higher in patients with CRC than in the control group (p = 0.0144, p < 0.0001, p < 0.0001, p < 0.0001) (Figure 1). ROC analysis was performed for evaluation of the diagnostic value of nitrosative stress parameters in the diagnostics of colorectal cancer (Table 2). Determination of NO, peroxynitrite, S-nitrosothiols, and nitrotyrosine showed a very high diagnostic value (AUC = 0.6545, p = 0.01423, AUC = 0.9176, p < 0.0001; AUC = 0.7771, p < 0.0001; AUC = 0.8740, p < 0.0001) in differentiating the group of patients with CRC from healthy people. The cutoff values with sensitivity and specificity values for determining nitrosative stress parameters have been shown in Table 2.
Protein Glycoxidation
We observed increased fluorescence of glycoxidation products (dityrosine, kynurenine, N-formylkynurenine, Amadori product, amyloid) in patients with CRC in comparison with control group. The differences were statistically significant, and p-values were followed: <0.0001, <0.0001, <0.0001, <0.0001, p = 0.0163 (Figure 2). AUC for dityrosine was 0.9171 with cutoff value >632.4 AFU/100mg protein, 87.78% sensitivity and 88.0% specificity, for kynurenine was 0.8707 with cutoff value >964.4 AFU/100mg protein, 80.49% sensitivity and 80.0% specificity whereas AUC for N-formylkynurenine was 0.8873 with cutoff value >744.4 AFU/100mg protein, 82.93% sensitivity and 82.0% specificity. We also demonstrated a very high diagnostic value of amyloid (AUC = 0.6492, p = 0.01615) and Amadori products (AUC = 0.9985, p < 0.0001) determination in the colorectal cancer patients (Table 2).
Oxidative Damage Products
We also assessed total thiols and 8-OHdG levels as a DNA and protein oxidation damages marker. There was a significant increase in total thiols and 8-OHdG levels in patients with colorectal cancer compared to healthy people (p < 0.05, p < 0.0001) (Figure 2). We demonstrated a very high diagnostic value of total thiols (AUC = 0.6333, p = 0.03802) and 8-OHdG (AUC = 0.8594, p < 0.0001) in differentiation of CRC patients from healthy control (Table 2).
Inflammatory Cytokines
All the analyzed cytokines (IL1α, IL1β, IL6, IL10, TNF-α) were significantly higher in the study group than in control. The differences between both groups in all analyzed parameters were statistically significant (Figure 3). ROC analysis demonstrated that each of the analyzed cytokines (IL1α, IL1β, IL6, IL10, TNF-α) might be useful for differentiation patients with colorectal cancer. AUC for IL1α was 0.7299, whereas the p-value was 0.0001054; for IL1β, these values were the following 0.7002 and 0.0009468, for IL6 AUC was 1.000 and p-value <0.0001. AUC for IL10 was 0.9564 and p-value <0.0001 and for TNF-α AUC was 0.9857 and p-value <0.0001 (Table 2).
Correlations
All statistically significant associations are presented in Figures 4 and 5. The analysis of the clinical parameters and analysed parameters revealed positive correlations between peroxynitrite (p = 0.015, R = 0.383) and nitrotyrosine (p = 0.018, R = 0.370) and tumour location. Interestingly, we demonstrated a negative correlation between IL1α and tumour size (p = 0.034, R=−0.263) and positive correlation between IL6 and histological type of the tumour (p = 0.043, R = 0.299). We observed correlations between CEA and dityrosine (p = 0.022, R = 0.484), total thiols (p = 0.05, R = 0.369), kynurenine (p = 0.026, r = 0.471), N-formylkynurenine (p = 0.036, R=0.448), and NO (p = 0.015, R = 0.511). Moreover, the level of dityrosine (p = 0.044, R = 0.342), kynurenine (p = 0.005, R=0.588), and N-formylkynurenine (p = 0.006, R = 0.562) correlated positively with CA19-9 (Figures 4 and 5). IL1α was positively correlated with Amadori products (p = 0.0013, R = 0.4852), total thiols (p = 0.0434, R = 0.317), kynurenine (p = 0.045, R = 0.313) and nitrotyrosine (p = 0.007, R = 0.410). Positive correlations were also present between IL1β and Amadori products (p = 0.001, R = 0.488), dityrosine (p = 0.075, R = 0.280), total thiols (p = 0.009, R = 0.401), kynurenine (p = 0.036, R = 0.328), S-nitrosothiols (p = 0.085, R=0.273), and nitrotyrosine (p = 0.001, R = 0.479). We showed positive correlations between IL6 and Amadori products (p = 0.003, R = 0.441), total thiols (p = 0.026, R=0.347), and nitrotyrosine (p = 0.017, R = 0.369). Moreover, there was a positive correlation between IL10 and Amadori products (p = 0.004, R = 0.438), total thiols (p = 0.029, R = 0.340), kynurenine (p = 0.027, R = 0.346) and nitrotyrosine (p = 0.004, R = 0.434). The significant associations were also found between TNFα and Amadori products (p = 0.0004, R = 0.522), dityrosine (p = 0.029, R = 0.340), total thiols (p = 0.005, R = 0.425), kynurenine (p = 0.02, R=0.360), and nitrotyrosine (p = 0.002, R = 0.453).
 |
Figure 4 Heat map of correlations redox and inflammatory parameters with chosen clinicopathological parameters. |
 |
Figure 5 Heat map of correlations redox and inflammatory parameters with chosen laboratory tests. |
Multiple Regression Analysis
In order to find a relationship between level of evaluated nitrosative stress parameters, protein and DNA oxidation/glycoxidation products, and pro- and anti-inflammatory cytokines and clinicopathological parameters including tumour’s location, tumour’s size, histological type, pT, pN and pM, we developed multiple linear regression models. The redox biomarkers and cytokine levels were used as independent/input variables and clinicopathological parameters were used as output/target/dependent variable. Multiple regression analysis showed that the right-side tumour was associated with the higher peroxynitrite level (p = 0.05). Moreover, the association between NO (p = 0.035) and amyloid (p = 0.028) concentration and presence of mucinous adenocarcinoma was also observed. Significant associations were also noticed between high stage of depth of tumour invasion (pT3) and NO (p = 0.045), kynurenine (p = 0.042), N-formylkynurenine (p = 0.049), dityrosine (p=0.015), and 8-OHdG (p = 0.049) (Table 3).
Discussion
For many years, scientists have tried to explain the significance of redox disturbances in malignant transformation. However, current reports do not indicate the impact of nitrosative stress in developing colorectal cancer. It is well known that nitrosative stress is caused by excessive RNS production induced by endogenous systems and exposure to different physicochemical or pathological factors like UV radiation, chemical carcinogens, lifestyle, diet, or chronic inflammatory conditions.24 Numerous, clinical and epidemiological research strongly support the thesis about the dual role of ROS and RNS.25 In high concentrations, RNS cause protein damages to amino acids, post-translational protein modifications, and eNOS-mediated Ras activation by S-nitrosylation.26 RNS also directly oxides polyunsaturated fatty acids in membrane lipids and inhibits lipid synthesis, fatty acid desaturation, or lipase activation.27 Reactive oxygen and nitrogen species are also responsible for nucleic acids oxidation, which is associated with the inability to repair DNA damage.28 Free radicals may cause point mutations in oncogenes associated with cancer, including p53, V-Raf murine sarcoma viral oncogene homolog hB (BRAF), or adenomatous polyposis coli (APC). RNS can also induce chromosomal abnormalities, DNA adduct formation, methylation, and acetylation, which may favor the transformation of normal cells into cancer cells.24
The primary source of nitrogen for RNS production in vivo is nitric oxide. NO is a product of the catalytic action of the nitric oxide synthase (NOS) on L-arginine. It can also be formed from nitrite derived from food or bacterial metabolism. NOS occur in three isoforms: neuronal (nNOS; type I NOS) and endothelial NOS (eNOS; type III NOS), which are regulated by the interaction of Ca2+ with calmodulin and the third isoform, inducible-NOS (iNOS; type II NOS) activated in response to inflammation, infection, or trauma. Moreover, iNOS is induced by different cells in response to cytokines and endotoxins, such as IL1, IFNγ, and TNFα.29 Although NO may contribute to the anti-inflammatory and antioxidant properties,30 at high concentrations NO promotes oxidation reactions and the formation of reactive nitrogen species.31 NO, and their metabolites like peroxynitrite can mediate the nitration of tyrosine to form 3-NT.32 NO via antiapoptotic and genotoxic mechanisms, and inhibition of host immune response against cancer may take part in malignant transformation.31 NO also participates in induction and promotion of angiogenesis and metastasis or suppression of proliferation and infiltration of leukocytes to the tumour site. Although NO’s role in many cancers has been described, few reports about its importance in colorectal carcinogenesis. Therefore, we determined NO concentration and other nitrosative stress parameters (peroxynitrite S-nitrosothiols, nitrotyrosine). We observed statistically and significantly increased NO, peroxynitrite, S-nitrosothiols, and nitrotyrosine concentration in the serum of patients with CRC compared to healthy control. It may be explained that cancer cells are characterized by enhanced oxygen consumption and its insufficient supply, which causes hypoxia. Therefore, tumour cells exposed to hypoxia contain higher NO levels and its metabolites.33,34 It is well known that NO may react rapidly to form peroxynitrite. Peroxynitrite may oxidize DNA and cause single-strand DNA breaks via an attack on the sugar-phosphate backbone, whereas other NO metabolites – N2O3 may take part in nitrosation amines to form N-nitrosamines and then alkylate DNA. Nitrosation of primary amines in DNA bases leads to the deamination and DNA-crosslinks.35 It may be confirmed that increased levels of reactive nitrogen species play an active role in colorectal carcinogenesis via numerous mechanisms, including DNA injury. On the other hand, increased concentration of S-nitrosothiols in CRC patients may be the body’s adaptive response expressed as cellular storage of NO.36 It is well-known that S-nitrosothiols preserve cells against the destructive effects of NO2, NO+, NO− and peroxynitrite.13 We also observed that NO concentration increased with CEA level, which may be a valuable tool in the early non-invasive diagnosis of CRC or monitor tumour recurrence in these patients after tumour resection.
Disturbances in ROS and RNS production and insufficient antioxidant barrier result in oxidative and nitrosative damages to proteins, lipids, and nucleic acids. Although ROS and RNS react with most biomolecules, proteins are the main target of action of free radicals. Protein oxidative modifications include mainly protein carbonylation and tyrosine nitration, known as irreversible modifications. In contrast, reversible oxidation includes mainly cysteine modifications such as sulfenic acid, nitrosothiols, protein disulfides, and s-glutathione.37 Total thiols belong to the group of organic compounds consisted of sulfhydryl group (-SH). Plasma thiols include the protein sulfhydryl groups and protein-mixed disulfides with homocysteine, cysteinyl glycine, and glutathione. Our work observed significantly higher concentrations of total thiols in patients with colorectal cancer. It may be an adaptive response to the enhanced production of free radicals. Moreover, the oxidation reaction of thiols with ROS/RNS leads to the formation of reversible disulfide bonds, which is the earliest indicator of radical-mediated protein oxidation. When oxidative/nitrosative stress condition ends, disulfide bond structures may be reduced to the thiol groups again. Thus, dynamic thiol-disulfide balance is maintained. Thiol/disulfide balance takes part in organizing antioxidant defense, apoptosis, detoxification, and cellular signalling.38 As stated above, ROS and RNS production may also cause nucleic acid damages. The 8-OHdG is the most common DNA damage because it is relatively quickly formed and has promutagenic properties.39,40 Therefore, it is not surprising that we observed a considerable increase of 8-OHdG in CRC patients. This indicates that cancer is inextricably linked with DNA oxidative damages. It has been observed that one of the causes associated with modulation of oxidative DNA damage is impairment of DNA repair mechanisms. Research-based on cultured cells and mice models revealed that overexpression of Ogg1 (mammalian glycosylases responsible for the initiation of the base excision repair pathway) increased the repair rate by several fold but did not decrease the steady-state level or the spontaneous mutation rate.39 Therefore, 8-OHdG seems to play a significant role in spontaneous mutagenesis accompanying malignant transformation.
We also demonstrated an increased level of protein glycoxidation products (kynurenine, N-formylkynurenine, dityrosine, Amadori products, amyloid) in patients with colorectal cancer compared to healthy controls. N-formylkynurenine and kynurenine are primary products of tryptophan metabolism, and as has been observed, colon cancer cells show greater tryptophan uptake and processing than normal colonic cells and tissues; therefore, the level of its metabolites was increased. It is known that kynurenine is a ligand for the transcription factor – AHR (aryl hydrocarbon receptor), and through the binding to this receptor, it may regulate growth-promoting genes in cancer cells. Some of the reports suggest that blocking the binding of kynurenine to AHR and inhibiting kynurenine production via tryptophan conversion may decrease the growth of cancer cells. Therefore, kynurenine may act as an oncometabolite.41
Glycoxidation products also can initiate the pro-inflammatory NF-κB pathway, which enhances the expression of pro-inflammatory cytokines and chemokines, adhesion molecules, growth factors, and nitric oxide synthases (mainly iNOS).13 Our work showed a relationship between pro-inflammatory (IL1α, IL1β, IL6, TNFα) and anti-inflammatory cytokines (IL10) with oxidation and glycoxidation products. Levels of pro-inflammatory and anti-inflammatory cytokines were considerably higher in patients with colorectal cancer than in the control group, indicating the intensification of inflammatory processes in patients with cancer. Chronic inflammation caused by NF-κB activation can be one of the essential promoters of tumour development. Inflammation may influence cancer initiation and progression by vascularization and remodeling the tumour microenvironment, crucial in tumour cell survival.42 Inflammatory tissues also release cytokines that activate NF-κB, which induces LOX, COX2, and iNOS, leading to overproduction of ROS and RNS. ROS and RNS-mediated MAPK (mitogen-activated protein kinases) contribute to the production of inflammatory cytokines, including pro-inflammatory mediators like TNFα and IL6, IL1β, or TGFβ. It has been confirmed that the NFκB pathway, oxidative/nitrosative stress, and the JAK-STAT pathway cooperate and intensify each other in cancer progression. However, NF-κB plays a vital role in promoting cancer by inducing abnormal cellular proliferation, cell survival, cell cycle regulation, and the development of resistance to drug therapies.43 Interestingly, mice with loss of NF-κB function in colonic epithelial cells developed fewer colon tumours after AOM/DSS application and had higher epithelial cell apoptosis without decreasing inflammation. In contrast, mice that lost function of NF-κB function in myeloid cells developed fewer tumours and tumours of smaller size and had decreased expression of cytokines and chemokines, especially IL1, IL6, and TNFα that are responsible for inflammation.8
In conclusion, we have shown that protein and DNA oxidation and glycoxidation products, nitrosative stress markers, and cytokines are significantly increased in patients with CRC. Therefore, nitrosative stress, protein oxidation, and carbonyl stress may play a significant role in colorectal carcinogenesis. Oxidation and glycoxidation products were positively correlated with pro-inflammatory (IL1α, IL1β, IL6, TNFα) and anti-inflammatory cytokines (IL10), indicating that redox damages may promote inflammation in CRC patients. Many redox biomarkers differentiate CRC patients from healthy individuals with high sensitivity and specificity. Thus, our research is a primary step for further clinical trials focusing on the evaluating the diagnostic utility of redox biomarkers in a larger population of colorectal cancer patients. Evaluation of the association between the intensity of nitrosative stress and the survival rate of CRC patients also seems to be advisable.
In the end, it is also worth considering some of limitations of our study. We carried out a research on a small group of patients; therefore, further study is required on a greater group of patients with CRC. The concentration of chosen redox parameters was assessed only in blood samples (serum/plasma), giving our results an approximate value. We evaluated only the chosen redox biomarkers, so we cannot completely describe the oxido-reductive disturbances in CRC patients. Nevertheless, the undoubted advantage of our work is a carefully selected group of CRC and control patients without any comorbidities.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
The study was approved by the Bioethics Committee of the Medical University of Bialystok, Poland (permission number APK.002.99.2021). After a detailed explanation of the purpose of our research and the possible risk, all the qualified patients agreed in writing to participate in the experiment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was granted by the Medical University of Bialystok, Poland (grant numbers: SUB/1/DN/19/001/2209/20; SUB/1/DN/20/005/2209). Dr Mateusz Maciejczyk was supported by the Foundation for Polish Science (FNP).
Disclosure
The authors declare that they have no competing interests.
References
1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer tomorrow. Lyon, France: International Agency for Research on Cancer; 2020. Available from: https://gco.iarc.fr/tomorrow/en.
2. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi:10.1001/jama.2021.0106
3. Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:1–13. doi:10.1155/2017/8416763
4. Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15. doi:10.1186/s12937-016-0186-5
5. Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:1–9. doi:10.1155/2016/7432797
6. Chatterjee S. Oxidative stress, inflammation, and disease. In: Oxidative Stress and Biomaterials. Elsevier; 2016:35–58. doi:10.1016/B978-0-12-803269-5.00002-4
7. Sabaawy HE, Ryan BM, Khiabanian H, Pine SR. JAK/STAT of all trades: linking inflammation with cancer development, tumor progression and therapy resistance. Carcinogenesis. 2021;42(12):1411–1419. doi:10.1093/CARCIN/BGAB075
8. Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–1816.e1. doi:10.1053/j.gastro.2011.01.057
9. Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186:57–62. doi:10.1007/s11845-016-1464-0
10. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):1–46. doi:10.1038/s41392-021-00658-5
11. Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System.
12. Amin MB. AJCC Cancer Staging System.
13. Choromańska B, Myśliwiec P, Łuba M, et al. Bariatric surgery normalizes protein glycoxidation and nitrosative stress in morbidly obese patients. Antioxidants. 2020;9:1087. doi:10.3390/antiox9111087
14. Grisham MB, Johnson GG, Lancaster JR. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996. doi:10.1016/s0076-6879(96)68026-4
15. Borys J, Maciejczyk M, Antonowicz B, et al. Glutathione metabolism, mitochondria activity, and nitrosative stress in patients treated for mandible fractures. J Clin Med. 2019;8:127. doi:10.3390/jcm8010127
16. Beckman JS, Ischiropoulos H, Zhu L, et al. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:438–445. doi:10.1016/0003-9861(92)90432-V
17. Wink DA, Kim S, Coffin D, et al. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999. doi:10.1016/S0076-6879(99)01083-6
18. Klimiuk A, Maciejczyk M, Choromańska M, Fejfer K, Waszkiewicz N, Zalewska A. Salivary redox biomarkers in different stages of dementia severity. J Clin Med. 2019;8(6):840. doi:10.3390/jcm8060840
19. Borys J, Maciejczyk M, Krȩtowski AJ, et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front Physiol. 2017;8. doi:10.3389/fphys.2017.00386
20. LeVine H. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi:10.1016/S0076-6879(99)09020-5
21. Johnson R, Baker J. Assay of serum fructosamine: internal vs external standardization. Clin Chem. 1987;33:1955–1956. doi:10.1093/clinchem/33.10.1955
22. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi:10.1016/0003-9861(59)90090-6
23. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779. doi:10.7326/0003-4819-113-10-779
24. Ortega ÁL, Mena S, Estrela JM. Oxidative and nitrosative stress in the metastatic microenvironment. Cancers. 2010;2:274–304. doi:10.3390/cancers2020274
25. Basak D, Uddin MN, Hancock J. The role of oxidative stress and its counteractive utility in Colorectal Cancer (CRC). Cancers. 2020;12:3336. doi:10.3390/cancers12113336
26. Lim K-H, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi:10.1038/nature06778
27. Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266(1):73–83. doi:10.1016/J.CANLET.2008.02.035
28. Lee J-H, Hwang I, Kang Y-N, Choi I-J, Kim D-K, Lo AWI. Genetic characteristics of mitochondrial DNA was associated with colorectal carcinogenesis and its prognosis. PLoS One. 2015;10:e0118612. doi:10.1371/journal.pone.0118612
29. Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin Diagn Labor Immunol. 1998;5:438–445. doi:10.1128/CDLI.5.4.438-445.1998
30. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Medicine and Cellular Longevity. 2016;2016:1–44. doi:10.1155/2016/1245049
31. Korde Choudhari S, Chaudhary M, Bagde S, Gadbail AR, Joshi V. Nitric oxide and cancer: a review. World J Surg Oncol. 2013;11. doi:10.1186/1477-7819-11-118
32. Ahsan H. 3-nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol. 2013;74(10):1392–1399. doi:10.1016/j.humimm.2013.06.009
33. Zińczuk J, Zaręba K, Kamińska J, et al. Association of tumour microenvironment with protein glycooxidation, DNA damage, and nitrosative stress in colorectal cancer. Cancer Manag Res. 2021;13:6329–6348. doi:10.2147/CMAR.S314940
34. Maciejczyk M, Zalewska A, Gryciuk M, et al. Effect of normobaric hypoxia on alterations in redox homeostasis, nitrosative stress, inflammation, and lysosomal function following acute physical exercise. Oxid Med Cell Longev. 2022;2022:1–18. doi:10.1155/2022/4048543
35. Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi:10.1038/sj.cr.7290133
36. Choromańska B, Myśliwiec P, Dadan J, Maleckas A, Zalewska A, Maciejczyk M. Effects of age and gender on the redox homeostasis of morbidly obese people. Free Radic Biol Med. 2021;175:108–120. doi:10.1016/J.FREERADBIOMED.2021.08.009
37. Cai Z, Yan L-J. Protein oxidative modifications: beneficial roles in disease and health. J Biochem Pharmacol Res. 2013;1(1):15–26.
38. Sönmez MG, Kozanhan B, Çigˇ Ç, et al. Is oxidative stress measured by thiol/ disulphide homeostasis status associated with prostate adenocarcinoma? Cent Eur J Immunol. 2018;43(2):174–179. doi:10.5114/ceji.2017.72285
39. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–139. doi:10.1080/10590500902885684
40. Sawczuk M, Sawczuk-Siemieniuk P, Zalewska C, et al. Salivary gland function, antioxidant defence and oxidative damage in the saliva of patients with breast cancer: does the BRCA1 mutation disturb the salivary redox profile? Cancers. 2019;11(10):1501. doi:10.3390/cancers11101501
41. Venkateswaran N, Conacci-Sorrell M. Kynurenine: an oncometabolite in colon cancer. Cell Stress. 2020;4:24–26. doi:10.15698/cst2020.01.210
42. Arfin S, Kumar Jha N, Kumar Jha S, et al. Antioxidants oxidative stress in cancer cell metabolism. Oxidative Stress Cancer Cell Metab Antioxidants. 2021;10. doi:10.3390/antiox10050642
43. Ahmed KM, Cao N, Li JJ. HER-2 and NF-kappaB as the targets for therapy-resistant breast cancer. Anticancer Res. 2006;26(6B):4235–4243.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





