Back to Journals » Journal of Inflammation Research » Volume 16
The Systemic Inflammation Score is Associated with the Survival of Patients with Prostate Cancer
Authors Xie J, Xiao X, Dong Z, Wang Q
Received 6 August 2022
Accepted for publication 2 February 2023
Published 7 March 2023 Volume 2023:16 Pages 963—975
DOI https://doi.org/10.2147/JIR.S385308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jie Xie,* Xu Xiao,* Zhenjia Dong, Qiangdong Wang
Department of Urology, the Fifth People’s Hospital of Huai’an, Huai’an City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiangdong Wang, Email [email protected]
Background: The systemic inflammation score (SIS) based on the albumin (Alb) level and lymphocyte-to-monocyte ratio (LMR), has been associated with survival in some cancers. However, its prognostic role in prostate cancer (PCa) remains unclear.
Methods: The associations between the SIS and the clinicopathological features of PCa were evaluated. The correlations between the SIS and overall survival (OS) and progression-free survival (PFS) were assessed using Kaplan-Meier analysis and the Log rank test. Univariate and multivariate Cox analyses were conducted to determine the prognostic factors for PCa. Hazard ratios and 95% confidence intervals were calculated.
Results: A total of 253 patients with PCa were included in this study. The Kaplan-Meier analysis and Log rank test suggested that patients with a higher Alb level, higher LMR, or a lower SIS had better 5-year OS and PFS compared with patients with a lower Alb level or lower LMR or higher SIS. Univariate and multivariate Cox analyses showed that drinking, prostate-specific antigen level > 100 ng/mL, and neutrophil-to-lymphocyte ratio > 2.09 were significant prognostic factors for OS and PFS in patients with PCa. Nomograms for 5-year OS and PFS were established with concordance index values of 0.888 and 0.824, respectively. The calibration curve was consistent between the actual observations and the prediction nomogram for OS and PFS probability at 5 years.
Conclusion: A high SIS is associated with unfavorable survival in patients with PCa. The SIS serves as a novel independent prognostic factor for OS in patients with PCa.
Keywords: systemic inflammation score, prostate cancer, progression-free survival, overall survival
Introduction
Prostate cancer (PCa) is a malignant tumor that occurs in males and affects millions of patients globally.1 PCa has the fourth highest incidence of all cancers worldwide.2 Prostate biopsies, imaging, and biomarkers are used to diagnose PCa.3 Approximately 1.3 million new PCa cases are diagnosed worldwide annually.4 Approximately 10 million people are currently diagnosed with PCa, of which approximately 700,000 have metastatic disease.5,6 Despite substantial advances in treatment for PCa, the prognosis of metastatic PCa is frustrating.7 Thus, novel prognostic biomarkers are needed to identify PCa patients with a higher probability of an adverse prognosis and to help to make an appropriate treatment plan.
Persistent chronic inflammation increases cancer risk and promotes the formation of tumors.8,9 Tumor-associated inflammation initiates tumorigenesis and drives malignant progression.10,11 A host of hematological inflammatory biomarkers, including the albumin (Alb) level, neutrophil, lymphocyte, and monocyte counts, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) are predictive markers in cancer patients.12–18 Tests for these biomarkers are available in routine clinical practice and help clinicians evaluate the clinical outcomes and survival of cancer patients. A new marker, called the systemic inflammation score (SIS), which is based on the Alb level and LMR, has been proposed.19 The SIS reflects nutrition and inflammation status, which was significantly associated with the clinical outcomes and prognosis of cancer.20–22 The preoperative SIS was shown to be an effective prognostic factor in many cancers.19,23–26 However, studies on the ability of the SIS system to predict the survival of PCa patients have not been reported. Thus, in this study, we elucidated the prognostic effect of the pretherapeutic SIS in patients with PCa in a Chinese Han population.
Patients and Methods
Subjects
In total, 253 patients with PCa were recruited from our hospital between January 2016 and December 2018. Inclusion criteria were as follows: 1. PCa patients came from Jiangsu province; 2. PCa was diagnosed by histology; 3. the survival time was more than one year. Exclusion criteria were as follows: 1. patients who received chemotherapy; 2. patients without complete laboratory parameters; 3. patients who was lost to follow-up; 4. patients with immunodeficiency diseases. Clinicopathological parameters for patients with PCa were collected from medical records. The blood test data were taken at the time of the initial visit before the biopsy. Informed consent was provided by all participants. This study was approved by the Ethics Committee of The Fifth People’s Hospital of Huai’an and was conducted following the Declaration of Helsinki.
Definition of Parameters
The definitions of NLR, PLR, LMR, and PNI were defined as follows: NLR = neutrophil/lymphocyte counts; LMR = lymphocyte/monocyte counts, PLR = platelet/lymphocyte counts, and prognostic nutritional index (PNI) = Alb (g/L) + 5 × total lymphocyte counts (109/L). The median Alb, NLR, PLR, PNI, and LMR values were 40.3 g/L, 2.09, 109.68, 49.1, and 4.05, respectively.
The SIS was established by determining the serum Alb level and LMR. The Alb level and LMR were analyzed as categorical variables and were dichotomized based on the median values of 40.3 g/L and 4.05, respectively. A SIS of 0 was defined as LMR ≥ 4.05 and Alb level ≥ 40.3 g/L, SIS of 1 as either Alb level ≥ 40.3 g/L or LMR ≥ 4.05, and SIS of 2 as Alb level < 40.3 g/L and LMR < 4.05 (Table 1). We divided the PCa patients according to the SIS into the low (score of 0 or 1) and high (score of 2) groups.
 |
Table 1 Evaluation Criteria of Systemic Inflammation Score |
Follow-Up
Overall survival (OS) was defined as the interval from the time of diagnosis to the time of death. Progression-free survival (PFS) was defined as the interval from the start of treatment to the date of progression, recurrence, or death. The follow-up investigation was performed every month during the first year, every three months during the second year, and every 6 months during the following years. Follow-up was conducted from January 2016 to December 2021.
Statistical Analysis
Continuous variables were evaluated by Shapiro–Wilk test for the normality tests. Continuous variables were compared using the Mann–Whitney U-test for non-normally distributed variables or Student’s t-test for normally distributed variables. The categorical variables were calculated using Fisher’s exact test or the chi square test, as appropriate. Univariate and multivariate Cox regression analyses were used to assess the prognostic factors for OS and PFS. The Log rank test and Kaplan-Meier method were to compare the survival curves of OS and PFS among the groups. Hazard ratios (HRs) and relative 95% confidence intervals (CIs) were calculated. Nomograms were established to predict the OS and PFS rates. The concordance index was used to evaluate the accuracy of the nomogram in predicting OS and PFS; the calibration curves were calculated to compare the consistency between the observed and predicted survivals. A two-tailed P-value < 0.05 indicated significant differences. SPSS software (version 21.0; SPSS Inc, Chicago, IL, USA), R (version 4.1.3) software, and MedCalc version 20 were used to analyze data.
Results
Clinical Characteristics of PCa
Patients with PCa were assessed for eligibility (n=276), and 253 patients with PCa were included (Figure 1). Table 2 presents the baseline clinicopathological characteristics of the PCa patients, including age, body mass index (BMI), drinking and smoking activity, hypertension, diabetes mellitus, tumor node metastasis (TNM) stage, ISUP grade, and treatments. In addition, pretherapeutic parameters, including the prostate-specific antigen (PSA) level, monocyte/neutrophil/platelet/lymphocyte counts, Alb level, NLR, PLR, PNI, LMR, and SIS were obtained. Regarding the SIS, 60, 117, and 76 PCa patients scored 0, 1, and 2 on the SIS system, respectively.
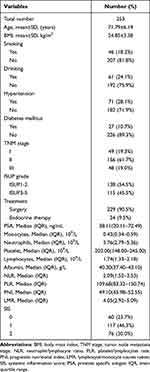 |
Table 2 Baseline Clinicopathological Characteristics of Prostate Cancer |
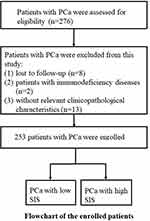 |
Figure 1 The flow diagram of this study. |
SIS and Patient Characteristics
The associations between the pretherapeutic SIS and baseline characteristics are described in Table 3. Patients with PCa were divided into low- and high-SIS groups according to the SIS. The high SIS group had high levels of PSA, monocytes, neutrophils, NLR, and PLR, but low levels of lymphocytes, Alb, PNI, and LMR, compared with the low SIS group. No differences in age, BMI, drinking, smoking, hypertension, ISUP grade, diabetes mellitus, TNM stage, or treatments were observed between the low and high SIS groups.
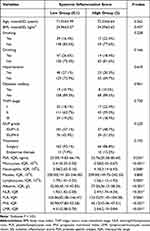 |
Table 3 Comparison of Baseline Clinicopathological Characteristics Based on Systemic Inflammation Score Status |
Associations of the SIS with Other Parameters and Survival in PCa
Patients with PCa were divided into the low and high SIS groups. Kaplan–Meier analysis indicated that PCa patients with an Alb level > 40.3 g/L (Figure 2) or LMR > 4.05 (Figure 3A, OS; Figure 3B, PFS) had a good prognosis. Patients with a low SIS had a better OS (P < 0.001, Figure 4A) and PFS (P = 0.001, Figure 4B) compared with those with a high SIS. In addition, a NLR < 2.09 (Supplementary Figure 1), PLR < 109.68 (Supplementary Figure 2), and PNI > 49.1 (Supplementary Figure 3) were associated with a better OS and PFS.
 |
Figure 2 Kaplan-Meier analysis for (A) OS and (B) PFS of PCa patients according to Alb. |
 |
Figure 3 Kaplan-Meier analysis for (A) OS and (B) PFS of PCa patients according to LMR. |
 |
Figure 4 Kaplan-Meier analysis for (A) OS and (B) PFS of PCa patients according to SIS. |
Association Between the SIS and the Prognosis of PCa
We determined whether the SIS was an independent predictor of OS and PFS in patients with PCa. Univariate and multivariate Cox analyses suggested that a high SIS (SIS = 2) was independently associated with a poorer OS (Table 4). Drinking, PSA level > 100 ng/mL, and NLR > 2.09 were associated with OS. The univariate Cox analysis revealed that a high SIS (SIS = 2) was significantly associated with a poorer PFS, but this association was not seen in the multivariate Cox analysis (Table 5). In addition, univariate and multivariate Cox analyses suggested that drinking, PSA level >100 ng/mL, and NLR > 2.09 were independently associated with a poorer PFS.
 |
Table 4 Univariate and Multivariate COX Regression Analysis for OS in Prostate Cancer |
 |
Table 5 Univariate and Multivariate COX Regression Analysis for PFS in Prostate Cancer |
Predictive Accuracy of the Prognostic Nomogram for OS and PFS
To predict OS (Figure 5A) and PFS (Figure 5B) in patients with PCa, nomograms were prepared using R software according to the results of the multivariate Cox analysis. Prognostic factors (drinking, PSA, SIS, and NLR) were included to develop the nomograms and predict the 5-year survival (Figure 5). The concordance index in the OS and PFS nomograms were 0.888 and 0.824, suggesting good predictive accuracy for the PCa prognosis. The calibration curve showed that the 5-year OS and PFS predictions were nearly consistent with the actual observations (Figure 6A and B).
 |
Figure 5 (A) Nomogram for 5-year OS of PCa; (B) Nomogram for 5-year PFS of PCa. |
 |
Figure 6 The calibration curves between predicted and observed PFS and OS outcomes. (A) The 5-year calibration curve for OS. (B) The 5-year calibration curve for PFS. |
Discussion
Our study showed that the SIS was associated with PSA, monocytes, neutrophils, NLR, PLR, lymphocytes, Alb, PNI, and LMR. The Kaplan–Meier analysis suggested that patients with a low SIS had better OS and PFS compared with those with a high SIS. Univariate and multivariate Cox analyses revealed that a high SIS was independently associated with a poorer OS in patients with PCa. To the best of our knowledge, this is the first study to determine that the SIS is a prognostic factor in patients with PCa.
The SIS is based on the Alb level and LMR,19 which can be easily tested in any clinical laboratory. This scoring system could reflect the host’s nutritional and inflammatory conditions. The SIS has been regarded as a novel risk stratification biomarker in a variety of cancers.
Chang et al first developed this SIS system to evaluate the prognosis of clear-cell renal cell carcinoma patients undergoing nephrectomy.19 They showed that a high SIS was a negative prognostic factor for OS.19 Then, Suzuki et al indicated that the SIS was an independent prognostic factor for OS in colorectal cancer (CRC) patients.23 However, this finding was inconsistent with other studies on CRC. Feng et al found that a higher SIS was an unfavorable predictor of OS, but not DFS, in patients with rectal cancer.24 Galizia et al suggested that the SIS system was not associated with the survival of CRC patients in a multivariate analysis.27 In addition, two studies25,26 utilized a modified SIS system with different cut-off values of Alb and LMR among CRC patients, which differed from the SIS system developed by Chang et al.19 However, those studies obtained conflicting results regarding OS.25,26 Martínez-Lago et al showed that the SIS was a prognostic factor for OS,26 while Wang et al found that the SIS was not associated with the survival of CRC.25 Several studies have used the SIS or modified SIS system to evaluate the prognosis of gastric cancer (GC). All indicated that a higher SIS was associated with poorer survival in GC patients.28–34 Additionally, the SIS system was related to survival in other cancers, including non-small-cell lung cancer,35 pancreatic cancer,36 cervical cancer,37 and breast cancer.38 However, to date, the utility of the SIS system in predicting the survival of PCa has never been evaluated.
To the best of our knowledge, this was the first study to assess the prognostic significance of the SIS system in patients with PCa. We modified Chang’s original SIS system19 by applying pretherapeutic cut-off values of the Alb level (40.3 g/L) and LMR (4.05). We divided all PCa patients into two groups according to their SIS (low SIS = 0 or 1; high SIS = 2). In this study, we found that a high SIS was a prognostic factor for adverse OS in patients with PCa, which was partly in line with findings for other cancers.19,26,28,35 We assumed that a higher SIS reflected persistent inflammation and impaired nutrition, which negatively affected PCa prognosis.
The SIS system is based on three laboratory markers: the Alb level and monocyte and lymphocyte counts. PCa with SIS=2 showed the worst prognosis in this study, which could be attributed to lymphocytopenia, monocytosis, and hypoalbuminemia they were suffering. We would explain this phenomenon from the following aspects. One, adaptive immunity is mediated by adaptive immune cells, called lymphocytes, which are significantly associated with tumor immunity. Studies have demonstrated that the lymphocyte count was associated with clinical outcomes and prognosis in PCa.39–42 A decreased lymphocyte count may cause an ineffective immune response to cancer progression.43,44 Two, monocyte are innate immune cells in the mononuclear phagocyte system that have emerged as pivotal regulators in cancer development and progression.45 Monocytes could be recruited to cancer tissues and differentiate into cancer-associated macrophages. These macrophages were reported to promote the proliferation and migration of tumor cells.46 Thus, a lower LMR was an indicator of a poor prognosis in many cancers.14,47–49 Three, the Alb level is closely associated with nutritional status, which could indicate immune status in cancer. Serum Alb level was an excellent predictor of cachexia and malnutrition in cancer patients.50–52 Almasaudi et al showed that hypoalbuminemia was independently related to survival in patients with CRC.50 Based on the evidence provided by abovementioned studies, we believed that the SIS is a good prognostic factor for the survival of PCa patients.
The SIS better reflects systemic inflammation and malnutrition status simultaneously in PCa patients. The SIS helped identify high-risk PCa patients. Suitable symptomatic therapies and nutritional support are essential in PCa patients with a higher pretherapeutic SIS. Additionally, the SIS is easily determined, which may be beneficial for clinicians to decide the optimal treatment strategy for PCa patients based on the SIS system.
This study had inevitable limitations. One, our study was retrospective; thus, some parameters were missing. Further prospective studies to evaluate the effects of SIS on the survival of PCa are needed. Further prospective studies are needed to evaluate the usefulness of the SIS in predicting the survival of PCa patients. Two, all PCa patients were from the same province, which may have led to selection bias. Three, the sample size in this study was not large enough. Four, other immune-nutritional biomarkers such as the hemoglobin level,53 and C-reactive protein level54 were not evaluated in this study. Five, the cut-off LMR and Alb level values varied between this study and others, and this discrepancy should be considered.
Totally, this study demonstrates that the SIS is significantly associated with the prognosis of patients with PCa. The SIS could serve as a prognostic indicator for OS in patients with PCa.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was approved by the Ethics Committee of The Fifth People’s Hospital of Huai’an, and was in according with the Helsinki declaration.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rebello RJ, Oing C, Knudsen KE, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7(1):9. doi:10.1038/s41572-020-00243-0
2. Bray F, Ferlay J. Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313. doi:10.3322/caac.21609
3. Lomas DJ, Ahmed HU. All change in the prostate cancer diagnostic pathway. Nat Rev Clin Oncol. 2020;17(6):372–381. doi:10.1038/s41571-020-0332-z
4. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398(10305):1075–1090. doi:10.1016/S0140-6736(21)
5. Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi:10.1016/S0140-6736(18)31694-5
6. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
7. Carneiro A, Baccaglini W, Glina FPA, et al. Impact of local treatment on overall survival of patients with metastatic prostate cancer: systematic review and meta-analysis. Int Braz J Urol. 2017;43(4):588–599. doi:10.1590/S1677-5538.IBJU.2016.0483
8. Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, Mechanisms, and Consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
9. Todoric J, Karin M. The Fire within: cell-Autonomous Mechanisms in Inflammation-Driven Cancer. Cancer Cell. 2019;35(5):714–720. doi:10.1016/j.ccell.2019.04.001
10. Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261–279. doi:10.1038/s41571-020-00459-9
11. Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974–982. doi:10.1038/s41590-020-0741-2
12. Tokumaru Y, Oshi M, Murthy V, et al. Low intratumoral genetic neutrophil-to-lymphocyte ratio (NLR) is associated with favorable tumor immune microenvironment and with survival in triple negative breast cancer (TNBC). Am J Cancer Res. 2021;11(11):5743–5755.
13. Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Transl Lung Cancer Res. 2021;10(2):866–877. doi:10.21037/tlcr-20-997
14. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
15. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram Based on Lactate Dehydrogenase-to-Albumin Ratio (LAR) and Platelet-to-Lymphocyte Ratio (PLR) for Predicting Survival in Nasopharyngeal Carcinoma. J Inflamm Res. 2021;14:4019–4033. doi:10.2147/JIR.S322475
16. Gupta V, Chaudhari V, Shrikhande SV, Bhandare MS. Does Preoperative Serum Neutrophil to Lymphocyte Ratio (NLR), Platelet to Lymphocyte Ratio (PLR), and Lymphocyte to Monocyte Ratio (LMR) Predict Prognosis Following Radical Surgery for Pancreatic Adenocarcinomas? Results of a Retrospective Study. J Gastrointest Cancer. 2021. doi:10.1007/s12029-021-00683-1
17. Guo Y, Wei L, Patel SH, et al. Serum Albumin: early Prognostic Marker of Benefit for Immune Checkpoint Inhibitor Monotherapy But Not Chemoimmunotherapy. Clin Lung Cancer. 2022. doi:10.1016/j.cllc.2021.12.010
18. Leibowitz-Amit R, Israel A, Gal M, et al. Association between the Absolute Baseline Lymphocyte Count and Response to Neoadjuvant Platinum-based Chemotherapy in Muscle-invasive Bladder Cancer. Clin Oncol. 2016;28(12):790–796. doi:10.1016/j.clon.2016.07.007
19. Chang Y, An H, Xu L, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–633. doi:10.1038/bjc.2015.241
20. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. doi:10.1038/ni.3754
21. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
22. Marazzi I, Greenbaum BD, Low DHP, Guccione E. Chromatin dependencies in cancer and inflammation. Nat Rev Mol Cell Biol. 2018;19(4):245–261. doi:10.1038/nrm.2017.113
23. Suzuki Y, Okabayashi K, Hasegawa H, et al. Comparison of Preoperative Inflammation-based Prognostic Scores in Patients With Colorectal Cancer. Ann Surg. 2018;267(3):527–531. doi:10.1097/SLA.0000000000002115
24. Feng Y, Liu L, Zhu Y. Systemic inflammation score in locally advanced rectal cancer patients following total mesorectal excision. Onco Targets Ther. 2019;12:6617–6622. doi:10.2147/OTT.S213720
25. Wang F, He W, Jiang C, et al. Prognostic value of inflammation-based scores in patients receiving radical resection for colorectal cancer. BMC Cancer. 2018;18(1):1102. doi:10.1186/s12885-018-4842-3
26. Martinez-Lago N, Fernandez-Montes A, Covela M, et al. Effect of antiangiogenic-based treatment and systemic inflammatory factors on outcomes in patients with BRAF v600-mutated metastatic colorectal cancer: a real-world study in Spain. BMC Cancer. 2021;21(1):64. doi:10.1186/s12885-020-07758-5
27. Galizia G, Lieto E, Auricchio A, et al. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, is an Independent Predictor of Long-term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis Colon Rectum. 2017;60(12):1273–1284. doi:10.1097/DCR.0000000000000961
28. Sato B, Kanda M, Tanaka C, et al. Significance of Preoperative Systemic Inflammation Score in Short-Term and Long-Term Outcomes of Patients with Pathological T2-4 Gastric Cancer After Radical Gastrectomy. World J Surg. 2018;42(10):3277–3285. doi:10.1007/s00268-018-4597-7
29. Lin JX, Lin JP, Xie JW, et al. Prognostic importance of the preoperative modified systemic inflammation score for patients with gastric cancer. Gastric Cancer. 2019;22(2):403–412. doi:10.1007/s10120-018-0854-6
30. Ma M, Weng M, Chen F, et al. Systemic inflammation score is a prognostic marker after curative resection in gastric cancer. ANZ J Surg. 2019;89(4):377–382. doi:10.1111/ans.15103
31. Hara K, Aoyama T, Yamada T, et al. The Prognostic Value of the Perioperative Systemic Inflammation Score for Patients With Advanced Gastric Cancer. Anticancer Res. 2020;40(3):1503–1512. doi:10.21873/anticanres.14095
32. Chen YR, Chen YL, Ouyang SS, et al. Prognostic efficacy of preoperative mGPS, SIS and LCS in patients with gastric cancer. Clin Chim Acta. 2020;511:81–89. doi:10.1016/j.cca.2020.09.027
33. Lin JX, Huang YQ, Wang ZK, et al. Prognostic importance of dynamic changes in systemic inflammatory markers for patients with gastric cancer. J Surg Oncol. 2021;124(3):282–292. doi:10.1002/jso.26498
34. Inagaki K, Kanda M, Nakanishi K, et al. Accurate Prediction of Prognosis After Radical Resection of Gastric Cancer by the Modified Systemic Inflammation Score; a Multicenter Dataset Analysis. World J Surg. 2021;45(8):2513–2520. doi:10.1007/s00268-021-06138-9
35. Li S, Zhang W, Yang Z, Li Y, Du H, Che G. Systemic Inflammation Score as a Novel Prognostic Indicator for Patients Undergoing Video-Assisted Thoracoscopic Surgery Lobectomy for Non-Small-Cell Lung Cancer. J Invest Surg. 2021;34(4):428–440. doi:10.1080/08941939.2019.1641169
36. Markus M, Abendroth A, Noureddine R, et al. Combined systemic inflammation score (SIS) correlates with prognosis in patients with advanced pancreatic cancer receiving palliative chemotherapy. J Cancer Res Clin Oncol. 2021;147(2):579–591. doi:10.1007/s00432-020-03361-0
37. Xu M, Wu Q, Cai L, Sun X, Xie X, Sun P. Systemic Inflammatory Score predicts Overall Survival in patients with Cervical Cancer. J Cancer. 2021;12(12):3671–3677. doi:10.7150/jca.56170
38. Huang ZZ, Hua X, Song CG, et al. The Prognostic Prediction Value of Systemic Inflammation Score and the Development of a Nomogram for Patients With Surgically Treated Breast Cancer. Front Oncol. 2020;10:563731. doi:10.3389/fonc.2020.563731
39. Buigues C, Navarro-Martinez R, Sanchez-Martinez V, Serrano-Carrascosa M, Rubio-Briones J, Cauli O. Interleukin-6 and Lymphocyte Count Associated and Predicted the Progression of Frailty Syndrome in Prostate Cancer Patients Undergoing Antiandrogen Therapy. Cancers. 2020;12:7. doi:10.3390/cancers12071716
40. Guo Y, Shi D, Zhang J, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score is a Novel Significant Prognostic Factor for Patients with Metastatic Prostate Cancer Undergoing Cytoreductive Radical Prostatectomy. J Cancer. 2019;10(1):81–91. doi:10.7150/jca.27210
41. Yang ZR, Zhao N, Meng J, et al. Peripheral lymphocyte subset variation predicts prostate cancer carbon ion radiotherapy outcomes. Oncotarget. 2016;7(18):26422–26435. doi:10.18632/oncotarget.8389
42. Roviello G, Nardone V, Bonetta A, et al. Effects of Whole Pelvic Radiotherapy on the Distribution of Lymphocyte Subpopulations in Prostate Cancer Patients. Am J Clin Oncol. 2022;45(3):105–111. doi:10.1097/COC.0000000000000894
43. Xu X, Wang D, Chen W, et al. A nomogram model based on peripheral blood lymphocyte subsets to assess the prognosis of non-small cell lung cancer patients treated with immune checkpoint inhibitors. Transl Lung Cancer Res. 2021;10(12):4511–4525. doi:10.21037/tlcr-21-899
44. Lee YJ, Park YS, Lee HW, Park TY, Lee JK, Heo EY. Peripheral lymphocyte count as a surrogate marker of immune checkpoint inhibitor therapy outcomes in patients with non-small-cell lung cancer. Sci Rep. 2022;12(1):626. doi:10.1038/s41598-021-04630-9
45. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi:10.1002/JLB.4RI0818-311R
46. Raposo TP, Pires I, Carvalho MI, Prada J, Argyle DJ, Queiroga FL. Tumour-associated macrophages are associated with vascular endothelial growth factor expression in canine mammary tumours. Vet Comp Oncol. 2015;13(4):464–474. doi:10.1111/vco.12067
47. Yasui K, Shida D, Nakamura Y, Ahiko Y, Tsukamoto S, Kanemitsu Y. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer. 2021;124(5):933–941. doi:10.1038/s41416-020-01189-6
48. Park JW, Chang HJ, Yeo HY, et al. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020;123(4):610–618. doi:10.1038/s41416-020-0924-5
49. Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139(1):220–231. doi:10.1002/ijc.30071
50. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers. 2020;12(7). doi:10.3390/cancers12071986
51. Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: nutritional marker or inflammatory surrogate? World J Gastrointest Surg. 2015;7(12):370–377. doi:10.4240/wjgs.v7.i12.370
52. Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36(5):1333–1338. doi:10.1016/j.clnu.2016.08.023
53. Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011;2(5):879–885. doi:10.3892/etm.2011.308
54. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC, Postoperative Systemic A. Inflammation Score Predicts Short- and Long-Term Outcomes in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg Oncol. 2017;24(4):1100–1109. doi:10.1245/s10434-016-5659-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
