Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Efficacy of Bevacizumab in High-Grade Meningiomas: A Retrospective Clinical Study
Received 6 April 2022
Accepted for publication 1 August 2022
Published 6 August 2022 Volume 2022:18 Pages 1619—1627
DOI https://doi.org/10.2147/NDT.S368740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xuexue Bai,1 Xiaomin Liu,2 Jun Wen1
1Neurosurgery, The First Affiliated Hospital, Jinan University, Guangzhou, People’s Republic of China; 2Neurosurgery, Tianjin Huanhu Hospital, Tianjin, People’s Republic of China
Correspondence: Xiaomin Liu; Jun Wen, Email [email protected]; [email protected]
Objective: We investigated the role of bevacizumab (BV) in high-grade meningiomas (HGMs) by retrospective analysis.
Methods: We retrospectively analyzed the clinical data of 139 patients with HGMs. The chi-square test was used to compare progression-free survival (PFS) and overall survival (OS) between patients who received BV and those who did not. According to whether they received BV treatment, we divided the patients into the BV group and non-BV group, and the effect of BV on PFS and OS was compared. In addition, we compared Karnofsky performance status (KPS) and steroid doses between the BV and non-BV groups.
Results: There were statistically differences in PFS and OS between the BV and non-BV groups at 12 and 36 months after surgery (P< 0.05). However, there was no significant difference in PFS and OS between the two groups at 60 months postoperatively (P> 0.05). Using survival curves drawn by the Kaplan Meier method, we found that the PFS and OS of the BV group were greater than those of the non-BV group, and the difference was statistically significant (P< 0.05).
Conclusion: BV could improve PFS and OS at 12 and 36 months after surgery in patients with HGMs. In addition, BV was associated with lower preoperative steroid use.
Keywords: bevacizumab, meningiomas, peritumoral brain edema, progression-free survival, overall survival
A Letter to the Editor has been published for this article.
Plain Language Summary
When we realized the miraculous efficacy of bevacizumab in gliomas, we wondered if it could be equally effective in high-grade meningiomas. Surprisingly, bevacizumab did improve survival in patients with meningiomas in the short term. The results may offer hope for patients who cannot be treated with surgery.
Introduction
Meningiomas are the most frequent intracranial tumors arising from the meninges of the brain, with a reported incidence that increases with age (median age of 65 years and an incidence of 7.86 per 100,000 population), and an overall 1% lifetime risk.1,2 According to the 2016 World Health Organization (WHO) Grading System, 80% of meningiomas are WHO I, 15–20% are WHO II and III, with the latter also called high-grade meningiomas (HGMs).1,2
Peritumoral brain edema (PTBE) is a common complication of meningiomas. PTBE may raise morbidity and mortality by increasing brain shift and intracranial pressure, making the surgical removal challenging, and it also is a predisposing factor to perioperative epilepsy.3,4 In addition, PTBE is a risk factor for postoperative intracranial hematoma and neurological deficit.5 Several studies have investigated mechanisms that support PTBE development in meningiomas.6 PTBE in meningiomas has been linked to the expression of aquaporin-4,7 aquaporin- 5,8 N-cadherin and β-catenin,9 collagen XVIII,10 matrix metalloproteinase-9,11 and vascular endothelial growth factor A (VEGF-A).12 In this process of tumor neovascularization, the signal protein VEGF contributes a vital role.13 Bevacizumab (BV) was engineered as a humanized monoclonal antibody to VEGF receptors and interferes with the binding and signal transduction necessary for tumor vascularization leading to a regression of tumor blood supply.14 Clinical trials15,16 have shown that BV, a monoclonal antibody against VEGF-A, provides an effective treatment for brain edema. Currently, several studies show promise regarding the efficacy of targeting VEGF‐regulated angiogenesis in meningiomas, especially in patients with higher-grade/recurrent meningiomas; however, data have not been conclusive.17,18 Therefore, we conducted a systematic review of clinical data of patients with HGMs to comprehensively evaluate the efficacy and safety of BV in the treatment of HGMs.
Methods
Patients
The inclusion criteria of patients were: (1) HGMs on pathological examination. (2) The patient had no history of other intracranial tumors. (3) Patients had radiographically confirmed cerebral edema, which was not relieved by treatment with steroids. (4) The patient does not have any history of anti-tumor treatment (5) The patient agrees to sign the written informed consent. Low-grade meningiomas have a good prognosis after craniotomy, so we did not include patients with low-grade meningiomas in this study. We retrospectively analyzed the clinical data of 139 patients with HGMs. The hospital academic ethics committee approved the study. This research follows the Declaration of Helsinki.
Imaging Examination
Edema volume was measured on FLAIR and T2-weighted imaging using a method previously described by Bitzer.19 The volume of cerebral edema is assumed to be an ellipsoid sphere. Therefore, V = π/6 × ABC calculates the volume. Volume is measured by drawing mutually perpendicular diameters (A & B) of the largest cross-section of cerebral edema in the axial plane and the maximum height of sagittal cerebral edema (C). These measurements are substituted into the formula above to complete the volume calculation.
Administration of BV
The recommended dose of BV in the previous literature is 5–10 mg/kg.16,20 A few believe that 15 mg/kg is reasonable.21 The usage of BV in this study was 10 mg/kg. The patient’s steroid refractory PTBE prompted us to use BV for clinical relief. All patients are suitable for BV therapy. However, it is ultimately up to the patient to decide whether to accept BV treatment. Considering the longer half-life of BV, patients in the BV group completed BV treatment one week before and two weeks after surgery, respectively.
Follow-Up Data
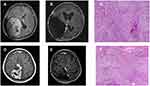
Overall survival (OS) represents from the first surgery to the last follow-up or death. Progression-free survival (PFS) is from the date of the first surgery to the date of recurrence or the last follow-up. Patients in the BV group received BV one week before and two weeks after surgery. We scored all patients with Karnofsky Performance Status (KPS) one week before surgery, one day before surgery, one week after surgery, and three weeks after surgery. In addition, we counted the steroid usage in the same period. All patients with meningioma underwent craniotomy for tumor resection. We evaluated the extent of surgical resection according to the Simpson grading scale.22 Gross total resection (GTR) represents Simpson grades I and II, and sub-total resection (STR) is Simpson grades III, IV, and V. Tumor recurrence indicates intracranial progression of the tumor at the primary and local sites. Follow-up MRI once a year after surgery. Figure 1 shows imaging and pathological results of HGMs. (Figure 1)
Statistical Analyses
Continuous variables were expressed as Median ± standard deviation (Median ± SD), and categorical variables were expressed as number (n) or percentage (%) of patients. The t-test or Wilcoxon rank-sum test was used to compare two groups of continuous variables, and the chi-square test or Fisher’s exact test was used to compare two groups of categorical variables. PFS and OS were compared between BV and non-BV groups using the chi-square test. The survival curves of OS and PFS of the patients were plotted using the Kaplan-Meier method, and the OS and PFS of the patients in the BV group and the groups without BV were compared using the log-rank. Univariate and multivariate Cox regression models were used to analyze the independent risk factors of each factor on patients’ PFS and OS. We used SPSS (Version 26.0, IBM) to perform our statistical analyses. P values < 0.05 were considered statistically significant.
Results
The Demographic Characteristics
64 of the 139 patients ultimately received BV therapy. Baseline characteristics have no statistical differences between the BV and non-BV groups (Table 1). Tumor recurrence occurred in 61 patients, including 24 in the BV group and 37 in the non-BV group. Twenty-two patients underwent reoperation after tumor recurrence, nine in the BV group and 13 in the non-BV group. Fifteen patients who relapsed received Gamma Knife radiosurgery (GRS), 8 in the BV group and 7 in the non-BV group. Eleven patients received reoperation combined with GRS after the recurrence of the lesions, 4 in the BV group and seven in the non-BV group. Thirteen patients refused treatment, including three in the BV group and 10 in the non-BV group.
 |
Table 1 Baseline Characteristics of All Patients |
Univariate and Multivariate Cox Regression Analysis
Univariate and multivariate Cox regression analysis showed that surgical resection extent (hazard ratio [HR]= 2.09, 95% confidence interval [CI], 1.15–3.81, P<0.05), pathological grade (HR= 0.56, 95% CI, 0.32–1.01, P <0.05) and PTBE (HR= 0.52, 95% CI, 0.29–0.94, P <0.05; Table 2) were independent risk factors for PFS in patients with HGMs. Meanwhile, the degree of surgical resection (HR=3.03, 95% CI, 1.27–7.24, P <0.05) and pathological grade (HR=0.47, 95% CI, 0.22–1.03, P <0.05; Table 3) were also independent risk factors for OS. Table 1 shows that there was no significant difference between the BV group and the non-BV group in the extent of surgical resection, PTBE and pathological grades (P>0.05).
 |
Table 2 Univariate and Multivariate Cox Regression Analysis for Progression-Free Survival |
 |
Table 3 Univariate and Multivariate Cox Regression Analysis for Overall Survival |
PFS and OS of the BV and the Non-BV Group
Table 4 reflects the PFS and OS levels of the BV and the non-BV group. Within six months after surgery, 3 patients in the BV group had tumor recurrence, and the progression-free survival at 6 months (PFS-6) was 95.3%, while 6 had tumor recurrence in the non-BV group, and the PFS-6 was 92.0% (P>0.05). At 12 months after surgery, the progression-free survival at 12 months (PFS-12) was 92.2% in the BV group and 80.0% in the non-BV group (P<0.05). At 36 months post-operatively, the progression-free survival at 36 months (PFS-36) was 84.4% in the BV group compared with 65.3% in the non-BV group (P<0.05). There was no statistical difference in the progression-free survival at 60 months (PFS-60) between the BV group and the non-BV group (P>0.05). Within 12 months after surgery, 1 patient died in the BV group and 8 in the non-BV group. The overall survival at 12 months (OS-12) were 98.4% and 89.3%, respectively (P<0.05). At 36 months after surgery, the overall survival at 36 months (OS-36) was 92.2% in the BV group and 80.0% in the non-BV group (P<0.05). There was no statistical difference in the overall survival at 60 months (OS-60) between the BV group and the non-BV group (P>0.05). The median PFS in the BV group was 35.83 ± 12.65 months compared to 24.63 ± 13.47 months in the non-BV group (P<0.05). The median OS was 41.25 ± 12.58 months in the BV group and 30.89 ± 14.10 months in the non-BV group (P<0.05). Therefore, we believe that BV can improve PFS and OS in patients with HGMs to some extent. Using survival curves drawn by the Kaplan Meier method, we found that the PFS and OS of the BV group were greater than those of the non-BV group, and the difference was statistically significant (P<0.05, Figure 2).
 |
Table 4 PFS and OS at Different Periods in BV and Non-BV Groups |
Changes in KPS and Steroid Dosage
Before BV treatment, the mean KPS of the BV group was 64.25±9.46, while that of the non-BV group was 61.89±11.58 (P>0.05). After one week of BV treatment, the KPS of the BV (75.42±8.09) group increased significantly, while the KPS of the non-BV (63.25±12.72) group had no change (P<0.05). One week after surgery, the KPS of the BV group was 84.37±7.03, while that of the non-BV group was 87.47±9.65 (P>0.05). There was no statistical difference in KPS between the BV group (92.45±2.98) and the non-BV group (95.46±3.47) 3 weeks after surgery (P>0.05). There were no significant differences in initial KPS and final KPS between the two groups. The KPS of the BV group was higher than that of the non-BV group after the first BV treatment, so BV may increase the KPS. There was no difference in KPS between the two groups after surgery. We believe that surgery is more effective in improving KPS than BV. Seven patients in this study cannot receive steroid treatment due to complications. All remaining patients received steroid therapy (40mg/d) to relieve symptoms of cerebral edema before enrollment. Despite the poor efficacy of steroids, we did not increase the dose due to possible complications. Patients in the BV group showed improvement in symptoms after BV treatment, so we reduced the steroid dose (20mg/d) for the BV group.
Tumor Recurrence
The 61 patients with tumor recurrence were divided into four groups based on the treatment method. Among the patients who received reoperation combined with GRS after tumor recurrence, the median OS of the BV group was 32.31±6.84 months, while that of the non-BV group was 33.62±4.69 months (P>0.05). Among patients who underwent reoperation, the OS was 30.51±5.27 months in the BV group and 22.49±7.75 months in the non-BV group (P<0.05). Among patients treated with GRS, the OS was 27.42±7.37 months in the BV group and 20.68±5.73 months in the non-BV group (P<0.05). Among patients who refused treatment, the OS was 10.47±4.52 months in the BV group and 8.63±3.48 months in the non-BV group (P>0.05). We found that OS in the BV group was significantly higher than that in the non-BV group in patients who underwent reoperation and GRS after tumor recurrence (P<0.05). The group that rejected treatment had the shortest OS, so we recommend that patients with HGMs receive aggressive treatment after tumor recurrence. We believe that BV can improve prognosis to a certain extent, but the benefit is significantly lower than surgery or GRS.
Adverse Reactions of BV
The adverse reactions of BV include hypertension, various bleeding, venous thrombus exfoliation, and albuminuria. In previous reports, all kinds of bleeding were 30%, including intracranial hemorrhage, epistaxis, gingival bleeding, conjunctival bleeding, injection-site bleeding, and hematuria.23 Besse reported in 2010 that the incidence of brain hemorrhage in patients with brain metastases after applicated BV was 0.8%-3.3%, while non-applicated was 1.0%.24 Khasraw reported in 2012 that the incidence of brain hemorrhage in patients with glioma or brain metastases after BV treatment was 3.7%, while the incidence of non-BV was 3.6%.25 Most studies reported a 1–10% rate of complications in wound healing for patients receiving BV who had previously undergone craniotomy for tumor resection or biopsy.26,27 Previous studies reported that the optimum time between cessation of BV therapy and surgery was 4 weeks. The timing for re-initiation of BV post-surgery was at least 2 weeks.28 The interval between surgical resection and BV in other studies ranged from 4 weeks to 18 months, but the complication rates were similar, suggesting that the risk of wound complications is always present in patients receiving BV.29–32 In our study, there was no significant difference in postoperative complications between the BV group and the non-BV group, which may be related to the fewer patients, the short course of treatment and the low dose of BV. Hypertension as the only complication occurred in 8 patients.
Discussion
Several older and newer antineoplastic agents have been tried in patients with relapsed or refractory meningioma after surgery or radiation. Disease stabilization was observed in some patients using these drugs, and only anecdotal evidence of significant tumor shrinkage was shown in isolated cases. Notwithstanding, their toxicity is not negligible, and the cost can be prohibitive. The VEGF-A isoform is an important regulator of angiogenesis.33 An angiogenic and an endothelial cell-specific mitogen, this molecule contributes to the creation of new blood vessels during embryonic life, after tissue injury, or to bypass blocked vessels via developing collateral circulation.34 VEGF-A, originally known as vascular permeability factor, acts as a vasodilator, increasing microvascular permeability.35 In addition, tumors expressing VEGF can grow and metastasize. PTBE is a consequence of high rates of angiogenesis in rapidly growing tumors and tissue hypoxia.12 PTBE has also been associated with increased risk of recurrence,36 increased Ki-67 proliferative index,37 and worse postoperative cognitive functioning38 and KPS.39 Meningiomas are highly vascularized tumors and, thus, potentially amenable to antiangiogenic therapy. A study by Lamszus et al found a correlation between VEGF content and meningioma grade, which was two times higher in atypical than in benign meningiomas and ten times higher in malignant than in benign meningiomas.34 These data suggest that antiangiogenic therapy may be particularly effective in HGMs.
A PFS-6 of greater than 40% for WHO I meningioma and greater than 30% for WHO II and III meningioma is currently viewed as an indicator of treatment efficacy.40 In 2015, Grimm et al presented Phase II clinical data of a cohort of 40 patients treated with BV for refractory meningioma after surgery or radiation therapy. PFS-6 was 87%, 77%, and 46% for WHO grade I, II, and III meningiomas, respectively.40 Another review demonstrated a median PFS of 16.8 months (range: 6.5‐22 months) and that 73% of patients were progression-free at six months (PFS‐6) (range: 44%‐93%).13 The PFS-6 in our study was superior to the above studies because surgery was the primary treatment, whereas BV was only used as an adjunct to surgery. Previous studies have mainly focused on PFS-6, while little attention has been paid to the long-term efficacy of BV in HGMs. Our study demonstrated that the PFS-12 and PFS-36 of the BV group were superior to those of the non-BV group (P<0.05). It provides new evidence for the long-term efficacy of BV in HGMs.
Approximately 15–20% of meningioma cases are WHO grade II or III that often demonstrate rapid growth, with cerebral or cerebellar invasion, or even remote metastases.2 Patients may present with headaches, dizziness, and frank seizures. These symptoms are due to local tumor expansion or PTBE. Steroids are a routine option for relieving symptoms of PTBE. Previous studies suggest that BV can improve the symptoms of steroid-refractory brain edema.41 After the first BV treatment, the patient’s clinical symptoms were relieved, so we reduced the steroid dose in the BV group. BV was associated with lower preoperative steroid use. For steroid-refractory brain edema, BV is an effective option.
The main limitation of our study is its retrospective nature. Second, this study is a single-center retrospective study. Therefore, in the follow-up study, we will conduct a multi-center, large sample prospective study with multiple medical centers to further demonstrate the value of BV in HGMs.
Conclusion
Previous studies have mainly focused on PFS-6 in patients with HGMs, while less attention has been paid to long-term efficacy. Our study provides new data on the long-term efficacy of BV in HGMs. This study retrospectively analyzed the clinical data of 139 patients and demonstrated that BV could improve PFS and OS at 12 and 36 months after surgery in patients with HGMs. In addition, BV can improve clinical symptoms before surgery and reduce steroid doses.
Ethics Approval
All persons gave their informed consent prior to their inclusion in the study. This study was performed in line with the principles of the Declaration of Helsinki. The academic and ethical committee of the First Affiliated Hospital of Jinan university approved the study.
Acknowledgments
The authors are grateful to all patients included in this study for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors did not receive support from any organization for the submitted work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
2. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. doi:10.1016/S1470-2045(17)30155-9
3. Lieu AS, Howng SL. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45–52. doi:10.1016/S0920-1211(99)00066-2
4. Vignes JR, Sesay M, Rezajooi K, et al. Peritumoral edema and prognosis in intracranial meningioma surgery. J Clin Neurosci. 2008;15(7):764–768. doi:10.1016/j.jocn.2007.06.001
5. Sindou MP, Alaywan M. Most intracranial meningiomas are not cleavable tumors: anatomic-surgical evidence and angiographic predictibility. Neurosurgery. 1998;42(3):476–480. doi:10.1097/00006123-199803000-00007
6. Gill CM, Loewenstern J, Rutland JW, et al. Peritumoral edema correlates with mutational burden in meningiomas. Neuroradiology. 2021;63(1):73–80. doi:10.1007/s00234-020-02515-8
7. Gawlitza M, Fiedler E, Schob S, et al. Peritumoral brain edema in meningiomas depends on aquaporin-4 expression and not on tumor grade, tumor volume, cell count, or Ki-67 labeling index. Mol Imaging Biol. 2017;19(2):298–304. doi:10.1007/s11307-016-1000-7
8. Lambertz N, Hindy NE, Adler C, et al. Expression of aquaporin 5 and the AQP5 polymorphism A(−1364) C in association with peritumoral brain edema in meningioma patients. J Neurooncol. 2013;112(2):297–305. doi:10.1007/s11060-013-1064-z
9. Rutkowski R, Chrzanowski R, Trwoga M, et al. Expression of N-cadherin and β-catenin in human meningioma in correlation with peritumoral edema. Int J Neurosci. 2018;128(9):805–810. doi:10.1080/00207454.2018.1424153
10. Salokorpi N, Yrjänä S, Tuominen H, et al. Expression of VEGF and collagen XVIII in meningiomas: correlations with histopathological and MRI characteristics. Acta Neurochir. 2013;155(6):989–996. doi:10.1007/s00701-013-1699-8
11. Reszec J, Hermanowicz A, Rutkowski R, et al. Expression of MMP-9 and VEGF in meningiomas and their correlation with peritumoral brain edema. Biomed Res Int. 2015;2015:646853. doi:10.1155/2015/646853
12. Hou J, Kshettry VR, Selman WR, et al. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013;35(6):E2. doi:10.3171/2013.8.FOCUS13301
13. Franke AJ, Skelton IV W, Woody L, et al. Role of bevacizumab for treatment-refractory meningiomas: a systematic analysis and literature review. Surg Neurol Int. 2018;9(1):133. doi:10.4103/sni.sni_264_17
14. Marty M, Pivot X. The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur J Cancer. 2008;44(7):912–920. doi:10.1016/j.ejca.2008.01.005
15. Lu VM, Ravindran K, Graffeo CS, et al. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J Neurooncol. 2019;144(2):239–248. doi:10.1007/s11060-019-03234-8
16. Pillay Smiley N, Alden T, Hartsell W, et al. Severe radiation necrosis successfully treated with bevacizumab in an infant with low-grade glioma and tumor-associated intractable trigeminal neuralgia. Pediatr Blood Cancer. 2016;63(9):1671–1673. doi:10.1002/pbc.26055
17. Pistolesi S, Boldrini L, Gisfredi S, et al. Angiogenesis in intracranial meningiomas: immunohistochemical and molecular study. Neuropathol Appl Neurobiol. 2004;30(2):118–125. doi:10.1046/j.0305-1846.2003.00516.x
18. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99(3):393–405. doi:10.1007/s11060-010-0343-1
19. Bitzer M, Opitz H, Popp J, et al. Angiogenesis and brain oedema in intracranial meningiomas: influence of vascular endothelial growth factor. Acta Neurochir. 1998;140(4):333–340. doi:10.1007/s007010050106
20. Hawasli AH, Rubin J, Tran D, et al. Antiangiogenic agents for nonmalignant brain tumors. J Neurol Surg B Skull Base. 2013;74(3):136–141. doi:10.1055/s-0033-1336173
21. Alanin MC, Klausen C, Caye-Thomasen P, et al. Effect of bevacizumab on intracranial meningiomas in patients with neurofibromatosis type 2 - A retrospective case series. Int J Neurosci. 2016;126(11):1002–1006. doi:10.3109/00207454.2015.1092443
22. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. doi:10.1136/jnnp.20.1.22
23. Kreisl TN, Zhang W, Odia Y, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13(10):1143–1150. doi:10.1093/neuonc/nor091
24. Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16(1):269–278. doi:10.1158/1078-0432.CCR-09-2439
25. Khasraw M, Holodny A, Goldlust SA, et al. Intracranial hemorrhage in patients with cancer treated with bevacizumab: the Memorial Sloan-Kettering experience. Ann Oncol. 2012;23(2):458–463. doi:10.1093/annonc/mdr148
26. Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–269. doi:10.1007/s11060-009-9957-6
27. Clark AJ, Butowski NA, Chang SM, et al. Impact of bevacizumab chemotherapy on craniotomy wound healing. J Neurosurg. 2011;114(6):1609–1616. doi:10.3171/2010.10.JNS101042
28. Abrams DA, Hanson JA, Brown JM, et al. Timing of surgery and bevacizumab therapy in neurosurgical patients with recurrent high grade glioma. J Clin Neurosci. 2015;22(1):35–39. doi:10.1016/j.jocn.2014.05.054
29. Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma. J Clin Oncol. 2008;26(6):1012–1013. doi:10.1200/JCO.2007.15.1605
30. Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. doi:10.1200/JCO.2010.30.2729
31. Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156–163. doi:10.1016/j.ijrobp.2008.10.043
32. Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110(1):173–180. doi:10.3171/2008.4.17492
33. Nassehi D, Dyrbye H, Andresen M, et al. Vascular endothelial growth factor A protein level and gene expression in intracranial meningiomas with brain edema. Apmis. 2011;119(12):831–843. doi:10.1111/j.1600-0463.2011.02764.x
34. Lamszus K, Lengler U, Schmidt NO, et al. Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery. 2000;46(4):938–947. doi:10.1097/00006123-200004000-00033
35. Panagopoulos AT, Lancellotti CLP, Veiga JCE, et al. Expression of cell adhesion proteins and proteins related to angiogenesis and fatty acid metabolism in benign, atypical, and anaplastic meningiomas. J Neurooncol. 2008;89(1):73–87. doi:10.1007/s11060-008-9588-3
36. Spille DC, Sporns PB, Heß K, et al. Prediction of high-grade histology and recurrence in meningiomas using routine preoperative magnetic resonance imaging: a systematic review. World Neurosurg. 2019;128:174–181. doi:10.1016/j.wneu.2019.05.017
37. Bečulić H, Skomorac R, Jusić A, et al. Correlation of peritumoral brain edema with morphological characteristics and Ki67 proliferative index in resected intracranial meningiomas. Acta Clin Croat. 2019;58(1):42–49. doi:10.20471/acc.2019.58.01.06
38. van Nieuwenhuizen D, Slot KM, Klein M, et al. The association between preoperative edema and postoperative cognitive functioning and health-related quality of life in WHO grade I meningioma patients. Acta Neurochir. 2019;161(3):579–588. doi:10.1007/s00701-019-03819-2
39. Loewenstern J, Aggarwal A, Pain M, et al. Peritumoral edema relative to meningioma size predicts functional outcomes after resection in older patients. Oper Neurosurg. 2019;16(3):281–291. doi:10.1093/ons/opy107
40. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. doi:10.1093/neuonc/not330
41. Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211–217. doi:10.1007/s11060-009-9948-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.