Back to Journals » Journal of Inflammation Research » Volume 15
Pro-Inflammatory and Anti-Inflammatory Cytokines Levels are Significantly Altered in Cerebrospinal Fluid of Unruptured Intracranial Aneurysm (UIA) Patients
Authors Kamińska J , Maciejczyk M , Ćwiklińska A , Matowicka-Karna J , Koper-Lenkiewicz OM
Received 29 June 2022
Accepted for publication 25 October 2022
Published 11 November 2022 Volume 2022:15 Pages 6245—6261
DOI https://doi.org/10.2147/JIR.S380524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Joanna Kamińska,1 Mateusz Maciejczyk,2 Agnieszka Ćwiklińska,3 Joanna Matowicka-Karna,1 Olga Martyna Koper-Lenkiewicz1
1Department of Clinical Laboratory Diagnostics, Medical University of Białystok, Białystok, Poland; 2Department of Hygiene, Epidemiology, and Ergonomics, Medical University of Białystok, Białystok, Poland; 3Department of Clinical Chemistry, Medical University of Gdańsk, Gdańsk, Poland
Correspondence: Joanna Kamińska, Department of Clinical Laboratory Diagnostics, Medical University of Białystok, 15A Jerzego Waszyngtona St, Białystok, 15-269S, Poland, Tel/Fax + 48 85 7468584, Email [email protected]
Introduction: Identifying all the relevant “players” in the formation and development of brain aneurysms may help understand the mechanisms responsible for the formation of an aneurysm, as well as in the search for non-invasive targets for aneurysm pharmacotherapy.
Aim: The evaluation of the concentration of pro-inflammatory and anti-inflammatory cytokines in cerebrospinal fluid (CSF) and serum of patients with unruptured intracranial aneurysms (UIA) in comparison to individuals without vascular lesions in the brain.
Methods: The concentration of 27 proteins in the CSF and serum of UIA patients (N = 40) and individuals without vascular lesions in the brain (N = 15) was evaluated using a multiplex ELISA kit (Bio-Plex Pro Human Cytokine 27-Plex Panel).
Results: In the CSF 13 out of 27 proteins evaluated presented a concentration 1.36-fold or greater in UIA patients in comparison to the control group. Significantly higher were IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-7, IL-8, IL-12, IL-13, TNF-α, INF-γ, MCP-1, and VEGF. In the serum none of the proteins evaluated significantly differ between UIA patients and the control group. The correlation coefficient analysis showed that CSF IL-1β, IL-8, and TNF-α positively, while IL-13 negatively correlated with the size of aneurysms. CSF IL-6 and MCP-1 concentrations positively correlated with the number of aneurysms.
Conclusion: In patients with UIA, pro-inflammatory and anti-inflammatory mechanisms are activated simultaneously, because the concentration of promoting and suppressing inflammatory response proteins was significantly higher in CSF of UIA patients compared to the control group. The preventive therapy of brain aneurysm development should be focused on IL-1β, IL-6, IL-8, MCP-1, and TNF-α, the concentration of which in CSF positively correlated with the size and number of aneurysms.
Keywords: cerebrospinal fluid, cytokines, inflammation, interleukins, intracranial aneurysm, unruptured intracranial aneurysms
Introduction
Inflammation within the arterial vessel due to hemodynamic changes is a critical element in the formation of an intracranial aneurysm (IA).1–8 Understanding the mechanisms leading to the formation of brain aneurysms and identifying all the relevant “players” in this process is essential for the development of an effective pharmacotherapy that could prevent life-threatening subarachnoid hemorrhage from rupture of IA.
Both the literature data and our previous research indicated, that the process of formation and development of brain aneurysms is multifactorial and dependent, among other things, on interleukins, cytokines, chemokines, growth factors, or matrix metalloproteinases.4,5,9–16 Researchers also highlighted the importance of immune cells in this process (monocytes/macrophages, neutrophils, T lymphocytes, natural killer cells (NK), mast cells) that infiltrate the vessel wall and promote its pathogenic remodeling.17–20 The most critical importance in the formation of brain aneurysms is attributed to macrophages secreting inflammatory cytokines,19,21 proteolytic enzymes, or reactive oxygen species, thus inducing apoptosis and fibrosis of smooth muscle cells within the IA wall.22–26 Persistent chronic inflammation has a modulating effect on the arterial wall, promoting its degradation, but also supports repair mechanisms.27 However, the advantage of degenerative over repair mechanisms contributes to the critical thinning of the vessel wall and eventual rupture, leading to aneurysmal subarachnoid hemorrage.28
A possible mechanism for the development of aneurysm-related inflammation has been attributed to the imbalance between the pro- and anti-inflammatory systemic response.10 Typical pro-inflammatory cytokines are interleukins (ILs) such as IL-1, −6, −8, −15, −17, −18, −23, tumor necrosis factor-alpha (TNF-α) and chemokines like monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and macrophage inflammatory protein-1β (MIP-1β).29,30 Also, IL-2, −5, −7, −12, interferon-γ (IFN- γ), granulocyte colony-stimulating factor (G-CSF), granulocyte monocyte colony-stimulating factor (GM-CSF), regulated on activation, normal T cell expressed and secreted (RANTES), interferon gamma-induced protein 10 (IP-10) are recognized as pleiotropic regulators of inflammation.31–36 Major anti-inflammatory cytokines encompass IL-1 receptor antagonist (IL-1ra), IL-4, −9, −10, −11, and IL-13.30,37
Studies that can “visualize” the body’s response to the formation of an aneurysm in the brain may help understand the mechanisms responsible for their formation as well as in the search for non-invasive targets for aneurysm pharmacotherapy. Therefore, the current study aimed to assess the concentrations of pro- and anti-inflammatory cytokines in cerebrospinal fluid (CSF) and serum of patients with unruptured intracranial aneurysms (UIA) in comparison to individuals without vascular changes in the brain. It should be highlighted that most of the conducted studies to date have been focused on animal models or performed in patients with ruptured aneurysms.16,18,38–41 The commercially available panel selected by us included 27 cytokines such as IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IL-1ra, TNF-α, IFN-γ, GM-CSF, G-CSF, Eotaxin, IP-10, MCP-1, MIP-1-α, MIP-1β, RANTES, VEGF, PDGF-BB, and bFGF. We also attempted to check if these proteins are related to the size and number of aneurysms.
Materials and Methods
Subjects
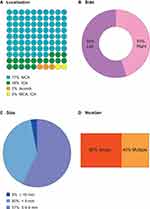
The study group consisted of 40 patients with unruptured intracranial aneurysms (UIA). The patients were operated on at the Department of Neurosurgery at the Clinical Hospital of the Medical University of Bialystok. All patients underwent craniotomy and direct surgical clipping of an unruptured intracranial aneurysm. Detailed information about the procedure can be found in our previous publications.4,5 The control group consisted of 15 patients suffering from trigeminal neuralgia because of an anatomical conflict between the trigeminal nerve and the cerebellar artery. All patients within this group were revealed to be unresponsive to conservative treatment and were qualified for posterior fossa craniotomy and microvascular decompression. Table 1 presents clinical characteristics and basic laboratory parameters in UIA patients and the control group. Figure 1A–D illustrates the localization and geometry of aneurysms in UIA patients. Table 2 presents modifiable risk factors of intracranial aneurysm development in the group of UIA patients and the control individuals without vascular lesions in the brain.
 |
Table 1 Clinical Characteristics and Basic Laboratory Parameters in UIA Patients and the Control Group |
 |
Table 2 Risk Factors of Intracranial Aneurysm Development in the Group of UIA Patients and the Control Group |
Exclusion criteria for both groups were comprised of neurodegenerative conditions like multiple sclerosis, neuroinfection, and a brain tumor in medical history, surgery or major trauma, antibiotics, anti-inflammatory, or corticosteroid administration in the preceding months.
The study was conducted according to the guidelines of the Declaration of Helsinki and the protocol was approved by the Bioethics Human Research Committee of the Medical University of Bialystok (Permission No. R-I-002/383/2015 and APK.002.9.2021). All subjects gave their informed consent for inclusion before they participated in the study.
Cerebrospinal Fluid (CSF) and Serum Collection and Storage
Neurosurgical procedures to obtain CSF were conducted in the standard manner. For details please refer to the publications of Kamińska et al4,5 Particular care was taken to prevent any contamination of the CSF with blood or warm saline solution used as irrigation. All the aforementioned steps were taken at the start of each procedure, before any bleeding might have occurred. Blood samples were collected into 5 mL tubes without anticoagulant (S-Monovette, SARSTEDT). Both the CSF and serum samples were centrifuged for 20 minutes at 1000 × g and in the next step aliquoted and placed in storage at −75°C until further analysis.
Methods
To detect the presence of 27 cytokines in CSF and serum in UIA patients and the control group the multiplex ELISA method was applied (Bio-Plex Pro Human Cytokine 27-Plex Panel, catalog no: #M500KCAF0Y, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Concentrations of the following molecules were determined: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IL-1ra, TNF-α, IFN-γ, GM-CSF, G-CSF, Eotaxin, IP-10, MCP-1, MIP-1-α, MIP-1β, RANTES, VEGF, PDGF-BB, and bFGF.
Statistical Analysis
The obtained results were analyzed with the use of the STATISTICA 12.0 PL software (StatSoft Inc., Tulsa, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, USA). In the first step, the normality of the distribution of the examined parameters was assessed using the Shapiro–Wilk test. When comparing measurable variables in two independent samples in the absence of a normal distribution, the non-parametric Mann–Whitney U-test was used. The Student’s t-test was used when normal distribution was present in both tested groups. The ANOVA rank Kruskal–Wallis test was used for the comparison of three samples. If significant differences were detected, the post-hoc test was conducted to assess which groups were different. Correlation coefficients were obtained by applying Spearman’s rank correlation and presented as a heat map. Differences were considered significant for p < 0.05.
Results
Interleukins, Cytokines, Chemokines, and Growth Factor Concentrations in UIA Patients in Comparison to the Control Group
Analysis of interleukins, cytokines, chemokines, and growth factor concentrations in the CSF showed that 13 out of 27 proteins evaluated presented a concentration 1.36-fold or greater in UIA patients when compared to the control group (Table 3). Significantly higher were: IL-1β, IL-1ra, IL-2, IL-4, IL- 5, IL-7, IL-8, IL-12, IL-13 (Figure 2A–I), TNF-α, INF-γ, MCP-1, and VEGF (Figure 3A–D). Analysis of serum interleukins, cytokines, chemokines, and growth factor concentrations showed that none of the 27 proteins evaluated significantly differ between UIA patients and the control group (Table 4).
 |
Table 3 CSF Concentrations of Interleukins, Cytokines, Chemokines, and Growth Factors in UIA Patients and the Control Group |
 |
Table 4 Serum Concentrations of Interleukins, Cytokines, Chemokines, and Growth Factors in UIA Patients and the Control Group |
Interleukins, Cytokines, Chemokines, and Growth Factor Concentrations in CSF When Compared to the Serum
In the group of UIA patients CSF IL-2, IL-6, IL-8, MIP-1α, and MCP-1 concentrations were significantly higher when compared to values obtained in the serum. In the control group, only MCP-1 concentration was significantly higher in the CSF when compared to the serum (Figure 4A–E).
Interleukins, Cytokines, Chemokines, and Growth Factor Concentrations Depending on the Aneurysm’s Location
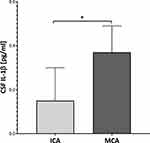
Proteins analysis depending on an aneurysm’s location showed that only CSF IL-1β significantly differ between UIA patients with MCA location compared to those with ICA location (Figure 5). Neither in serum, nor in the CSF did we find any other differences regarding the aneurysm’s location.
Correlation Coefficient Results in the CSF
The complex relationship between interleukins, cytokines, chemokines, and growth factors in the CSF of UIA patients is presented on the heat map (Figure 6). We also analyzed the correlations between protein concentrations and the size and number of aneurysms. The correlation coefficient analysis showed that CSF IL-1β, IL-8, and TNF-α positively, while IL-13 negatively correlated with the size of aneurysms. Only CSF IL-6 and MCP-1 concentrations positively correlated with the number of aneurysms (Figure 7A–F).
CSF Interleukins, Cytokines, Chemokines, and Growth Factor Concentrations Depending on Confounding Factors
We also analyzed whether CSF concentrations of analyzed proteins may differ depending on confounding factors such as sex, age, smoking, cardiovascular disease, drinking, obesity, or hypertension. We demonstrated that in the UIA group CSF IFN-γ concentration was significantly higher in females compared to males (Supplementary Table 1). CSF TNF-α concentration was significantly higher in UIA patients >60 years old compared to patients ≤60 years old (Supplementary Table 2). CSF IL-8 concentration was significantly lower in UIA patients who smoke compared to non-smokers (Supplementary Table 3). CSF IL-1ra and IL-13 concentrations were significantly lower in UIA patients with cardiovascular disease compared to those without cardiovascular disease (Supplementary Table 4). Drinking, obesity, and hypertension had no influence on the CSF protein concentrations (Supplementary Tables 5–7).
Discussion
The formation and development of intracranial aneurysms is a complex process and depends on the interaction of many molecules, including interleukins, cytokines, chemokines, growth factors, or matrix metalloproteinases.42,43 Alteration of the biomechanical properties of the arterial wall, apparently associated with inflammation and repair mechanisms, leads to formation and rupture of intracranial aneurysms.44 Therefore, studies evaluating the protein panel in unruptured intracranial aneurysm (UIA) patients seem to be extremely valuable. To the best of our knowledge, our study is the first to assess 27 proteins in CSF and serum that can positively and negatively modulate the inflammatory response in UIA patients.
Our study showed that in the CSF of UIA patients, the concentration of 13 out of 27 tested proteins was significantly higher compared to individuals without vascular lesions in the brain. High CSF concentrations of these proteins indicated their local synthesis within the central nervous system (CNS), and can be alternatively regarded as evidence of inflammatory response at the site of an aneurysm.45,46 Thus, the increased CSF concentration of IL-1β, IL-1ra, IL-2, IL-4, IL- 5, IL-7, IL-8, IL-12, IL-13, TNF-α, INF-γ, MCP-1, and VEGF in UIA patients as compared to individuals without vascular lesions in the brain may indicate that these molecules can modulate UIA development. The results of our research also draw attention to the cytokines IL-1ra, IL-2, IL-4, IL-5, IL-7, and IL-13, whose role in the formation and development of brain aneurysms has so far, not been widely studied. In this context, our study also provides new insight into understanding the process of intracranial aneurysm formation. Additionally, in UIA patients, contrary to subjects without vascular lesions in the brain, IL-2, IL-6, IL-8, and MIP-1α concentrations were significantly higher in CSF when compared to the serum values, which indicates that these molecules are synthesized locally within the CNS,4,5,47 possibly in response to the development of a brain aneurysm. Current research also strengthens our previous findings indicating the significant role of IL-6, IL-8, and MCP-1 in the formation and development of brain aneurysms in humans.4,5
Studies in animal models showed increased mRNA and protein expression of IL-1β, IL-8, TNF-α, and MCP-1 in endothelial cells in intracranial aneurysms (IA).16,18,40,41,48 Chalouhi et al12 showed significantly higher concentrations of IL-8, IL-17, MCP-1, RANTES, MIG, IP-10, in UIA patients and Eotaxin in plasma obtained from the lumen of the cerebral aneurysm compared to the plasma obtained from the femoral artery. These studies suggest the involvement of the above-mentioned cytokines in the pathomechanism of aneurysm formation. Although it is difficult to indicate beyond doubt, whether these cytokines are the cause or the result of aneurysm development, it is certain that, along with immunocompetent cells, they play a part in the pathological remodeling of the cerebral vessel. Pro-inflammatory cytokines IL-1β, IL-6, IL-8, TNF-α, and IFN-γ are mainly secreted by macrophages, having the greatest contribution to the formation of brain aneurysms.23,49 Additionally, studies conducted in our population of UIA patients confirmed the involvement of these cytokines in the formation of brain aneurysms. However, apart from interleukins and TNF-α, our study showed a significantly higher concentration of VEGF in CSF in patients with UIA compared to those without vascular lesions in the brain. VEGF is one of the most potent angiogenic factors involved in physiological and pathological angiogenesis by inducing a proliferation of endothelial cells. Our research, therefore, may suggest a possible role for VEGF in the pathogenesis of brain aneurysms.
Moriwaki et al40 in an experimental mouse model of IA showed that IL-1β expression is induced in the aneurysmal wall in the early stages of its development. Moreover, they also found increased expression of IL-1β in smooth muscle cells (SMC), which promoted their apoptosis and thus the process of brain aneurysm formation. In addition, the progression of the aneurysm was slower in the mutant mice (IL-1β -/-) compared to the wild-type mice.40 The relationship between IL-1β and aneurysm development was also confirmed in our study, which showed a positive correlation between the concentration of IL-1β in CSF and the size of the aneurysm. Zhang et al10 showed a significantly higher concentration of MCP-1 and TNF-α in the plasma of patients with ruptured brain aneurysms compared to patients with unruptured brain aneurysms and patients with multiple brain aneurysms, thus indicating the participation of these cytokines primarily in the progression of the vascular lesion. This finding provides a link between ultrastructural alterations and modifications of the biomechanical properties at the rupture zone. The above-mentioned hypothesis is confirmed by the results of our study, which showed that the concentration of MCP-1 in CSF positively correlated with the number of aneurysms, and the concentration of TNF-α in CSF with the size of the aneurysm. It should be emphasized that IL-1β promotes the synthesis of the chemokine MCP-1.49 Another protein, that upregulates the expression of MCP-1, is tissue-type plasminogen activator (tPA), which stimulates the infiltration of macrophages within the aneurysmal wall. Thus, tPA indirectly contributes to the IA formation and rupture through a pro-inflammatory pathway.50 All this data taken together suggests the presence of a feedback loop between cytokines in patients with UIA, which was also confirmed by our current research showing many significant correlations between the concentrations of the analyzed cytokines in the CSF.
It should also be noted that the positive correlation of CSF IL-1β, IL-8, and TNF-α levels with aneurysms’ size and CSF IL-6 and MCP-1 levels with aneurysms’ number might be due to the volume of the lesions (the total number of cells producing cytokines). Undoubtedly, the issue of whether increased protein concentrations are associated with the presence of smaller or larger lesions, requires further analysis.
Brain aneurysm development, like atherosclerosis, is the result of chronic inflammation involving Th1 lymphocytes,49,51 the differentiation of which is dependent on IL-12 and IFN-γ. Th1 lymphocytes mainly produce IFN-γ and TNF-α, thus stimulating NK cells to secrete IFN-γ, IL-4, and IL-13, and activate macrophages to the pro-inflammatory phenotype M1.52 IFN-γ produced by Th1 lymphocytes and activated M1 macrophages inhibits both SMC proliferation and collagen production, contributing to the apoptosis of smooth muscle cells.49,51,53–55 Moreover, IFN-γ, acting synergistically with IL-1β and TNF-α, induces the expression of leukocyte adhesion molecules, which also promotes inflammatory remodeling of the arterial vessel.56 In our study, the concentration of IL-12, IFN-γ, IL-4, and IL-13 in CSF was significantly higher in UIA patients compared to those without vascular lesions in the brain, which indirectly indicates the involvement of Th1-dependent cellular response in the pathogenesis and development of brain aneurysms.
The results of our studies also revealed that in UIA patients there is a significant increase in the secretion of anti-inflammatory cytokines. We showed for the first time that in UIA patients, the concentration of IL-1ra, which inhibits the binding of both IL-1α and IL-1β to cell surface receptors, was significantly higher compared to individuals without vascular lesions in the brain. Arend et al57 indicated that inhibition of a biological IL-1 response requires at least a 100-fold excess of IL-1ra over IL-1. In our study, we demonstrated an over 380-fold excess of IL-1ra to IL-1β. We also discovered that the concentrations of IL-4, influencing the differentiation of T helper cells into anti-inflammatory Th2 cells,58 and IL-5, inhibiting the differentiation of pro-inflammatory Th1 and Th17 lymphocytes,59 were significantly higher in UIA patients compared to individuals without vascular lesions in the brain. This allows us to hypothesize that in UIA patients mechanisms aimed to inhibit the development of the aneurysm are also activated. However, the anti-inflammatory effects of cytokines may be annulled by the pleiotropism of the pro-inflammatory cytokines and the predominance of cells with an inflammatory over an anti-inflammatory potential. Especially since Jayaraman et al23 suggested that although anti-inflammatory Th2 lymphocytes are present in the wall of the brain aneurysm, this is not where they are activated, and thus they do not achieve their function.
IL-2 is a cytokine, whose role in the formation and progression of brain aneurysms has not been fully discovered.60 In our study, the concentration of IL-2 was significantly higher in CSF of UIA patients compared to individuals without vascular lesions in the brain. Zhang et al in animal IA model studies showed that administration of IL-2 significantly increased the expression of the checkpoint molecule Tim-3 in Treg cells, thereby boosting their proliferation.60 IL-2 not only ensures the homeostasis of Treg lymphocytes but also influences the differentiation of T helper lymphocytes into Th1 and Th2 subpopulations. Indirectly, it may promote pro and anti-inflammatory mechanisms, and thus the evaluation of IL-2 importance in the formation of brain aneurysms requires further study.61,62
Study Limitation
We would like to stress, that CSF collection via lumbar puncture is not routine in patients with UIA. Thus, the Local Bioethics Committee gave permission for CSF collection only within neurosurgery. In order to reliably assess the concentration of the analyzed molecules in the CSF, the comparative group needed to have the fluid taken from the same interspace in order to make the samples relatively homogenous. For this reason, the control group was not composed of entirely healthy people, but patients suffering from trigeminal neuralgia. Nevertheless, we were still able to demonstrate statistically significant differences.
Another study limitation could be the number of cases included to the study. However, we would like to clarify that only CSF samples with a red blood cell count that equaled 0/μL were used for further analysis, which significantly limited the number of patients included to the study. Moreover, only trigeminal neuralgia patients, who did not respond to conservative treatment, were qualified for posterior fossa craniotomy and microvascular decompression.
Conclusions
The current study showed, that in the CSF 13 out of 27 proteins evaluated were significantly higher in UIA patients in comparison to individuals without vascular lesions in the brain. On the contrary, in the serum none of the proteins tested significantly differ between UIA patients and the comparison group. These results indicated, that CSF is a better material for searching molecules implicated in IA formation and development. High CSF concentrations of these proteins indicated their local synthesis within the CNS, and can be alternatively regarded as evidence of inflammatory response at the site of an aneurysm. Our results also suggest that in patients with UIA, pro-inflammatory and anti-inflammatory mechanisms are activated simultaneously, because the concentration of both promoting and suppressing the inflammatory response proteins was significantly higher in UIA patients compared to individuals without vascular lesions in the brain. We suggest that the molecules responsible for the formation of IA are primarily IL-2, IL-6, IL-8, MIP-1α, and MCP-1, the concentration of which in UIA patients was significantly higher in CSF compared to serum, indicating their local synthesis within the central nervous system. On the other hand, the preventive therapy of brain aneurysm development should be focused on IL-1β, IL-6, IL-8, MCP-1, and TNF-α, whose concentration in CSF positively correlated with the size and number of aneurysms.
Abbreviations
APTT, activated partial thromboplastin; bFGF, basic fibroblast growth factor; BMI, body mass index; CNS, central nervous system; CSF, cerebrospinal fluid; eGFR, estimated glomerular filtration rate; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte monocyte colony-stimulating factor; HCT, hematocrit; HGB, hemoglobin; IA, intracranial aneurysm; IFN-γ, interferon-γ; IL, interleukin; IL-1ra, IL-1 receptor antagonist; INR, International Normalized Ratio Time; IP-10, interferon gamma-induced protein 10; K+, potassium ion; M1, macrophages 1; MCP-1, monocyte chemoattractant protein-1; MIG, chemokine monokine induced by interferon (IFN)-γ; MIP-1-α, macrophage inflammatory protein-1α; MIP-1β, macrophage inflammatory protein-1β; MPV, mean platelet volume; mRNA, messenger ribonucleic acid; Na+, sodium ion; NK, natural killer; PDGF-BB, platelet-derived growth factor-BB; P-LCR, platelet‐large cell ratio; PLT, platelet count; PT, prothrombin time; RANTES, regulated on activation, normal T cell expressed and secreted; RBC, red blood cell count; SD, standard deviation; SMC, smooth muscle cells; Th, T helper cells; Tim-3, T-cell immunoglobulin and mucin domain-3; TNF-α, tumor necrosis factor-α; UIA, unruptured intracranial aneurysms; VEGF, vascular endothelial growth factor; WBC, white blood cells count.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author (J.K.) on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki and the protocol was approved by the Bioethics Human Research Committee of the Medical University of Bialystok (Permission No. APK.002.9.2021). All subjects gave their informed consent for inclusion before they participated in the study.
Acknowledgments
We are grateful to Martin Lenkiewicz, MSc and Jan Dobrodumow for their language assistance. We are grateful to Zenon Mariak, Karol Sawicki, Robert Chrzanowski, and Marek Jadeszko from the Department of Neurosurgery of the Clinical Hospital of the Medical University of Bialystok for their help with material collection. We are grateful to Piotr Abramowicz for his technical help with preparing figures.
Funding
This research received no external funding. This work was supported by the Medical University of Bialystok (research project no: SUB/1/DN/22/002/2209).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Aoki T, Nishimura M, Matsuoka T, et al. PGE 2-EP 2 signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol. 2011;163(6):1237–1249. doi:10.1111/j.1476-5381.2011.01358.x
2. Hashimoto T, Meng H, Young WL. Intracranial aneurysms: links among inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28(4):372–380. doi:10.1179/016164106X14973
3. Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659–1676. doi:10.1038/jcbfm.2012.84
4. Kamińska J, Lyson T, Chrzanowski R, et al. Ratio of IL-8 in CSF versus serum is elevated in patients with unruptured brain aneurysm. J Clin Med. 2020;9(6):1761. doi:10.3390/jcm9061761
5. Kamińska J, Dymicka-Piekarska V, Chrzanowski R, et al. Il-6 quotient (the ratio of cerebrospinal fluid il-6 to serum il-6) as a biomarker of an unruptured intracranial aneurysm. J Inflamm Res. 2021;14:6103–6114. doi:10.2147/JIR.S335618
6. Yang S, Liu Q, Yang J, Wu J, Wang S. Increased levels of serum IL-15 and TNF-β indicate the progression of human intracranial aneurysm. Front Aging Neurosci. 2022;14. doi:10.3389/fnagi.2022.903619
7. Liu Q, Zhang Y, Yang J, et al. The relationship of morphological-hemodynamic characteristics, inflammation, and remodeling of aneurysm wall in unruptured intracranial aneurysms. Transl Stroke Res. 2022;13(1):88–99. doi:10.1007/s12975-021-00917-1
8. Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019;47(1):E21. doi:10.3171/2019.5.FOCUS19234
9. Chen Z, Song S, Zhu J, Lai X. Regulatory mechanism of MiR-21 in formation and rupture of intracranial aneurysm through JNK signaling pathway-mediated inflammatory response. Int J Clin Exp Pathol. 2020;13(7):1834–1841.
10. Zhang H-F, Zhao M-G, Liang G-B, Song Z-Q, Li Z-Q. Expression of pro-inflammatory cytokines and the risk of intracranial aneurysm. Inflammation. 2013;36(6):1195–1200. doi:10.1007/s10753-013-9655-6
11. Aoki T, Kataoka H, Shimamura M, et al. NF-κB is a key mediator of cerebral aneurysm formation. Circulation. 2007;116(24):2830–2840. doi:10.1161/CIRCULATIONAHA.107.728303
12. Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013;44(9):2594–2597. doi:10.1161/STROKEAHA.113.002361
13. Aoki T, Fukuda M, Nishimura M, Nozaki K, Narumiya S. Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation. Acta Neuropathol Commun. 2014;2(1):34. doi:10.1186/2051-5960-2-34
14. Yi C, Katina W, He G, et al. Myeloperoxidase is increased in human cerebral aneurysms and increases formation and rupture of cerebral aneurysms in mice. Stroke. 2015;46(6):1651–1656. doi:10.1161/STROKEAHA.114.008589.Myeloperoxidase
15. Cheng WT, Wang N. Correlation between MMP-2 and NF-κ B expression of intracranial aneurysm. Asian Pac J Trop Med. 2013;6(7):570–573. doi:10.1016/S1995-7645(13)60098-X
16. Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40(3):942–951. doi:10.1161/STROKEAHA.108.532556
17. Hosaka K, Hoh BL. Inflammation and cerebral aneurysms. Transl Stroke Res. 2014;5(2):190–198. doi:10.1007/s12975-013-0313-y
18. Nowicki KW, Hosaka K, He Y, McFetridge PS, Scott EW, Hoh BL. Novel high-throughput in vitro model for identifying hemodynamic-induced inflammatory mediators of cerebral aneurysm formation. Hypertension. 2014;64(6):1306–1313. doi:10.1161/HYPERTENSIONAHA.114.03775
19. Nowicki KW, Hosaka K, Walch FJ, Scott EW, Hoh BL. M1 macrophages are required for murine cerebral aneurysm formation. J Neurointerv Surg. 2018;10(1):93–97. doi:10.1136/neurintsurg-2016-012911
20. Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53(4):399–406. doi:10.1097/00005072-199407000-00012
21. Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42(1):173–178. doi:10.1161/STROKEAHA.110.590976
22. Starke R, Chalouhi N, Ali M, et al. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013;10(3):247–255. doi:10.2174/15672026113109990003
23. Jayaraman T. TNF-α-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4(24):805–817. doi:10.2147/VHRM.S2700
24. Fukuda S, Hashimoto N, Naritomi H, et al. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation. 2000;101(21):2532–2538. doi:10.1161/01.CIR.101.21.2532
25. Pentimalli L, Modesti A, Vignati A, et al. Role of apoptosis in intracranial aneurysm rupture. J Neurosurg. 2004;101(6):1018–1025. doi:10.3171/jns.2004.101.6.1018
26. Oka M, Shimo S, Ohno N, et al. Dedifferentiation of smooth muscle cells in intracranial aneurysms and its potential contribution to the pathogenesis. Sci Rep. 2020;10(1):1–5. doi:10.1038/s41598-020-65361-x
27. Ducruet AF. Commentary on’Inflammatory changes in the aneurysm wall: a review’. J Neurointerv Surg. 2018;10(Suppl 1):i56–i56. doi:10.1136/neurintsurg-2018-014090
28. Tawk RG, Hasan TF, D’Souza CE, Peel JB, Freeman WD. Diagnosis and treatment of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. Mayo Clin Proc. 2021;96(7):1970–2000. doi:10.1016/j.mayocp.2021.01.005
29. Koper-Lenkiewicz OM, Sutkowska K, Wawrusiewicz-Kurylonek N, Kowalewska E, Matowicka-Karna J. Proinflammatory cytokines (IL-1, −6, −8, −15, −17, −18, −23, TNF-α) single nucleotide polymorphisms in rheumatoid arthritis—a literature review. Int J Mol Sci. 2022;23(4):2106. doi:10.3390/ijms23042106
30. Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi:10.1097/AIA.0b013e318034194e
31. Capobianco MP, Cassiano GC, da Cruz Furini AA, Storti de Melo LM, Bonini Domingos CR, Dantas Machado RL. Human interleukin 2 (IL-2) promotion of immune regulation and clinical outcomes: a review. J Cytokine Biol. 2016;01(02):14–17. doi:10.4172/2576-3881.1000109
32. Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50(4):796–811. doi:10.1016/j.immuni.2019.03.022
33. Hartgring SAY, Willis CR, Bijlsma JWJ, Lafeber FPJG, van Roon JAG. Interleukin-7-aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation. Arthritis Res Ther. 2012;14(3):R137. doi:10.1186/ar3870
34. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:1–12. doi:10.1155/2014/292376
35. Mello JDC, Gomes LEM, Silva JF, et al. The role of chemokines and adipokines as biomarkers of Crohn’s disease activity: a systematic review of the literature. Am J Transl Res. 2021;13(8):8561–8574.
36. Serna-Rodríguez MF, Bernal-Vega S, de la Barquera JAO-S, Camacho-Morales A, Pérez-Maya AA. The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation. J Neuroimmunol. 2022;371:577951. doi:10.1016/j.jneuroim.2022.577951
37. Cuneo A, Autieri M. Expression and function of anti-inflammatory interleukins: the other side of the vascular response to injury. Curr Vasc Pharmacol. 2009;7(3):267–276. doi:10.2174/157016109788340721
38. Kao HW, Lee KW, Kuo CL, et al. Interleukin-6 as a prognostic biomarker in ruptured intracranial aneurysms. PLoS One. 2015;10(7):6–13. doi:10.1371/journal.pone.0132115
39. Hendryk S, Jarzab B, Josko J. Increase of the IL-1 beta and IL-6 levels in CSF in patients with vasospasm following aneurysmal SAH. Neuro Endocrinol Lett. 2004;25(1–2):141–147.
40. Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N. Impaired progression of cerebral aneurysms in interleukin-1β-deficient mice. Stroke. 2006;37(3):900–905. doi:10.1161/01.STR.0000204028.39783.d9
41. Aoki T, Yamamoto K, Fukuda M, Shimogonya Y, Fukuda S, Narumiya S. Sustained expression of MCP-1 by low wall shear stress loading concomitant with turbulent flow on endothelial cells of intracranial aneurysm. Acta Neuropathol Commun. 2016;4(1):48. doi:10.1186/s40478-016-0318-3
42. Signorelli F, Sela S, Gesualdo L, et al. Hemodynamic stress, inflammation, and intracranial aneurysm development and rupture: a systematic review. World Neurosurg. 2018;115:234–244. doi:10.1016/j.wneu.2018.04.143
43. Signorelli F, Turjman F, Gory B, Labeyrie P-E, Pelissou-Guyotat I, Riva R. Hemodynamics, inflammation, vascular remodeling, and the development and rupture of intracranial aneurysms: a review. Neuroimmunol Neuroinflamm. 2015;2(2):59. doi:10.4103/2347-8659.154885
44. Signorelli F, Pailler-Mattei C, Gory B, et al. Biomechanical characterization of intracranial aneurysm wall: a multiscale study. World Neurosurg. 2018;119:e882–e889. doi:10.1016/j.wneu.2018.07.290
45. Koper OM, Kamińska J, Grygorczuk S, Zajkowska J, Kemona H. CXCL9 concentrations in cerebrospinal fluid and serum of patients with tick-borne encephalitis. Arch Med Sci. 2018;14(2):313–320. doi:10.5114/aoms.2016.58667
46. Koper-Lenkiewicz OM, Kamińska J, Milewska A, et al. Serum and cerebrospinal fluid Neudesin concentration and Neudesin quotient as potential circulating biomarkers of a primary brain tumor. BMC Cancer. 2019;19(1):319. doi:10.1186/s12885-019-5525-4
47. Koper OM, Kamińska J, Sawicki K, et al. Cerebrospinal fluid and serum IL-8, CCL2, and ICAM-1 concentrations in astrocytic brain tumor patients. Ir J Med Sci. 2018;187(3):767–775. doi:10.1007/s11845-017-1695-8
48. Cao Y, Zhao J, Wang S, Zhong H, Wu B. Monocyte chemoattractant protein-1 mRNA in human intracranial aneurysm walls. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36(7):519–521. doi:10.1136/jnnp-2011-302068
49. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi:10.1038/nri1882
50. Labeyrie P-E, Goulay R, Martinez de Lizarrondo S, et al. Vascular tissue-type plasminogen activator promotes intracranial aneurysm formation. Stroke. 2017;48(9):2574–2582. doi:10.1161/STROKEAHA.117.017305
51. Zhang H-F, Zhao M-G, Liang G-B, et al. Dysregulation of CD4 + T cell subsets in intracranial aneurysm. DNA Cell Biol. 2016;35(2):96–103. doi:10.1089/dna.2015.3105
52. Dymicka-Piekarska V, Koper-Lenkiewicz OM, Zińczuk J, Kratz E, Kamińska J. Inflammatory cell-associated tumors. Not only macrophages (TAMs), fibroblasts (TAFs) and neutrophils (TANs) can infiltrate the tumor microenvironment. The unique role of tumor associated platelets (TAPs). Cancer Immunol Immunother. 2020;70:1497–1510. doi:10.1007/s00262-020-02758-7
53. Miyata H, Koseki H, Takizawa K, et al. T cell function is dispensable for intracranial aneurysm formation and progression. PLoS One. 2017;12(4):1–13. doi:10.1371/journal.pone.0175421
54. Sawyer DM, Amenta PS, Medel R, Dumont AS. Inflammatory mediators in vascular disease: identifying promising targets for intracranial aneurysm research. Mediators Inflamm. 2015;2015:1–10. doi:10.1155/2015/896283
55. Sawyer DM, Pace LA, Pascale CL, et al. Lymphocytes influence intracranial aneurysm formation and rupture: role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. J Neuroinflammation. 2016;13(1):185. doi:10.1186/s12974-016-0654-z
56. Fan XJ, Zhao HD, Yu G, Zhong XL, Yao H, Yang QD. Role of inflammatory responses in the pathogenesis of human cerebral aneurysm. Genet Mol Res. 2015;14(3):9062–9070. doi:10.4238/2015.August.7.15
57. Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi:10.1016/S0065-2776(08)60535-0
58. Jinfang Z. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–29. doi:10.1016/j.cyto.2015.05.010.T
59. Ye D, Wang Z, Ye J, et al. Interleukin-5 levels are decreased in the plasma of coronary artery disease patients and inhibit Th1 and Th17 differentiation in vitro. Rev Esp Cardiol. 2020;73(5):393–402. doi:10.1016/j.recesp.2019.07.013
60. Zhang H-F, Liang G-B, Zhao M-G, Zhao G-F, Luo Y-H. Regulatory T cells demonstrate significantly increased functions following stimulation with IL-2 in a Tim-3-dependent manner in intracranial aneurysms. Int Immunopharmacol. 2018;65:342–347. doi:10.1016/j.intimp.2018.10.029
61. Read KA, Powell MD, McDonald PW, Oestreich KJ. IL-2, IL-7, and IL-15: multistage regulators of CD4+ T helper cell differentiation. Exp Hematol. 2016;44(9):799–808. doi:10.1016/j.exphem.2016.06.003
62. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi:10.1038/nri3156
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.