Back to Journals » International Journal of General Medicine » Volume 16
Usability of Serum Stanniocalcin-1 as a Prognostic Biochemical Marker of Acute Supratentorial Intracerebral Hemorrhage: A Prospective Cohort Study
Authors Gao CF, Zhang GH, Ye ZH, Xu YY, Li Z
Received 19 May 2023
Accepted for publication 23 June 2023
Published 3 July 2023 Volume 2023:16 Pages 2791—2803
DOI https://doi.org/10.2147/IJGM.S420245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Chun-Fang Gao,1 Guo-Hai Zhang,1 Zhe-Hao Ye,1 Yu-Yu Xu,2 Zhao Li1
1Department of Neurosurgery, The Shengzhou Hospital of Traditional Chinese Medicine, Shengzhou, Zhejiang Province, People’s Republic of China; 2Department of Neurology, The Shengzhou Hospital of Traditional Chinese Medicine, Shengzhou, Zhejiang Province, People’s Republic of China
Correspondence: Guo-Hai Zhang, Department of Neurosurgery, The Shengzhou Hospital of Traditional Chinese Medicine, Shengzhou, Zhejiang Province, People’s Republic of China, Email [email protected]
Objective: Stanniocalcin-1 (STC1) may be neuroprotective. This study aimed to evaluate the prognostic role of serum STC1 levels in intracerebral hemorrhage (ICH).
Methods: This prospective observational study was assigned in two parts. In the first part, blood samples of 48 patients with ICH were acquired on admission and on days 1, 2, 3, 5, and 7 after ICH, and those of 48 controls were collected at their entry into the study. In the second part, blood samples of 141 patients with ICH were obtained upon admission. Serum STC1 levels were measured, and the National Institutes of Health Stroke Scale (NIHSS), hematoma volume, and poststroke 6-month modified Rankin Scale (mRS) scores were recorded. Dynamic changes in serum STC levels and their correlation with disease severity and prognosis were investigated.
Results: Serum STC1 levels were elevated after ICH, peaked on day 1, plateaued on day 2, declined gradually afterwards, and were significantly higher than those in controls. Serum STC1 levels were independently correlated with NIHSS scores, hematoma volume, and the 6-month post-injury mRS scores. Serum STC1 levels, NIHSS scores, and hematoma volume independently predicted a poor prognosis (mRS scores of 3– 6). The model integrating serum STC1 levels, NIHSS scores, and hematoma volume was visually displayed using a nomogram and was relatively stable using the Hosmer–Lemeshow test and calibration curve analysis. Under the receiver operating characteristic curve, serum STC1 levels efficiently predicted a poor prognosis and showed similar prognostic ability to NIHSS scores and hematoma volume. The preceding model had significantly higher prognostic capability than NIHSS scores and hematoma volume alone and their combination.
Conclusion: Substantial enhancement of serum STC1 levels after ICH, which is strongly correlated with severity, independently distinguished the risk of poor prognosis, assuming that serum STC1, as a prognostic parameter, may be clinically valuable in ICH.
Keywords: stanniocalcin-1, intracerebral hemorrhage, prognosis, severity, biomarkers
Introduction
Primary intracerebral hemorrhage (ICH) mainly results from rupture of a microartery with hypertension or cerebral amyloid angiopathy.1 ICH has received considerable attention as a serious cerebrovascular disease globally because of its high incidence, disability, and mortality rates.2,3 Therefore, it is essential to evaluate the severity and prognosis of ICH.4 Accumulating data have screened several closely relevant prognostic predictors, among which the National Institutes of Health Stroke Scale (NIHSS) score and hematoma volume are two highly recommended prognostic determinants.5 The modified Rankin Scale (mRS) is usually used to assess neurological outcomes in patients with ICH.6 Secondary brain injury after ICH involves a series of molecules pertinent to inflammatory reactions, oxidative stress, and cellular apoptosis.7 Biochemical markers, such as S100B, matrix metalloproteinase 9, interleukin-6 and tumor necrosis factor-alpha, have attracted the interest of neurological research for the severity assessment and prognostic prediction of ICH.8
Stanniocalcin-1 (STC1) is a homodimeric glycoprotein hormone that was initially found to be involved in calcium regulation, and has been implicated in cell proliferation, differentiation, migration, survival, apoptosis, and vascular remodeling.9 Interestingly, STC1 expression is upregulated under hypoxic conditions, and STC1 expression induced by hypoxia exerts anti-apoptotic, anti-inflammatory, and antioxidative effects.10–12 Hypoxia can increase STC1 expression in neurons and glial cells.13,14 In animal models of acute ischemic stroke or sepsis-associated encephalopathy, STC1 significantly improves neurological function by inhibiting oxidative stress and inflammatory reactions.15,16 Thus, STC1 might have neuroprotective effects. A recent study showed that serum STC1 may be a potential prognostic predictor because it was highly correlated with clinical severity and independently predicted neurological poor outcome in a cohort of patients with aneurysmal subarachnoid hemorrhage.17 This finding prompted us to further explore the prognostic value of serum STC1 in human ICH.
Materials and Methods
Participants
In this single-center prospective observational cohort study, we consecutively enrolled patients with first-ever primary supratentorial ICH who were conservatively treated at the Shengzhou Hospital of Traditional Chinese Medicine between April 2018 and August 2021, aged 18 years or older, and hospitalized at 24 h or shorter since the onset of stroke symptoms. Furthermore, we excluded patients with a history of neurological diseases (such as moderate-to-severe head trauma, stroke, and tumors) or severe diseases in other organs (such as malignancies, heart failure, cirrhosis, uremia, and lung failure). Controls were recruited from among healthy volunteers at our hospital between August 2020 and August 2021. Controls did not have chronic diseases and had normal blood glucose levels and white blood cell, erythrocyte, and platelet counts. The study protocol complied with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Shengzhou Hospital of Traditional Chinese Medicine (No. SZH2018-12). Informed voluntary consent was signed from patients’ relatives or controls themselves.
Data Collection
The inquired materials included demographic information, comorbidities, adverse lifestyle habits, vascular risk factors, and medications. The registered data included the time since the onset of stroke symptoms and the time from stroke onset to blood sampling. The recorded vital signs included noninvasively measured systolic and diastolic arterial blood pressures. The clinical severity of ICH was assessed using the NIHSS score. Hematoma-relevant parameters included hematoma volume, hematoma location (lobar or deep), and the presence of intraventricular or subarachnoid hemorrhage. The hematoma size was estimated using the following formula:0.5×A×B×C formula.18 Two common complications were recorded: pneumonia and seizures. Post-stroke six month modified Rankin scale (mRS) scores of 3–6 was designated as poor prognosis.19
Immune Analysis
Peripheral venous blood samples of the enrolled patients, who agreed to blood collection at multiple time points, were acquired on admission and on days 1, 2, 3, 5, and 7 after ICH. Venous blood samples of the remaining patients were collected upon admission. Blood samples from controls were collected at their entrance into the study. Venous blood drawn via the median cubital vein was immediately put in 5 mL gel-containing biochemistry tubes and centrifuged. Separated serum samples were stored at −80°C until analysis. Frozen blood samples were thawed in batches every three to six months and serum STC1 levels were measured using a sandwich enzyme-linked immunosorbent assay kit (Wuhan Fine Biotech Co., Ltd., Wuhan, China). The limit of detection of the assay ranged from 78.125 to 5000 pg/mL, the sensitivity was 46.875 pg/mL, the intra-assay coefficient of variation was less than 8%, and the inter-assay coefficient of variation was < 10%. Following the manufacturer’s instructions (Catalogue no.: EH12661), measurements were performed in duplicate by the same experienced technician who was inaccessible to the study materials. The two measurements were averaged for the final analysis.
Statistical Analysis
In this study, the used statistical and graphing softwares were Statistical Package for the Social Sciences (version 19.0; SPSS Inc., Chicago, IL, USA), R software (version 3.5.1; https://www.r-project.org), MedCalc (version 9.6.4.0; MedCalc Software, Mariakerke, Belgium) and GraphPad Prism (version 8; GraphPad Prism Software Inc., San Diego, CA). Count variables were reported as frequencies (percentages). The Kolmogorov–Smirnov test was used to test the normal distribution of the measurement variables. They were reported as means ± standard deviations (SDs) for patterns of normal distribution or medians with percentiles 25th-75th for pattern of non-normal distribution. Comparisons between the two groups were performed using the Mann–Whitney U-test for nonparametric variables, the independent sample t-test for parametric variables, and the Fisher’s exact test or Pearson’s Chi-square test for categorical variables. Because serum STC1 levels were non-normally distributed and therefore Friedman test was used for comparisons among multiple groups. Bivariate correlations were analyzed using Spearman correlation analysis. Multivariate models included binary multiple linear regression and multiple logistic regression. A nomogram was established to visually describe the prognostic prediction model. The prediction model fit was assessed using Hosmer-Lemeshow goodness of fit statistics and calibration curve analysis. Predictive efficiency was evaluated using a receiver operating characteristic (ROC) curve. Statistical significance was set at P <0.05.
Results
Participant Selection and Characteristics
Based on the inclusion criteria, we initially included 170 patients with acute primary supratentorial ICH and then excluded 29 patients because they had a history of neurological disease (12 cases), severe diseases in other organs (13 cases), or other specific conditions, such as refusal to participate, loss to follow-up, unavailable samples, hemolysis, and incomplete clinical materials (4 cases). Ultimately, 141 patients were enrolled in this study. Blood samples were collected from 48 patients at all designated six time points. Those 48 patients were aged from 42 to 84 years (mean, 60.4 years; SD, 12.5 years), included 26 males and 22 females, body mass index (BMI) ranged from 18.9 to 28.1 kg/m2 (mean, 22.7 kg/m2; SD, 2.3 kg/m2), and contained 12 cigarette smokers and 20 alcohol drinkers. There were 48 healthy controls, who were aged from 26 to 85 years (mean, 60.7 years; SD, 14.9 years), included 28 males and 20 females, BMI ranged from 17.1 to 33.3 kg/m2 (mean, 23.6 kg/m2; SD, 4.5 kg/m2), and contained 17 cigarette smokers and 16 alcohol drinkers. None of the preceding five variables was significantly different between the 48 patients and 48 controls (all P>0.05).
Among 141 patients, there were 77 males and 64 females. They were aged from 42 to 86 years (mean, 63.3 years; SD, 12.5 years) and their BMI varied from 18.6 to 29.7 kg/m2 (mean, 23.1 kg/m2; SD, 2.6 kg/m2). There were 45 cigarette smokers, 52 alcohol consumers, 92 hypertensive sufferers, 28 diabetic subjects, 44 patients with dyslipidemia, and 16 individuals with coronary heart disease. Regarding medication, 34, 8, 19, 78, and 23 patients had previously been administered oral statins, anticoagulants, antiplatelet agents, antihypertensive drugs, and hypoglycemic drugs or insulin, respectively. Patients were hospitalized from 1.0 to 24.0 h (median, 9.9 h; percentiles 25th- 75th, 4.5–13.6 h) and blood samples were collected from 1.5 to 25.8 h (median, 11.6 h; percentiles 25th- 75th, 6.0–15.4 h). Systolic arterial pressure, diastolic arterial pressure and mean arterial pressure varied from 94 to 210 mmHg (mean, 143.9 mmHg; SD, 22.8 mmHg) and from 66 to 113 mmHg (mean, 88.0 mmHg; SD, 10.1 mmHg) and from 75 to 145 mmHg (mean, 106.6 mmHg; SD, 13.9 mmHg) respectively. As regards hematoma locations, deep and lobar hematomas were found in 96 and 45 patients respectively. There were 37 patients with extension of the hematoma into the intraventricular cavity and 13 patients with bleeding inside the subarachnoid cavity. The main complications were seizures (n = 13) and pneumonia (n = 29). Regarding severity, NIHSS scores and hematoma volumes varied from 0 to 16 (median, 5; lower-upper quartiles, 2–12) and from 2 to 44 mL (median, 12 mL; lower-upper quartiles, 6–22 mL), respectively.
Change of Serum STC1 Levels After ICH and Its Correlation with the Severity
As shown in Figure 1, serum STC1 levels were significantly elevated after ICH, peaked on day 1, plateaued on day 2, declined gradually on days 3, 5, and 7, and were substantially higher than those in healthy controls (all P<0.05). There was a close correlation between serum STC1 levels and NIHSS scores (P<0.001; Figure 2) as well as between serum STC1 levels and hematoma volumes (P<0.001; Figure 3). In addition to NIHSS scores and hematoma volumes, other variables strongly correlated with serum STC1 levels were blood glucose levels and intraventricular hemorrhage (both P<0.05; Table 1). In the multivariate linear regression model, NIHSS scores, hematoma volume, blood glucose levels, and intraventricular hemorrhage were included. NIHSS scores (β, 0.247; 95% confidence interval (CI), 0.065–0.430; VIF, 4.939; t=2.686; P=0.008) and hematoma volume (β, 0.127; 95% CI, 0.087–0.166; VIF, 2.114; t=2.128; P=0.010) were the two independent variables correlated with serum STC1 levels.
 |
Table 1 Bivariate Correlation Analysis of Serum Stanniocalcin-1 Levels After Acute Intracerebral Hemorrhage |
Relation of Serum STC1 Levels to mRS Scores at Six Months After ICH
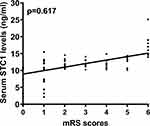
The mRS scores varied from 0 to 6 (median, 2; lower-upper quartiles, 1–4); and mRS scores from 0 to 6 were revealed in 12, 30, 37, 15, 22, 12, and 13 patients, respectively. Serum STC1 levels were highly and positively correlated with mRS scores (P<0.001; Figure 4), and were significantly elevated in the order of mRS scores from 0 to 6 (P<0.001; Figure 5). In addition, the mRS scores were closely related to other data, including the NIHSS scores, hematoma volume, blood glucose levels, and intraventricular hemorrhage (all P<0.01; Table 2). Multivariate linear regression analysis revealed that serum STC1 levels, NIHSS scores, hematoma volume, blood glucose levels, intraventricular hemorrhage, serum STC1 levels (β, 0.179; 95% CI, 0.097–0.261; VIF, 1.506; t=2.884; P=0.009), NIHSS score (β, 0.132; 95% CI, 0.041–0.222; VIF, 5.202; t=4.323; P=0.001), and hematoma volume (β, 0.206; 95% CI, 0.124–0.288; VIF, 1.430; t=2.547; P=0.012) were independently correlated with mRS scores.
 |
Table 2 Bivariate Correlation Analysis of Modified Rankin Scale Scores 6 Months After Acute Intracerebral Hemorrhage |
Relationship Between Serum STC1 Levels and Six-Month Poor Prognosis After ICH
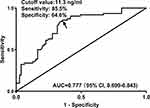
Six months after ICH, 62 patients had poor prognosis (mRS scores of 3–6). Compared to patients with good prognosis, those with poor prognosis exhibited significantly higher serum STC1 levels (P<0.001; Figure 6). Using the Youden method, serum STC1 levels > 11.3 ng/mL could efficiently discriminate patients at risk of poor prognosis (Figure 7). As shown in Figure 8, the AUC of serum STC1 levels was equivalent to that of NIHSS scores (P=0.170) and hematoma volume (P=0.320). The AUC of serum STC1 levels combined with NIHSS scores and hematoma volume was significantly superior to that of NIHSS scores (P=0.017), hematoma volume (P=0.011), serum STC1 levels (P=0.005), and the combination of NIHSS scores and hematoma volume (P=0.026).
Univariate analysis showed differences in serum STC1 levels, intraventricular hemorrhage, NIHSS scores, hematoma volume, and blood glucose levels between patients with good and poor prognosis (Table 3; all P<0.05). These significantly different variables were incorporated into the multivariate model, and it was shown that serum STC1 level (odds ratio, 1.428; 95% CI, 1.071–1.906; P=0.024), NIHSS score (odds ratio, 1.228; 95% CI, 1.027–1.469; P=0.007), and hematoma volume (odds ratio, 1.102; 95% CI, 1.048–1.159; P=0.015) were independent predictors of poor prognosis. Using the Hosmer-Lemeshow test, the prediction model integrating them was comparatively stable and reliable (P=0.470). Alternatively, a nomogram was constructed to outline the prediction model (Figure 9). Under the calibration curve, the prediction model was relatively stable and reliable (Figure 10).
 |
Table 3 Factors Related to a Poor Prognosis 6 Months After Acute Intracerebral Hemorrhage |
Discussion
Since a clinical epidemiological investigatory study published in 2021 showed that a significant enhancement of serum STC1 levels was highly correlated with the severity and was substantially associated with a poor functional outcome of patients with aneurysmal subarachnoid hemorrhage,17 our study, to the best of our knowledge, has been the first series for determining whether serum STC1 levels are altered after acute ICH. Our interesting findings were that (1) Serum STC1 levels were elevated until day 7 after ICH, and were significantly higher than those in healthy controls; (2) Serum STC1 levels were independently related to stroke severity, as estimated by NIHSS scores and hematoma volume; (3) Serum STC1 levels were independently associated with post-stroke 6-month mRS, which was regarded as a categorical variable or a continuous variable; and (4) Serum STC1 levels combined with NIHSS scores and hematoma volume displayed a more efficient predictive ability than either of them alone or NIHSS scores combined with hematoma volume. Overall, serum STC1 may represent a potential prognostic biochemical marker with clinical value for acute ICH.
STC1 is a homodimeric glycoprotein hormone that exerts neuroprotective effects by inhibiting the inflammatory responses and oxidative reactions. Specifically, STC1 markedly attenuates the cerebral ischemic brain of rats with global cerebral ischemia/perfusion by reducing oxidative stress and improving blood-brain barrier permeability.20 An in vitro experiment showed that altered STC1 expression could significantly protect cerebral neurons from hypoxic and ischemic damage.14 Another in vitro experiment demonstrated that STC1 contributes to the protection of astrocytes against hypoxic damage by regulating glycolysis and redox homeostasis.21 Similarly, using in vitro and in vivo experiments, STC1 was confirmed to exert neuroprotective effects against sepsis-associated encephalopathy by depressing the inflammatory response in rat microglia.15 Overall, STC1 may exhibit brain-protective effects and may, therefore, be a therapeutic target for brain injury.
Compelling evidence has shown that STC1 expression in brain tissue can be induced under hypoxic conditions.13,14 Specifically, hypoxic preconditioning upregulates STC1 expression in the mouse brains.13 Similarly, STC1 was significantly expressed in the human neural-crest-derived cell line Paju when exposed to a hypercalcemic culture medium, and substantial upregulation of STC1 expression was found in neurons surrounding the core of acute infarcts in human and rat brains.14 In rats with traumatic brain injury, significantly enhanced expression of STC1 occurred.22 In addition, there is increased expression of STC1 in astrocyte cell lines and astrocytes in the brain tissues of mice under hypoxic conditions.21 Serum STC1 levels are substantially elevated after aneurysmal subarachnoid hemorrhage in humans.17 Consistent with the above data,17 we found a dramatic increase in serum STC1 levels in this group of patients with ICH compared with healthy controls. Cerebral blood flow is substantially reduced after ICH.23 Presumably, this may lead to STCI in brain tissues after ICH. In addition, STC1 in the peripheral blood of patients might have at least partly originated from the brain tissue damaged by hemorrhage.
To the best of our knowledge, this is the first report on the relationship between serum STC1 levels, severity and prognosis in patients with acute ICH. On the one hand, this study demonstrated that serum STC1 levels were independently correlated with NIHSS scores and hematoma volumes of ICH patients, and in other words, serum STC1 could reflect hemorrhagic severity of ICH. Furthermore, serum STC1 levels were independently associated with the mRS at 6 months after ICH, which was regarded as a continuous variable or a categorical variable (mRS scores 0–2 versus 3–6). These data indicate that serum STC1 may be a biomarker of poor long-term prognosis. The results of our study are in line with those of another study in which serum STC1 was highly correlated with the severity and 1-year functional outcome indicated by the Glasgow outcome scale in a group of patients with aneurysmal subarachnoid hemorrhage.17 In summary, serum STC1 levels may be closely related to the severity and poor outcome of patients with acute brain injury such as ICH and subarachnoid hemorrhage. An intriguing finding in this study was that serum STC1 levels combined with NIHSS scores and hematoma volume showed higher prognostic predictive ability than other predictors, namely NIHSS scores, hematoma volume and even combination of NIHSS scores and hematoma volume; moreover, the prediction model containing NIHSS scores, hematoma volume and serum STC1 levels showed rather stability and reliability, which was verified using calibration curve analysis and Hosmer-Lemeshow goodness of fit statistics. Thus, serum STC1 might be a promising biomarker for ICH prognosis.
Several strengths and weaknesses should be mentioned in this study. The strengths are displayed below: (1) To the best of our knowledge, this is a first series for discerning serum STC1 levels in a cohort of ICH patients and subsequently it is not only found that there was a close relation of serum STC1 to severity and prognosis of ICH, but also it is confirmed that prognosis prediction model composed of serum STC1, NIHSS score and hematoma volume had a very strong administrative efficiency in ICH; (2) The preceding associations were verified using various statistical methods, including ROC curve analysis, multivariate analysis, calibration curve analysis and Hosmer-Lemeshow test and therefore the conclusions are relatively reliable, rational and believable. However, there were three weaknesses or perspectives of study in future. (1) All patients were conservatively treated in this study. So, it may be interesting to explore whether successful surgical treatment may affect serum STC1 levels of patients with ICH. (2) In the light of combinative ability of serum STC1 with NIHSS scores and hematoma volumes, which has been demonstrated in the present study, it is conceivably assumed that serum STC1 may be a useful prognostic biomarker of ICH. However, it is possibly intriguing that the additive effect of serum STC1 will be compared to those of other inflammatory biomarkers, such as C-reactive protein, interleukin-6 and tumor necrosis factor-alpha. (3) In acute ischemic stroke, serum STC1 has not been determined and therefore, it is a good topic regarding whether serum STC1 may be related to outcome of acute ischemic stroke.
Conclusions
In the present study, we performed a clinical investigation to determine the prognostic role of serum STC1 levels in acute ICH. We found a dynamic change in serum STC1 levels after ICH, and elevated admission serum STC1 levels were highly correlated with stroke severity and tightly associated with long-term functional outcome of ICH. In addition, serum STC1 levels combined with NIHSS scores and hematoma volume have strong prognostic predictive efficiency for ICH. The prognostic significance of serum STC1 level may be assumed in the clinical management of ICH.
Abbreviations
ICH, intracerebral hemorrhage; NIHSS, National Institutes of Health Stroke Scale; ROC, receiver operating characteristic; mRS, modified Rankin scale; STC1, stanniocalcin-1; SD, standard deviation.
Acknowledgments
We thank all the study participants, their relatives, and staff at the recruitment centers for their invaluable contributions.
Disclosure
The authors declare that there were no conflicts of interest.
References
1. Sutherland GR, Auer RN. Primary intracerebral hemorrhage. J Clin Neurosci. 2006;13(5):511–517. doi:10.1016/j.jocn.2004.12.012
2. Sheth KN, Ropper AH. Spontaneous intracerebral hemorrhage. N Engl J Med. 2022;387(17):1589–1596. doi:10.1056/NEJMra2201449
3. Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
4. Ziai WC, Carhuapoma JR. Intracerebral hemorrhage. Continuum. 2018;24(6):1603–1622. doi:10.1212/CON.0000000000000672
5. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi:10.1161/STR.0000000000000069
6. Asuzu D, Nystrom K, Amin H, et al. Modest association between the discharge modified Rankin scale score and symptomatic intracerebral hemorrhage after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2015;24(3):548–553. doi:10.1016/j.jstrokecerebrovasdis.2014.09.034
7. Shao Z, Tu S, Shao A. Pathophysiological mechanisms and potential therapeutic targets in intracerebral hemorrhage. Front Pharmacol. 2019;10:1079. doi:10.3389/fphar.2019.01079
8. Guo H, Zhang Y, Hu Z, Wang L, Du H. Screening and identification of biomarkers associated with the immune infiltration of intracerebral hemorrhage. J Clin Lab Anal. 2022;36(5):e24361. doi:10.1002/jcla.24361
9. Yoshiko Y, Aubin JE. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides. 2004;25(10):1663–1669. doi:10.1016/j.peptides.2004.04.015
10. Tang SE, Wu CP, Wu SY, et al. Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med. 2014;71:321–331. doi:10.1016/j.freeradbiomed.2014.03.034
11. Ito Y, Zemans R, Correll K, et al. Stanniocalcin-1 is induced by hypoxia inducible factor in rat alveolar epithelial cells. Biochem Biophys Res Commun. 2014;452(4):1091–1097. doi:10.1016/j.bbrc.2014.09.060
12. Huang L, Garcia G, Lou Y, et al. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174(4):1368–1378. doi:10.2353/ajpath.2009.080476
13. Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38(3):1025–1030. doi:10.1161/01.STR.0000258113.67252.fa
14. Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A. 2000;97(7):3637–3642. doi:10.1073/pnas.97.7.3637
15. Bonfante S, Joaquim L, Fileti ME, et al. Stanniocalcin 1 inhibits the inflammatory response in microglia and protects against sepsis-associated encephalopathy. Neurotox Res. 2021;39(2):119–132. doi:10.1007/s12640-020-00293-y
16. Durukan Tolvanen A, Westberg JA, Serlachius M, et al. stanniocalcin 1 is important for poststroke functionality, but dispensable for ischemic tolerance. Neuroscience. 2013;229:49–54. doi:10.1016/j.neuroscience.2012.10.062
17. Jun Q, Luo W. Early-stage serum stanniocalcin 1 as a predictor of outcome in patients with aneurysmal subarachnoid hemorrhage. Medicine. 2021;100(51):e28222. doi:10.1097/MD.0000000000028222
18. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. doi:10.1161/01.str.27.8.1304
19. Han X, You S, Huang Z, et al. Prognostic significance of serum magnesium in acute intracerebral hemorrhage patients. Curr Neurovasc Res. 2019;16(2):123–128. doi:10.2174/1567202616666190412124539
20. Bonfante S, Della Giustina A, Danielski LG, et al. Stanniocalcin-1 ameliorates cerebral ischemia by decrease oxidative stress and blood brain barrier permeability. Microvasc Res. 2020;128:103956. doi:10.1016/j.mvr.2019.103956
21. Sun B, He S, Liu B, et al. Stanniocalcin-1 protected astrocytes from hypoxic damage through the AMPK pathway. Neurochem Res. 2021;46(11):2948–2957. doi:10.1007/s11064-021-03393-z
22. Long Y, Zou L, Liu H, et al. Altered expression of randomly selected genes in mouse hippocampus after traumatic brain injury. J Neurosci Res. 2003;71(5):710–720. doi:10.1002/jnr.10524
23. Patel TR, Schielke GP, Hoff JT, Keep RF, Lorris Betz A. Comparison of cerebral blood flow and injury following intracerebral and subdural hematoma in the rat. Brain Res. 1999;829(1–2):125–133. doi:10.1016/s0006-8993(99)01378-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.