Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Serum Sulfonylurea Receptor-1 Levels After Acute Supratentorial Intracerebral Hemorrhage: Implication for Prognosis
Authors Zhuge CJ, Zhan CP, Wang KW, Yan XJ, Yu GF
Received 26 March 2022
Accepted for publication 25 May 2022
Published 3 June 2022 Volume 2022:18 Pages 1117—1126
DOI https://doi.org/10.2147/NDT.S368123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Cheng-Jun Zhuge,1 Cheng-Peng Zhan,2 Ke-Wei Wang,1 Xin-Jiang Yan,2 Guo-Feng Yu2
1The Second School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Department of Neurosurgery, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, People’s Republic of China
Correspondence: Guo-Feng Yu, Tel +86 570 3055179, Email [email protected]
Objective: Sulfonylurea receptor-1 (SUR1) is implicated in acute brain injury. This study was designed to determine relationship between serum SUR1 levels and severity, early neurologic deterioration (END) plus clinical outcome after intracerebral hemorrhage (ICH).
Methods: Serum SUR1 levels of 131 ICH patients and 131 healthy controls were quantified in this prospective, observational study. END was defined as an increase of 4 or more points in the National Institutes of Health Stroke Scale (NIHSS) score or death within 24 hours after admission. Patients with a modified Rankin scale (mRS) score of 3– 6 at 90 days following onset were considered to experience a poor outcome.
Results: Serum SUR1 levels were substantially higher in patients than in controls. Serum SUR1 levels of patients were highly correlated with NIHSS score, Glasgow Coma Scale score, hematoma volume and ICH score. Compared with patients with END or mRS score of 0– 2, other remainders had significantly elevated serum SUR1 levels. Serum SUR1 levels independently predicted END and 90-day poor outcome. Under receiver operating characteristic curve, serum SUR1 levels significantly predicted END and a poor outcome at 90 days after hemorrhagic stroke and its predictive value was similar to those of NIHSS score, Glasgow coma scale score, hematoma volume and ICH score.
Conclusion: Serum SUR1 levels are highly correlated with severity, END and poor outcome after hemorrhagic stroke, indicating that serum SUR1 may be useful for risk stratification and prognostic prediction of ICH.
Keywords: Sulfonylurea receptor-1, intracerebral hemorrhage, early neurologic deterioration, functional outcome, severity
Introduction
Spontaneous non-traumatic intracerebral hemorrhage (ICH) is very frequently caused by small vessel diseases of brain, mainly including hypertensive arteriopathy or cerebral amyloid angiopathy.1 Although the incidence of ICH is much lower than that of ischemic stroke, it causes higher stroke mortality and morbidity.2 National Institutes of Health Stroke Scale (NIHSS) score, Glasgow coma scale (GCS) score, hematoma volume and ICH score were very commonly used to assess hemorrhagic severity following ICH.3–6 Early neurologic deterioration (END) often occurs and ICH patients with END are at high risk of poor outcome and death.7 Thus, early discriminating patients with possible development of END can be of considerable clinical value.
Sulfonylurea receptor 1 (SUR1) is recognized as a member of the large superfamily of ATP-binding cassette proteins, with involvement in ion channel formation.8 Expression of SUR1 is up-regulated obviously in injured cells from brain tissues, including neurons and astrocytes.9–11 SUR1 may contribute to astrocyte swelling or brain edema via the transient receptor potential melastatin 4 (TRPM4) channel.12 Inhibiting SUR1 activity could decrease brain edema, inhibit neuroinflammation, recover permeability of blood-brain barrier, and lessen neuronal apoptosis, thereby improving neurological function in various clinical studies and animal experiments, which were pertinent to ischemic stroke, ICH, subarachnoid hemorrhage and traumatic brain injury.13–16 Cerebrospinal fluid SUR1 was detected in humans after severe traumatic brain injury, as well as its elevated levels were correlated with brain edema and intracranial pressure.17 Also, SUR1 levels in cerebrospinal fluid after traumatic brain injury were significantly higher in pediatric patients with a poor prognosis than in other remainders.18 Moreover, patients with aneurysmal subarachnoid hemorrhage had substantially higher serum SUR1 levels than healthy individuals; and serum SUR1 levels of patients were closely correlated with severity and prognosis.19 A more recent study has showed the close relationship between serum SUR1 levels and traumatic severity plus 3-month poor neurological outcome of patients with moderate to severe traumatic brain injury.20 Overall, serum SUR1 may be a potential prognostic biomarker of acute brain injury. In this study, we recruited a group of ICH patients and attempted to determine whether serum SUR1 levels are correlated with hemorrhagic severity and clinical outcomes after stroke.
Materials and Methods
Study Design, Patient Population and Control Participants
This is an observational study performed between April 2017 and October 2020 at the Quzhou Affiliated Hospital of Wenzhou Medical University. This study conformed to the tenets of the Declaration of Helsinki and the ethical board at the Quzhou Affiliated Hospital of Wenzhou Medical University approved the protocol of this study (opinion number: LW2021-004). The data utilized in the current study were collected consecutively and prospectively without any risk to the patients. Relative of each patient signed informed consent to participate in this study. All spontaneous ICH patients were screened according to the inclusion criteria and exclusion criteria below. Inclusion criteria included (1) first-ever stroke; (2) supratentorial ICH confirmed by head computerized tomography (CT) scan; (3) hospitalization within 24 hours after the onset of stroke symptom and (4) age of 18 years or greater. Exclusion criteria encompassed (1) secondary brain bleeding as a result of congenital or acquired coagulation abnormalities, hemorrhagic transformation of cerebral infarction, moyamoya disease, cerebral aneurysm, arteriovenous malformation or tumor; (2) a surgical evacuation of hematoma; (3) primary intraventricular bleeding; (4) presence of previous neurological diseases, such as stroke, brain tumors and severe head trauma; and (5) coexistence with severe systemic diseases, for example, malignancies, immune deficiency syndromes and severe heart, liver, lung or kidney dysfunction. A group of healthy volunteers constituted controls, who were also enrolled at the Quzhou Affiliated Hospital of Wenzhou Medical University from April 2017 to October 2020. The controls were informed of the protocol of this study and written consent was obtained from them.
Data Collection, Clinical and Radiologic Assessment and Follow-Up
All patients presented to the emergency department and the diagnosis of ICH was
confirmed on head CT scan. All head CT scans were performed according to the radiology department protocol. Radiologists who read them were blinded to clinical information. We inquired about demographical information (age and gender), adverse life habits (cigarette smoking history and alcohol drinking history), medication history (previous use of statins, anticoagulation drugs or antiplatelet drugs) and medical history (hypertension, diabetes mellitus and hyperlipidemia). Data regarding non-invasive blood pressure measurements were acquired at emergency department. The neurological status of all patients was assessed using the GCS and NIHSS, and the scores were recorded. Diagnostic CT scan findings were assessed and hematoma size was calculated according to the formula 0.5×a×b×c.21 The ICH topography was classified as lobar or deep. Deep ICH was referred to as hematoma located in putamen, caudate, internal capsule and thalamus. We observed whether bleeding was extended into subarachnoidal or intraventricular cavity. ICH score was estimated based on age, GCS score, hematoma volume, hematoma locations and extension of hematoma into intraventricular space.6 END was defined as an increase of 4 or more points in the NIHSS score or death within 24 hours after admission.22 Patients were followed until the completion of 90 days after stroke. The neurological status was evaluated at post-injury 90 days using modified Rankin scale (mRS). All patients were divided into two groups as follows: a poor outcome with mRS score of 3–6 and a good outcome with mRS score of 0–2.23
Blood Sample Collection and Determination of Serum SUR1 Levels
5 mL venous blood was drawn from each patient via the antecubital vein at emergency department and those of controls were obtained at the entrance into study. The acquired blood samples were promptly preserved in biochemistry tubes; and thereafter, serum was collected after centrifugation at 3500 g for 10 min. The separated serum was frozen immediately at −80℃ until further measurements. Serum SUR1 quantification was completed using enzyme linked immunosorbent assay (Bioassay Technology Laboratory, Shanghai, China) in accordance with the manufacturer’s instructions. Results were reported in ng/mL. Measurements were done by the same technician inaccessible to clinical data.
Statistical Analysis
Statistical software used in the current study was SPSS version 19.0 (IBM for Windows). Categorical variables were reported as numbers (proportions). After test of normal distribution, continuous variables were presented as means ± standard deviations (SDs) if normally distributed and as medians (percentiles 25th-75th) if non-normally distributed. Comparisons of data were done between two groups using the Chi-square test, Fisher exact test, Student’s t-test or Mann–Whitney test as appropriate. Comparisons of continuous data among multiple groups were performed using the Kruskal–Wallis H-test. Bivariate correlation analyses were completed using the Spearman correlation coefficient. The binary logistic regression model was constructed to discriminate independent predictors of END and 90-day poor outcome after stroke. The receiver operating characteristic (ROC) curve was built to assess distinguishable ability for END and poor 90-day outcome following stroke. A two-sided p < 0.05 was considered statistically significant.
Results
Participant Characteristics
During the study period, we at first assessed 186 patients with first-ever, supratentorial ICH, who were admitted within 24 hours after symptom onset and aged at 18 years or greater. Finally, a total of 131 patients were analyzed after excluding 55 patients because of the following criteria: secondary brain bleeding (10 cases), a surgical evacuation of hematoma (8 cases), primary intraventricular bleeding (12 cases), presence of previous neurological diseases (11 cases) and coexistence of severe systemic diseases (14 cases).
Patients were composed of 73 males and 58 females, as well as were aged from 38 to 87 years (mean, 63.9 years; SD, 12.1 years). One hundred and thirty-one controls consisted of 69 males and 62 females, as well as were aged from 25 to 88 years (mean, 58.3 years; SD, 13.9 years). There were not statistically significant differences of gender percentage and mean age between patients and controls (both P > 0.05).
Among this cohort of patients, 83 (63.4%) suffered from hypertension, 22 (16.8%) were inflicted with diabetes mellitus, 40 (30.5%) had hyperlipidemia, 47 (35.7%) smoked cigarettes and 49 (37.4%) consumed alcohol. A total of 29 (22.1%), 9 (6.9%) and 18 (13.7%) patients took orally statins, anticoagulation drugs and antiplatelet drugs, respectively, prior to admission to hospital. Patients were hospitalized from 0.5 to 24.0 h (median, 6.3 h; percentiles 25th-75th, 6.6–15.1 h) after stroke and blood samples were obtained from 1.0 to 25.5 h (median, 11.2 h; lower-upper quartiles, 7.9–16.2 h) following onset of symptom. Non-invasive arterial blood pressure measurement showed that mean systolic and diastolic arterial pressures were 157.0 mmHg (range, 108–224 mmHg; SD, 24.1 mmHg) and 88.0 mmHg (range, 67–116 mmHg; SD, 10.8 mmHg), respectively. By calculation, mean arterial pressure ranged from 81 to 149 mmHg (mean, 111.1 mmHg; SD, 14.8 mmHg). Regarding locations of hematoma, ratio of lobar to deep ICH was 0.32 (32/99). There were 28 patients (21.4%) with their hematomas extended into intraventricular cavity and 10 patients (7.6%) with their hematomas leaked into subarachnoidal space. Median baseline NIHSS, GCS and ICH scores were 8 (range, 0–19; upper-lower quartiles, 4–11), 13 (range, 5–15; upper-lower quartiles, 10–14) and 1 (range, 0–4; upper-lower quartiles, 0–2), respectively, as well as median admission ICH volume was 14 mL (range, 4–49 mL; upper-lower quartiles, 9–23 mL). In total, 32 patients (24.4%) experienced END. At 90 days after stroke, 10, 25, 37, 35, 9, 10 and 5 patients had mRS scores of 0, 1, 2, 3, 4, 5 and 6, respectively; and therefore 59 patients (45.0%) suffered from a poor outcome (mRS score of 3–6).
Serum SUR1 Levels and Its Correlation with Hemorrhagic Severity
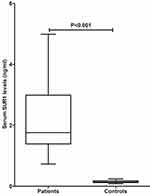
ICH patients had significantly higher serum SUR1 levels than controls (Figure 1, P < 0.001). In addition, serum SUR1 levels of patients were highly correlated with NIHSS score, GCS score, hematoma volume and ICH score, showing that serum SUR1 levels were substantially elevated with increasing NIHSS score, hematoma volume and ICH score, as well as decreasing GCS score (Figure 2A–D; all P < 0.001).
Serum SUR1 Levels and the Development of Poor 90-Day Outcome
Figure 3 displays that serum SUR1 levels of patients were substantially raised with rising mRS score (P < 0.001). Just as shown in Table 1, as compared to patients at no risk of poor outcome, those possibly experiencing poor outcome had significantly higher percentages of extension of hematomas into intraventricular and subarachnoid spaces (both P < 0.01), were more likely to have substantially raised NIHSS score, hematoma volume and ICH score (all P < 0.001), were more prone to exhibit markedly decreased GCS score (P < 0.001), as well as tended to show profoundly higher blood glucose levels (P < 0.01), serum C-reactive protein levels (P < 0.05) and serum SUR1 levels (P < 0.001). The afore-mentioned variables were entered into the binary logistic regression model and subsequently it was revealed that NIHSS score (odds ratio, 1.402; 95% confidence interval, 1.198–1.640; P = 0.003), hematoma volume (odds ratio, 1.113; 95% confidence interval, 1.050–1.181; P = 0.009) and serum SUR1 levels (odds ratio, 1.970; 95% confidence interval, 1.141–3.399; P = 0.015) were still the independent predictors of poor outcome at 90 days after stroke.
 |
Table 1 Factors Related to 90-Day Functional Outcome After Acute Intracerebral Hemorrhage |
Using maximum Youden index under ROC curve, we chose an optimal value of serum SUR1 levels, which discriminated patients at risk of 90-day poor outcome with medium-high sensitivity and specificity values (Figure 4A). In Figure 4B, the predictive ability of serum SUR1 levels was in range of NIHSS score, GCS score, ICH score and hematoma volume (all P > 0.05).
Serum SUR1 Levels and Occurrence of END
Just as listed in Table 2, as compared to patients without the risk of END, those with possible development of END showed significantly higher proportions of diabetes mellitus (P < 0.01), extension of hematomas into intraventricular cavity (P < 0.001) and leakage of bleeding into subarachnoid space (P < 0.05), had substantially higher NIHSS score, hematoma volume and ICH score (all P < 0.001), displayed markedly lower GCS score (P < 0.001), as well as exhibited pronouncedly higher blood glucose levels (P < 0.01), serum C-reactive protein levels (P < 0.05) and serum SUR1 levels (P < 0.001). The binary logistic regression model, which encompassed the above-mentioned factors significant in univariate analysis, showed that predictors independently associated with occurrence of END after stroke were NIHSS score (odds ratio, 1.398; 95% confidence interval, 1.231–1.588; P = 0.001), hematoma volume (odds ratio, 1.123; 95% confidence interval, 1.063–1.185; P = 0.006) and serum SUR1 levels (odds ratio, 1.636; 95% confidence interval, 1.034–2.589; P = 0.036).
 |
Table 2 Factors Pertinent to Early Neurologic Deterioration After Acute Intracerebral Hemorrhage |
A ROC curve was configured and then maximum Youden method was used to yield an optimal value of serum SUR1 levels, which differentiated between patients with development of END and those without END with medium-high sensitivity and specificity values (Figure 4C). Figure 4D displays that the predictive capability of serum SUR1 levels was equivalent to those of NIHSS score, GCS score, ICH score and hematoma volume (all P > 0.05).
Discussion
To the best of our knowledge, two previous clinical studies showed elevated cerebrospinal fluid SUR1 levels may be associated with prognosis and traumatic severity of humans with severe traumatic brain injury,17,18 and there is a clinical investigation displaying raised serum SUR1 levels may be related to outcome and hemorrhagic severity of patients with aneurysmal subarachnoid hemorrhage.19 Although those studies enrolled a small number of patients (≤44 cases) and did not use multivariate analyses,17–19 a more recent study recruited 100 healthy controls and 138 patients with moderate to severe traumatic brain injury and found that elevated serum SUR1 levels, in close correlation with traumatic severity, were independently associated with poor prognosis after traumatic brain injury.20 The preceding data have indicated that serum SUR1 may be a potential prognostic biomarker of diseases with acute brain injury.
This is a first series for detecting serum SUR1 levels in patients with ICH and further determining its relation to hemorrhagic severity and clinical outcome after hemorrhagic stroke. Our main findings were as follows: (1) serum SUR1 levels were markedly elevated after acute ICH, as compared to healthy controls; (2) serum SUR1 levels were intimately correlated with admission NIHSS score, GCS score, hematoma volume and ICH score; (3) serum SUR1 retained as an independent predictor of 90-day poor outcome and END after ICH; and (4) serum SUR1 levels displayed similar area under ROC curve, as compared to NIHSS score, GCS score, hematoma volume and ICH score. In summary, serum SUR1 may serve as a potential prognostic biomarker of ICH.
SUR1 is a member of the adenosine triphosphate-binding cassette protein superfamily.24 In some animal models with acute brain injury, SUR1 was greatly upregulated in neurons, astrocytes, and capillary endothelial cells after ischemic stroke9 as well as in neurons and endothelial cells after brain contusions10 and subarachnoid hemorrhage.11 Interestingly, SUR1 was obviously expressed in human injured brain tissues.25,26 Elevated SUR1 levels in cerebrospinal fluid have been revealed in adults or children with severe traumatic brain injury.17,18 Also, serum SUR1 levels were substantially increased in patients with aneurysmal subarachnoid hemorrhage19 or moderate to severe traumatic brain injury.20 In line with the preceding studies,17–20 our study confirmed that ICH patients had substantially higher serum SURE1 levels than healthy individuals. Overall, it is hypothesized that SUR1 in peripheral blood of ICH patients may at least in part be derived from injured brain tissues.
SUR1 is a key mediator of central nervous system cellular swelling via the TRPM4 channel.8 Accumulating evidence has shown that SUR1-TRPM4 contributed to brain edema and secondary brain injury across several types of central nervous system injury, including ICH, ischemic stroke, traumatic brain injury and subarachnoid hemorrhage.13–16 Genetic silencing or pharmacological inhibition of SUR1-TRPM4 channel lessened brain edema, hemorrhage progression, neuroinflammation and/or cell death, thereby improving neurologic function in some experimental and early clinical studies across a spectrum of acute brain injury, such as ICH, ischemic stroke and traumatic brain injury.13–16 In conclusion, SUR1-TRPM4 channel may be as a translatable therapeutic target of central nervous system injury.
A previous study of 28 patients with severe traumatic brain injury and 15 controls with normal pressure hydrocephalus showed that elevated SUR1 levels in cerebrospinal fluid after head trauma were closely correlated with CT-edema and preceded peak intracranial pressure, as well as SUR1 trajectories between 48–72h were associated with outcome indicated by Glasgow outcome scale score at 3 months after trauma.17 In another study recruiting 16 pediatric patients with severe traumatic brain injury, cerebrospinal fluid SUR1 levels were found to be associated with increased intracranial pressure over seven days and worse extended Glasgow outcome scale score over the initial year after injury.18 Also, an epidemiological investigation of 44 patients with aneurysmal subarachnoid hemorrhage showed that serum SUR1 levels, in correlation with World Federation of Neurosurgical Societies score, were associated with Glasgow outcome scale score at 14 days after hemorrhagic stroke.19 The biggest number of cases in the preceding studies was only 44, so no multivariate analyses were used.17–19 Of note, in a clinical study of 100 healthy controls and 138 patients with moderate to severe traumatic brain injury, rising serum SUR1 levels were intimately related to disease severity and independently predicted poor prognosis after traumatic brain injury.20 Alternatively, our study enrolled 131 patients and 131 healthy controls and found that rising serum SUR1 levels were highly correlated with hemorrhagic severity indicated by NIHSS score, GCS score, hematoma volume and ICH score, as well as were independently associated with END and poor outcome at 90 days after ICH; moreover, this biomarker displayed similar predictive capability, as compared to NIHSS score, GCS score, hematoma volume and ICH score. Taken together, serum SUR1 may represent a potential biomarker of ICH.
There are two limitations in the current study. First, this study recruited a total of 131 patients and 131 healthy individuals and therefore, sample size conformed to statistical requirements. Moreover, multivariate analysis has been used for analyzing prognosis and the four commonest ICH severity assessment grading system, namely NIHSS score, GCS score, hematoma volume and ICH score, have been used for severity assessment. So, there is sufficient evidence to support the conclusions. However, it is better that a larger cohort study be implemented to validate such results. Second, because we have excluded patients with a surgical evacuation of hematoma based on the exclusion criteria, hematoma volume is generally small. Nevertheless, another clinical study is warranted to demonstrate whether such conclusions can be extended to patients with larger hematoma volume.
Conclusions
This is the first series for ascertain the relationship between serum SUR1 levels and hemorrhagic severity, END plus functional outcome after acute ICH. The current study demonstrated that, elevated serum SUR1 levels, which are significantly correlated positively with NIHSS score, hematoma volume and ICH score, as well as inversely with GCS score, are independently associated with END and poor 90-day outcome, indicating that serum SUR1 may represent a potential prognostic biomarker of acute ICH.
Abbreviations
END, early neurologic deterioration; CT, computerized tomography; ICH, intracerebral hemorrhage; NIHSS, National Institutes of Health Stroke Scale; ROC, receiver operating characteristic; GCS, Glasgow Coma scale; SUR1, sulfonylurea receptor-1; mRS, modified Rankin scale; TRPM4, transient receptor potential melastatin 4; SD, standard deviation.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
The authors gratefully thank all study participants, their relatives, and the staffs at the recruitment centers for their invaluable contributions.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–142. doi:10.1016/S0140-6736(20)31179-X
2. Rodgers H. Stroke. Handb Clin Neurol. 2013;110:427–433.
3. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60(1):61. doi:10.1016/j.jphys.2013.12.012
4. Middleton PM. Practical use of the Glasgow Coma Scale; a comprehensive narrative review of GCS methodology. Australas Emerg Nurs J. 2012;15(3):170–183. doi:10.1016/j.aenj.2012.06.002
5. LoPresti MA, Bruce SS, Camacho E, et al. Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage. J Neurol Sci. 2014;345(1–2):3–7. doi:10.1016/j.jns.2014.06.057
6. Hemphill JC
7. Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461–467. doi:10.1212/01.WNL.0000133204.81153.AC
8. Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013;288(5):3655–3667. doi:10.1074/jbc.M112.428219
9. Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12(4):433–440. doi:10.1038/nm1390
10. Simard JM, Kilbourne M, Tsymbalyuk O, et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J Neurotrauma. 2009;26(12):2257–2267. doi:10.1089/neu.2009.1021
11. Simard JM, Geng Z, Woo SK, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29(2):317–330. doi:10.1038/jcbfm.2008.120
12. Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32(9):1699–1717. doi:10.1038/jcbfm.2012.91
13. Caffes N, Kurland DB, Gerzanich V, Simard JM. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int J Mol Sci. 2015;16(3):4973–4984. doi:10.3390/ijms16034973
14. Tosun C, Kurland DB, Mehta R, et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke. 2013;44(12):3522–3528. doi:10.1161/STROKEAHA.113.002904
15. Kimberly WT, Battey TW, Pham L, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20(2):193–201. doi:10.1007/s12028-013-9917-z
16. Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69(12):1177–1190. doi:10.1097/NEN.0b013e3181fbf6d6
17. Jha RM, Puccio AM, Chou SH, et al. Sulfonylurea receptor-1: a novel biomarker for cerebral edema in severe traumatic brain injury. Crit Care Med. 2017;45(3):e255–e264. doi:10.1097/CCM.0000000000002079
18. Zusman BE, Kochanek PM, Bell MJ, et al. Cerebrospinal fluid sulfonylurea receptor-1 is associated with intracranial pressure and outcome after pediatric TBI: an exploratory analysis of the Cool Kids Trial. J Neurotrauma. 2021;38(12):1615–1619. doi:10.1089/neu.2020.7501
19. Dundar TT, Abdallah A, Yurtsever I, Guler EM, Ozer OF, Uysal O. Serum SUR1 and TRPM4 in patients with subarachnoid hemorrhage. Neurosurg Rev. 2020;43(6):1595–1603. doi:10.1007/s10143-019-01200-6
20. Ying X, Chen M, Zhang J, Sun CF, Zhou J. Serum sulfonylurea receptor-1 as a biomarker of clinical severity and prognosis in patients with traumatic brain injury. Clin Chim Acta. 2022;528:65–73. doi:10.1016/j.cca.2022.01.018
21. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1395. doi:10.1161/01.STR.27.8.1304
22. Du Q, Yang DB, Shen YF, et al. Plasma leptin level predicts hematoma growth and early neurological deterioration after acute intracerebral hemorrhage. Peptides. 2013;45:35–39. doi:10.1016/j.peptides.2013.04.017
23. Chen B, Shen J, Zheng GR, et al. Serum cyclophilin A concentrations and prognosis of acute intracerebral hemorrhage. Clin Chim Acta. 2018;486:162–167. doi:10.1016/j.cca.2018.08.002
24. Jha RM, Rani A, Desai SM, et al. Sulfonylurea receptor 1 in central nervous system injury: an updated review. Int J Mol Sci. 2021;22(21):11899. doi:10.3390/ijms222111899
25. Martínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, et al. Sulfonylurea receptor 1 in humans with post-traumatic brain contusions. J Neurotrauma. 2015;32(19):1478–1487. doi:10.1089/neu.2014.3706
26. Mehta RI, Ivanova S, Tosun C, Castellani RJ, Gerzanich V, Simard JM. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2013;72(9):871–883. doi:10.1097/NEN.0b013e3182a32e40
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Plasma SIRT3 as a Biomarker of Severity and Prognosis After Acute Intracerebral Hemorrhage: A Prospective Cohort Study
Yan T, Wang ZF, Wu XY, Du Q, Yu WH, Hu W, Zheng YK, Wang KY, Dong XQ
Neuropsychiatric Disease and Treatment 2022, 18:2199-2210
Published Date: 26 September 2022
Usability of Serum Stanniocalcin-1 as a Prognostic Biochemical Marker of Acute Supratentorial Intracerebral Hemorrhage: A Prospective Cohort Study
Gao CF, Zhang GH, Ye ZH, Xu YY, Li Z
International Journal of General Medicine 2023, 16:2791-2803
Published Date: 3 July 2023
A Prospective Cohort Study of Inter-Alpha-Trypsin Inhibitor Heavy Chain 4 as a Serologic Marker in Relation to Severity and Functional Outcome of Acute Intracerebral Hemorrhage
Shentu HS, Chen YH, Cheng ZY, Fu B, Fu YH, Zheng SF, Li C
Neuropsychiatric Disease and Treatment 2023, 19:2363-2379
Published Date: 6 November 2023
Serum Secreted Protein Acidic and Rich in Cysteine-Like 1 as a Biochemical Predictor for Prognosticating Clinical Outcomes After Acute Supratentorial Intracerebral Hemorrhage: A Prospective Cohort Study
Huang J, Shao F, Chen B, Zheng G, Shen J, Qiu S
Neuropsychiatric Disease and Treatment 2023, 19:2709-2728
Published Date: 5 December 2023