Back to Journals » Infection and Drug Resistance » Volume 15
Epidemiological Characteristics of Chronic Viral Hepatitis in Kazakhstan: Data from Unified Nationwide Electronic Healthcare System 2014–2019
Authors Ashimkhanova A, Syssoyev D , Gusmanov A , Yesmembetov K, Yespotayeva A, Abbay A, Nurpeissova A, Sarria-Santamera A , Gaipov A
Received 10 March 2022
Accepted for publication 9 June 2022
Published 27 June 2022 Volume 2022:15 Pages 3333—3346
DOI https://doi.org/10.2147/IDR.S363609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Aiymkul Ashimkhanova,1,* Dmitriy Syssoyev,1,* Arnur Gusmanov,1 Kakharman Yesmembetov,2 Arina Yespotayeva,3 Anara Abbay,1 Aiymzhan Nurpeissova,4 Antonio Sarria-Santamera,1 Abduzhappar Gaipov1
1Department of Medicine, Nazarbayev University School of Medicine, Nur-Sultan, Kazakhstan; 2Department of Medicine III, University Medical Center Aachen, Aachen, Germany; 3Al-Farabi University, Faculty of Medicine and Healthcare, Almaty, Kazakhstan; 4Department of Medical Information Analysis of Outpatient and Polyclinic Care, Republican Center of Electronic Healthcare, Nur-Sultan, Kazakhstan
*These authors contributed equally to this work
Correspondence: Abduzhappar Gaipov, Department of Medicine, Nazarbayev University School of Medicine, Kerey and Zhanibek Khans Street 5/1, Nur-Sultan, 010000, Kazakhstan, Tel +7 7172 706 297, Email [email protected]
Background: Viral hepatitis is the leading cause of hepatic cirrhosis and liver-related mortality, yet there are no countrywide epidemiological studies available to date in Kazakhstan. The aim of the study was to perform an estimation of mortality, prevalence and incidence of Hepatitis B and C infections and liver-related complications.
Methods: Using centralized healthcare data from the Unified National Electronic Health System (UNEHS) for the period 2014– 2019, a total of 82,700 registered patients with chronic viral hepatitis B (HBV), C (HCV) and D (HDV) have been extracted based on ICD − 10 codes. Crude rates of incidence, prevalence and mortality, as well as age-, sex- and year-specific rates of incidence and mortality per 100,000 population were estimated. Unadjusted and adjusted hazard ratios were estimated using Cox proportional hazards regression modeling.
Results: For the total number of 82,700 patients, 56.6% were represented by chronic HCV infection and 43.4% by HBV infection. The prevalence of coinfection was 10% for HBV+HDV and 3.5% for HBV+HCV. Both HBV and HCV were more prevalent among female patients (56%) and among Kazakh ethnic group (64.8%). Males with HBV had a higher probability of death than females; this trend was stronger among male patients with HCV. Russian ethnic groups infected with HBV had a higher risk of death compared to Kazakh and other ethnic groups. Whereas in HCV-infected patients, Russian ethnic group and other ethnic group had similar risk for death, but higher compared to Kazakhs.
Conclusion: During the 2014– 2019 period, prevalence, incidence and mortality from chronic HBV and HCV infections increased. Despite the disproportionately higher infection rate among females with chronic viral hepatitis, all-cause mortality was more than two-fold higher among males. Higher death rates in Russian ethnic group compared to other ethnicities need to be evaluated in further studies for other confounding factors and associated comorbidities in this group.
Keywords: HBV, HCV, mortality, incidence, prevalence, survival
Introduction
Blood-borne viral hepatitis B (HBV), D (HDV), and C (HCV) are the major causes of liver-related mortality worldwide and continue to be a global health concern.1 According to the WHO report on combating viral hepatitis, the projected mortality could be as high as 19 million hepatitis-related deaths from 2015 to 2030. Hepatitis elimination proposal by the WHO has been aimed to eliminate new viral hepatitis infections by 90% and reduce mortality by 65% by 2030.2
Up to date, there are over 250 million individuals infected with chronic viral hepatitis B worldwide. HBV remains the leading cause of liver cirrhosis and liver failure, hepatocellular carcinoma and other liver-related mortalities.3 HDV is a defective virus, which requires HBV in order to propagate in liver cells. In endemic areas, viral HDV adds a greater risk to HBV coinfection- and superinfection-related morbidity and mortality.4 Although an estimated prevalence of HDV among HBsAg-positive people is in the 10–13% range, the rates of liver-related complications and overall outcomes in HBV/HDV people are significantly worse.5,6 There is a limited number of studies showing the proportion of deaths related to HDV coinfection among total HBV-related deaths.
The global prevalence of viral hepatitis C is estimated to be at 130–170 million persons. HCV is one of the major causes of hepatocellular carcinoma (HCC) and liver transplantation referrals worldwide, and also in Kazakhstan.7 The revolutionary role of direct acting antivirals (DAA) in treatment of HCV and its affordability made the goals set by the WHO more achievable, with 9.4 million cured cases over 2015–2019.8 In the United States, the age-adjusted HCV mortality rate in 2013 was 5.03 per 100,000 persons. With the introduction of DAA, this number was reduced to 3.3 per 100,000 persons in 2019. In order to meet the WHO aim of reducing HCV-related deaths to 3 per 100,000 persons by 2025, mortality rate should be reduced by 9.9%.9 For developing countries with higher prevalence of HCV, this ambitious goal of mortality reduction could be challenging, as the national economies with healthcare expenditure limits, social, political and other factors need to be taken into consideration. In the example of Egypt, where the nationwide seroprevalence of HCV is estimated at 14%, the rate of HCV-related mortality was reported to be 33 per 100,000 in 2013, with the aim of reducing this to 7.5 per 100,000 by 2030 through the implementation of National elimination program.10
Kazakhstan, as one of the post-Soviet Union countries, faced many challenges in reforming the healthcare system, including prevention and control of communicable diseases, such as viral hepatitis.11 Epidemiological data on viral hepatitis in Kazakhstan is scattered and inconsistent. Review on HBV prevention and treatment in Central Asian countries reported the estimated prevalence of HBV infection as 3.4%.12 According to the recent systematic review on prevalence of HIV, HCV and HBV in Central Asian countries, the highest prevalence of HCV was reported in Kazakhstan with 90.1% among Intravenous Drug Users (IVDU); and HBV among IVDU to be 74.2% and 85.3% in two regions of the country.13 Nationwide universal vaccination program was implemented in 1998 and is expected to decrease the burden of HBV infection in future decades.14
Currently, all available data is either from single-center analysis or from studies performed within high-risk populations.15–17 For example, two major Liver transplant centers in Kazakhstan performed a little over 200 cases of orthotopic liver transplants with half of the referrals due to viral hepatitis B, C and D. Among 131 liver transplantation cases at Syzganov Center, Almaty, 47 cases were due to HBV and HBV+HDV infection, and 12 cases – due to HCV-induced liver cirrhosis.18 Another study from the National Oncology Center in Nur-Sultan reported 30 patients with liver cirrhosis due to HBV and HBV+HDV infections, and four cases of HCV infection among 64 transplantations carried out at the center.19,20 These reports with high rates of viral hepatitis among transplant referrals indicate the need to identify the true prevalence of viral hepatitis to implement policies for reducing the burden of disease.
Due to scarce data on viral hepatitis in the country, there is a demand for collective data addressing the epidemiology of viral hepatitis and its burden in Kazakhstan at a country scale. The establishment of the Unified National Electronic Health System (UNEHS) in 2014 has given us the opportunity to widen the possibility of learning the impact of this problem. Therefore, the objective of the current study is to estimate the prevalence, incidence and all-cause mortality from chronic viral hepatitis B and C infections in Kazakhstan for the 5-year period by using large-scale data from UNEHS for the period of 2014–2019.
Methods
Study Population and Design
The initial study sample consisted of 20,810,911 patient records received from UNEHS for the period of 2014–2019 as 2 separate registries – inpatient and outpatient. Out of 11,157,509 records in the outpatient registry, 69,560 corresponded to unique patients with hepatitis according to the International Classification of Diseases (ICD) 10th Revision (ICD-10 codes B15-B19). The inpatient registry contained 9,653,402 total records, corresponding to 20,170 unique hepatitis patients. The patients in the registry had either been diagnosed with hepatitis between 2014 and 2019 or had died while under outpatient care in this period. The flowchart in Figure 1 shows stepwise cohort selection.
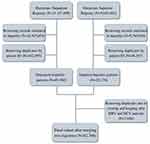 |
Figure 1 The flowchart of cohort selection. |
After merging two registries, duplicate observations had been removed. Only patients with HBV or HCV as their primary diagnosis were enrolled into the study. Inclusion or exclusion was determined based on ICD-10 codes only. Patients with ICD-10 codes B16.2, B16.9, and B18.1 were classified as having HBV without HDV superinfection, patients with ICD-10 codes B16.0, B16.1, B17.0, and B18.0 – as having HBV with HDV superinfection, while patients with ICD-10 codes B17.1 and B18.2 were classified as having HCV. The final cohort consisted of 82,700 unique patient records.
Exposures and Covariates
The individual patient data used in the analysis included age, sex, ethnicity, coinfections, progression type, outcomes (including complications or death), hepatitis type, date of diagnosis, date of death and follow-up duration. Age was divided into 5 categories (0–18 years, 19–34, 35–50, 51–70, 71+), ethnicity was categorized as Kazakh, Russian, and others. Coinfections were defined as HBV patients concurrently diagnosed with HCV, HDV, or both. Progression type was defined as either “acute” or “chronic”. The outcomes included relevant complications, namely liver failure (K72.9), liver cirrhosis (K74.4, K74.6), portal hypertension (K76.6), hepatorenal syndrome (K76.7), hepatocellular carcinoma (C22.0), intrahepatic bile duct carcinoma (B22.1), ascites (R18), and all-cause deaths. Complications were identified using ICD-10 codes. The dates of birth and death (if applicable) were obtained from the Population Registry based on population registry number (RPN). The follow-up time was calculated as either the difference between the earliest diagnosis of hepatitis and the time of death or December 31, 2019, which is the end of the follow-up period for which the data were obtained.
Outcome Assessment
Incidence, all-cause mortality and period prevalence of HBV and HCV patients were assessed. For each year of observation between 2014 and 2019, the crude rates per 100,000 population were estimated using Kazakhstan population data obtained from Information-Analytical System of Statistical Government.21 Incidence and mortality rates were calculated by dividing the number of new cases or the number of deaths at the end of the year, respectively, by total population of Kazakhstan at that year. Period prevalence was calculated by dividing the cumulative number of patients diagnosed with HBV or HCV alive at the end of the year by the total population for that year. The sex-specific incidence and mortality rates were subdivided by hepatitis type (HBV or HCV) and calculated by categorization by year or age category (with an increment of 5 years from 0 to 100 years of age). Year- and sex-specific incidence rate (IR) was calculated by dividing the number of new cases of a given hepatitis type and sex at the end of the year by the total population count of that sex at that year. Age- and sex-specific incidence was calculated by dividing the number of new cases of a specific hepatitis type within a given age category and sex by the median population size of that sex in the period of 2014–2019.
Statistical Analysis
In descriptive analysis tables, the categorical data were presented as numbers of corresponding patients and their percentages. The continuous variables are presented as means with standard deviation. Bivariable analysis was conducted using t-test, chi-square test or Fisher’s exact test where appropriate. Cox proportional hazards regression analysis was used to obtain crude and adjusted hazard ratios. The final adjusted model included hepatitis type, age category, ethnicity, and sex. These were chosen for significant differences in HBV and HCV patients during the bivariable analysis. While also significantly different, progression type was not included due to the number of diagnosed acute cases being possibly unrepresentative due to poor capture compared to chronic hepatitis. Complications were not included as well, due to possible incomplete capture. Interaction between the covariates were assessed, and the significant interaction terms were included in the final model. The two assumptions of the Cox proportional hazards regression modelling were tested using Schoenfeld residuals and the log–log plot. Kaplan–Meier estimation was used to calculate and depict the adjusted failure functions by age category, sex, and ethnicity. Log rank test was used to assess the significance of the difference between the curves. Statistical analysis, data cleaning (identifying and removing duplicate cases) and data management (labeling all data, creating and categorizing new variables) were performed using STATA 15 MP2 Version (STATA Corporation, College Station, TX). P values are two-sided and reported as statistically significant at <0.05 for all analyses.
Ethical Approval
The study was conducted in compliance with the Declaration of Helsinki and approved by the Nazarbayev University Institutional Review Ethics Committee (NU-IREC 315/21092020 on 23/09/2020). Exemption from informed consent was granted due to the retrospective nature of the study in which only the anonymous data were analyzed.
Results
Demographics and General Characteristics
The demographic information about the cohort is presented in Table 1. The mean age of the cohort was 43.3 ± 15.3 years, with higher mean age among chronic HCV patients, compared to chronic HBV patients. Chronic HBV is more prevalent in the younger category of 19–34 y.o. with 38.6%, compared to chronic HCV infection with 19.3% in this age group. In the age category of 51–70 y.o., chronic HCV infection was found in 34.9% of the patients, while chronic HBV infection – in 22.2%. Females constituted 56% of the cohort with equal gender distribution in HBV and HCV groups. The Kazakh ethnic group was represented by 64.8% of the cohort, Russian ethnic group – by 19.6%, and all others – by 15.6%. Among the 82,700 registered cases, 56.6% correspond to chronic HCV, and 43.3% – to chronic HBV infection. The proportions of coinfections with chronic HBV+HDV, HBV+HCV and HBV+HDV+HCV were 10%, 3.5%, and 0.5%, respectively. Chronic cases constituted 96.6% of the sample in the HBV group, and 99.0% in the HCV group. Hepatic failure events in acute patients were registered only in HBV patients. On the contrary, liver failure events superimposed on chronic infection were similar in both groups. Chronic HCV patients had a higher proportion of portal hypertension, compared to HBV patients (2.3% vs 1.9%). Other complications such as ascites, hepatorenal syndrome, and liver cancer – HCC and CCC – were similar in both HCV and HBV patients. Chronic HCV patients had higher death rates compared to chronic HBV patients (5.3% vs 3.2%).
 |
Table 1 General Characteristics of Patients Subdivided by Hepatitis Type |
Prevalence and Incidence
Among people with coinfections, the HBV+HDV group had the highest prevalence of hepatocellular carcinoma (1.1%) compared to HBV+HCV coinfected patients (0.7%), whereas HBV+HDV+HCV coinfected patients had none. Liver cirrhosis prevalence was similar in all coinfection groups (Supplementary Table 1).
The crude prevalence and incidence rates for both HBV and HCV among registered cases during 2014–2019 period was calculated per 100,000 population. Prevalence of HBV infection, as shown in Figure 2A, had a dramatic increase from 47.6 per 100,000 population in 2014 and 191.1 per 100,000 population in 2018. Prevalence of HCV infection similarly has a substantial increase for the period of 2014–2019 from 72.5 per 100,000 population to 245.6 per 100,000 population over the years (Figure 2B). Incidence and mortality rates for both HBV and HCV show a trend of moderate increase with each year. Absolute numbers of prevalent and incident cases, as well as deaths are given in Figure 2C for HBV patients and in Figure 2D for HCV patients.
Year- and sex-specific incidence rate (IR) for HBV shows a steady increase predominantly for the female group up to 40 per 100,000 population per year; it shows a slight decline to 30 per 100,000 population in 2019. In comparison, HCV has higher incidence rates with a similar trend among females with almost 50 per 100,000; around 40 per 100,000 among males with a plateau by 2019 without decline in cases (Figure 3A). Age- and sex-specific incidence rate for the entire period of 2014–2019 per 100,000 median population was calculated. HBV incidence rate peaked around age 35 for females with a gradual decline in later ages. For males, the rate remained level between ages 35 and 75. Among HCV patients, incidence rates among females reached over 500 in age category of 55–75 with a drop after that. Among males, incidence rate peaked at 500 in age 45, plateauing at 400 in ages 50–70 and then declining (Figure 3B).
 |
Figure 3 Incidence rates of HBV and HCV per 100,000 population over 2014–2019 ((A) – crude year- and sex-specific, (B) – crude age- and sex-specific). |
Similar approaches were used in the estimation of year- and sex-specific, and age- and sex-specific mortality rates (MR) per 100,000 population. A steady rise in MR from under one to up to four per 100,000 between 2014 and 2019 can be seen in all groups, with HCV patients showing higher mortality rates than HBV patients, and males demonstrating higher mortality rates than females for both hepatitis types (Figure 4A). Similar trends across both hepatitis types are seen in age categorization, with the highest peak MRs at age 75 reaching over 60 per 100,000 in females with HCV, down to 20 per 100,000 in males with HBV (Figure 4B).
 |
Figure 4 Mortality rates of HBV and HCV per 100,000 population over 2014–2019 ((A) – crude year- and sex-specific, (B) – crude age- and sex-specific). |
Table 2 compares the demographics and outcomes of these patients. The development of HCC occurred in older age of 60.1 ± 10.5, compared to cirrhosis age of 50.9 ±12.3. Male patients had higher rates of cancer - 58.3%, compared to the female patients; however, liver cirrhosis was prevalent among females at 51.8%. Kazakh ethnic group showed higher rates of cancer at 70.6%, compared to Russian and other ethnic groups. Liver failure incidents were prevalent in the cirrhosis subgroup with 2.6% vs 0.8% in the cancer subgroup. The prevalence of complications was higher in the cirrhosis subgroup compared to cancer subgroups, namely portal hypertension (61.2% vs 21.4%), hepatorenal syndrome (3.8% vs 0.8%) and ascites (9.8% vs 2.7%). Among 374 cases of cancer, 2.7% were attributable to cholangiocarcinoma. Death rates were highest in the cancer subgroup, reaching 55.9%.
 |
Table 2 General Characteristics of Patients Subdivided by Presence of Cirrhosis and Hepatocellular Carcinoma |
Mortality Rate Among Chronic Hepatitis Patients
Stratification of mortality rates per 1000 person-years by hepatitis type is shown in Table 3. Male patients had a higher crude mortality rate compared to females in the HBV cohort (61.3% vs 38.7%) and in the HCV cohort (59.3% vs 40.6%). Deaths from cirrhosis and HCC were higher in the HBV cohort with 32.2% and 7.6%, respectively. In the HCV cohort, death from cirrhosis and HCC was observed in 26.1% and 4.9% patients, respectively. Deaths among patients with ascites were also higher in the HBV cohort with 5.1% compared to HCV with 4.7%. With increasing age, mortality was also rising sharply in both HBV and HCV cohorts.
 |
Table 3 Death Counts and Mortality Rates per 1000 Person-Years Stratified by Hepatitis Type |
In Kaplan–Meier analysis in Figure 5, the estimates of probability of death were obtained. According to these estimates, males with HBV infection had higher probability of death than females (Figure 5A). Among male patients with HCV infection, this gender-based trend is even stronger (Figure 5B). The Russian ethnic group was found to be at a higher risk of death among HBV patients, compared to Kazakhs and other ethnic groups (Figure 5C); in Hepatitis C patients, Russian and other ethnicities had a similar risk of death, but higher compared to Kazakhs (Figure 5D).
 |
Figure 5 Mortality estimates in HBV patients ((A) – based on sex, (C) – based on ethnicity), and in HCV patients ((B) – based on sex, (D) – based on ethnicity). |
Mortality estimates by age category adjusted for sex and ethnicity among patients diagnosed with HBV or HCV are shown in Supplementary Figure 1. Among people with HBV infection, the mortality rate in the age category of 71+ has the marked increase in mortality. HCV infection has similar trends in mortality rates in the age category of 71+.
In Cox proportional hazard regression analysis (Table 4), the older age was associated with higher all-cause mortality across the age categories and this trend increased further after adjustments. Age category of 0–18 was excluded for better comparability since it contributed to disproportionality of hazards. Male patients with chronic hepatitis had 1.99-fold higher risk of death (unadjusted HR 1.99; 95% CI: 1.85–2.13) compared to female patients, and this risk increased after adjustments (adjusted HR 2.44; 95% CI: 1.90–3.13). The Russian ethnic group had a higher hazard ratio compared to the Kazakh ethnic group with unadjusted HR of 1.57 (95% CI: 1.45–1.69) and adjusted HR of 1.63 (95% CI: 1.25–2.12). Similarly, other ethnic groups had higher HR of 1.37 (95% CI: 1.13–1.37), and after adjustment HR it remained at 1.37 (95% CI: 0.99–1.89), compared to Kazakh ethnicity. HCV patients had 71% higher risk of all-cause death (unadjusted HR 1.71; 95% CI: 1.59–1.84) compared to HBV infected patients, and this number increased to HR of 2.01 (95% CI: 1.56–2.61) after adjustments.
 |
Table 4 Adjusted and Unadjusted Hazard Ratios with 95% Confidence Intervals for All-Cause Mortality Among Hepatitis Patients |
Discussion
Using the information from the large representative data of UNEHS for the analysis of viral hepatitis in the 5-year period from 2014 to 2019, we found that the prevalence and incidence of viral hepatitis B and C had increased in this period. Prevalence of HBV infection had a dramatic increase of more than 4 times for the 5-year period, whereas HCV prevalence increased more than 3 times. Although the prevalence of both HBV and HCV infections was higher in female patients, survival estimates were significantly lower for males having HBV and HCV infections. Furthermore, Russian ethnic group was found to be at higher risk of death from HBV infection compared to Kazakh ethnic group and other ethnicities after adjustments for age and gender.
The findings in all-cause mortality rates presented by increase in both HCV- and HBV-related deaths during the 5-year period are similar to those in the Eurasian continent countries neighboring Kazakhstan. According to the Russian Federal Statistics Agency from Saint Petersburg, all-cause mortality from Hepatitis C for the period of 2013–2017 had an increasing tendency. If the number of deaths per 100,000 was 1446 from HCV infection in 2013, it went up to 5696 in 2017.22 Uzbekistan estimated the death and overall trends of HBV using a mathematical simulation model. The estimation showed 3074 deaths per year from HBV-related causes. This could be attributed to the higher prevalence of HBsAg carriers in the country compared to worldwide statistics predicted by the WHO.23 With the introduction of free DAA access in the country, there is an expectation of a decrease in the HCV burden in future years.24
The trend of increase in incidence of HCV that has been observed between 2014 and 2019 can be explained by several factors. Donor blood screening has been improved with 6-month serum preservation and highly closed-system PCR machines for screening of donor blood. Another explanation is better surveillance and awareness starting from the early 2010s. In 2014, the Ministry of Health established medical centers for hepatitis follow-up and treatment in all 14 regions of the country. Age peaks for both male and female patients at 60 and 75 years can possibly be explained by the time of their infection, which is prior to the introduction of HCV screening and safety. As for the peak at age 40 among males, this could correspond to the population at risk (IVDUs, HIV-coinfected, hemodialysis, or other).
This analysis demonstrates that the prevalence of HBV+HDV coinfection is 10% among registered patients, and HBV+HCV coinfection – 3.5%. Moreover, HBV+HDV coinfection patients had a higher prevalence of HCC compared to HBV+HCV coinfection. Most published reviews report the prevalence of HDV coinfection to be 10–13% among HBV patients.6,25 In this study, the prevalence of HBV+HDV coinfection showed 10% prevalence that is comparable to the reported world database. Moreover, HBV+HDV coinfection is associated with the most severe form of chronic hepatitis and high risk of cirrhosis and HCC.26
Furthermore, there was a gender-dependent trend in prevalence of HCC development in Kazakhstan. Our study showed a higher prevalence of HCC, but a lower prevalence of liver cirrhosis in male patients. Ethnic Kazakhs had a higher prevalence of HCC compared to Russians and other groups. In the United States, the HCC incidence was more than 4 times higher among Asian Pacific Islanders compared to Caucasians.27 A hypothesis that could be raised in view of this data is the possible effect of genetic determinants in Asian populations, which may make them more susceptible compared to Russians in the development of HCC.
The estimation of probability of death showed that males with either HBV or HCV infection had a higher risk of death compared to females. This sex disparity in the survival outcome is proposed to be multifactorial as male patients have other risk factors apart from chronic viral hepatitis. One of the possible mechanisms was attributed to sex hormone differences in HBV progression.28 A possible protective effect of better immune clearance to prevent chronic HCV progression in females has been rejected due to potential bias from the study.29
In addition, the results revealed that the Russian ethnic group had higher death rates with both unadjusted and after adjustment hazard ratios. This finding has never been addressed before in Central Asian studies, concerning ethnicity disparities within the country. There has been a report from European CDC analysis looking at the prevalence of chronic HBV and HCV infections among immigrants in European countries, where Russian immigrants had around 50–60 thousand cases and Kazakhstani accounted for about 20 thousand cases.30
As in all retrospective studies, there are potential limitations that should be considered. Foremost important is the inability to analyze cause-specific mortality among chronic hepatitis patients based on this database. Another downside is the possible errors in data coding: some cirrhosis patients with decompensation or acute exacerbations might be coded without prior hepatitis codes, thus some patients might be out of the count. In addition, this database does not provide seroprevalence of viral hepatitis or information about medication and treatment regimens; the study population identification was based only on ICD-10 codes. One downside of ICD-based data excavation in our case may be the limited ability to recognize other contributing factors of liver disease, such as alcohol, which could possibly shed light on the increased mortality in certain subgroups. Confounding factors could be more than what this study was able to adjust for, so generalization of some results will not be attempted. Despite these aforementioned limitations, this study is the first to report from the Central Asian region the epidemiology and complications related to chronic viral hepatitis. This UNEHS system provides a reliable platform to investigate important questions enabling the researchers to analyze overall mortality from chronic viral hepatitis and the proportion of related complications based on ICD-10 codes such as HCC, CCC, PH, HRS and others. With a breakthrough in DAA treatment of HCV infection, this data might provide essential information for future policy making towards elimination of HCV in this region.
Conclusion
In conclusion, this retrospective cohort study provides evidence for the increase in prevalence, incidence and all-cause mortality from HBV and HCV infections in Kazakhstan. Use of administrative healthcare data allowed analyzing data for the period of 2014–2019. Despite the higher infection rate among females with chronic viral hepatitis, male patients showed mortality rates twice higher than females. Death rates depending on ethnicity also showed a difference in survival that needs further validation studies.
Abbreviations
HBV, viral hepatitis B; HCV, viral hepatitis C; HDV, viral hepatitis D; HCC, hepatocellular carcinoma; CCC, cholangiocarcinoma; PH, portal hypertension; HRS, hepatorenal syndrome; IVDU, intravenous drug users; WHO, World Health Organization; DAA, direct acting antivirals; CI, confidence interval; HR, hazard ratio; UNEHS, Unified National Electronic Health System; RPN-ID, population registry number; ICD, International Code of Diseases; PY, person-years; MR, mortality rates; IR, incidence rates.
Data Sharing Statement
All data related to this study are available from the Republican Center for Electronic Health of the Ministry of Health of the Republic of Kazakhstan, but restrictions apply to the availability of these data, which were used under the contract agreement for the current study, and so are not publicly available. Data are, however, available from the authors ([email protected]) upon reasonable request and with permission from the Ministry of Health of the Republic of Kazakhstan.
Acknowledgment
Aiymkul Ashimkhanova and Dmitriy Syssoyev equally contributed as a first author.
Funding
This study was funded by the Ministry of Education and Science of the Republic of Kazakhstan grant funding [Funder Project Reference: #AP09259016, title: Epidemiology and Forecasting of Infectious Diseases in Kazakhstan Using Big Healthcare Data, Mathematical Modeling, and Machine Learning.] The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. A. Gaipov is the PI of the projects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Easterbrook P, Luhmann N, Newman M, et al. New WHO guidance for country validation of viral hepatitis B and C elimination. Lancet Gastroenterol Hepatol. 2021;6(10):778–780. doi:10.1016/S2468-1253(21)00267-3
2. World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. World Health Organization; 2021.
3. Cox AL, El-Sayed MH, Kao J-H, et al. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17(9):533–542. doi:10.1038/s41575-020-0332-6
4. Papatheodoridi M, Papatheodoridis GV. Current status of hepatitis delta. Curr Opin Pharmacol. 2021;58:62–67. doi:10.1016/j.coph.2021.03.008
5. Miao Z, Zhang S, Ou X, et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. 2019;221(10):1677–1687. doi:10.1093/infdis/jiz633
6. Hou C, Hua Z, Xu P, et al. Estimating the prevalence of hepatitis B by wastewater-based epidemiology in 19 cities in China. Sci Total Environ. 2020;740:139696. doi:10.1016/j.scitotenv.2020.139696
7. Petruzziello A, Marigliano S, Loquercio G, et al. Global epidemiology of hepatitis C virus infection: an update of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–7840. doi:10.3748/wjg.v22.i34.7824
8. World Health Organization. New report highlights global progress on reducing HIV, viral hepatitis and sexually transmitted infections and signals need for renewed efforts to reach 2030 targets. Geneva; 2021. Available from: https://www.who.int/news/item/20-05-2021-new-report-highlights-global-progress-on-reducing-hiv-viral-hepatitis-and-sexually-transmitted-infections-and-signals-need-for-renewed-efforts-to-reach-2030-targets.
9. CDC WONDER Online Database, r.i., Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999–2018 on CDC WONDER Online Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999–2018, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. 2020.
10. Katsaga A, Kulzhanov M, Karanikolos M, et al. Kazakhstan health system review. Health Syst Transit. 2012;14(4):1–154.
11. Igissinov N, Kulmirzayeva D, Sibinga CTS, et al. Epidemiological aspects of hepatitis B and C markers in blood donors in Kazakhstan; 2000–2011. Iran J Public Health. 2014;43(2):156–161.
12. Amerzhanov D, Suleimenova I, Davlidova S, et al. HBV prevention and treatment in countries of Central Asia and the Caucasus. Viruses. 2020;12(10):1112. doi:10.3390/v12101112
13. Davlidova S, Haley-Johnson Z, Nyhan K, et al. Prevalence of HIV, HCV and HBV in Central Asia and the Caucasus: a systematic review. Int J Infect Dis. 2021;104:510–525. doi:10.1016/j.ijid.2020.12.068
14. Konysbekova A. Chronic viral hepatitis D – the current state of a problem. J Clin Med Kazakh. 2017;1(43):11–14. doi:10.23950/1812-2892-JCMK-00388
15. Mukhatayeva A, Mustafa A, Dzissyuk N, et al. Author correction: hepatitis B, hepatitis C, tuberculosis and sexually-transmitted infections among HIV positive patients in Kazakhstan. Sci Rep. 2021;11(1):18123. doi:10.1038/s41598-021-97673-x
16. Almadiyeva A, Ibrayev S, Turgambayeva A, et al. Cost-effectiveness of oral protease inhibitors co-administration versus pegylated interferon-Alpha2b and ribavirin only for the patients with hepatitis C genotype 1 in Kazakhstan Health Care Settings. Iran J Public Health. 2018;47(12):1845–1853.
17. Yesmembetov K, Ashimkhanova A, Kaliaskarova K. First case in Kazakhstan of successful therapy with 2 consecutive direct-acting antiviral regimens in a patient with hepatitis C virus-induced decompensated liver cirrhosis on a liver transplant wait list. Exp Clin Transplant. 2016;14(Suppl 3):112–113.
18. Baimakhanov Z, Kaniev S, Doskhanov M, et al. Evolution of liver transplantation in Kazakhstan: two-era experience of a single center, the first report. Transplant Proc. 2019;51(10):3360–3363. doi:10.1016/j.transproceed.2019.07.023
19. Sultanaliev T, Zhexembayev A, Mukazhanov A, et al. Liver transplant outcomes in a newly started program. Exp Clin Transplant. 2015;13(Suppl 3):120–122. doi:10.6002/ect.tdtd2015.P66
20. Saparbay J, Spatayev J, Sharmenov A, et al. Liver transplantation: a 10-year low-volume transplant center experience in Kazakhstan. Ann Transplant. 2021;26:e931786. doi:10.12659/AOT.931786
21. Agency, B.o.N.s.O.t. Taldau statistical database. 2014–2019. 2019.
22. POvkovskiy VI. Viral Hepatitis in Russian Federation 2018.
23. Beutels P, Musabaev EI, Van Damme P, et al. The disease burden of hepatitis B in Uzbekistan. J Infect. 2000;40(3):234–241. doi:10.1053/jinf.1998.0666
24. WHO. WHO progress report on access to hepatitis C treatment. 2018.
25. Chen HY, Shen D-T, Ji D-Z, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68(3):512–521. doi:10.1136/gutjnl-2018-316601
26. Bockmann J-H, Grube M, Hamed V, et al. High rates of cirrhosis and severe clinical events in patients with HBV/HDV co-infection: longitudinal analysis of a German cohort. BMC Gastroenterol. 2020;20(1):24. doi:10.1186/s12876-020-1168-9
27. Ochner M, Wong L, Wimmer-Kunitomo K. Hepatocellular cancer: risk factors and survival in Pacific Islanders compared to Caucasians in Hawaii. Ethn Dis. 2010;20:169–173.
28. Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136(5):1629–1638. doi:10.1053/j.gastro.2009.01.052
29. Stroffolini T, Rapicetta M, Di Stefano R. Hepatitis C virus clearance and gender. Gut. 2007;56(6):884.
30. ECDC. Epidemiological Assessment of Hepatitis B and C Among Migrants in the EU/EEA. Stockholm: ECDC; 2016.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.