Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Prevalence of HIV and Its Co-Infection with Hepatitis B/C Virus Among Chronic Liver Disease Patients in Ethiopia
Authors Tassachew Y , Abebe T , Belyhun Y , Teffera T, Shewaye AB, Desalegn H , Andualem H , Kinfu A , Mulu A, Mihret A , Howe R, Aseffa A
Received 11 March 2022
Accepted for publication 6 May 2022
Published 13 May 2022 Volume 2022:14 Pages 67—77
DOI https://doi.org/10.2147/HMER.S365443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Yayehyirad Tassachew,1– 3 Tamrat Abebe,1 Yeshambel Belyhun,4 Tezazu Teffera,5 Abate Bane Shewaye,6,7 Hailemichael Desalegn,8 Henok Andualem,9 Abiy Kinfu,10 Andargachew Mulu,2 Adane Mihret,1,2 Rawleigh Howe,2 Abraham Aseffa2
1Department of Microbiology, Immunology, and Parasitology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia; 3School of Medical Laboratory Sciences, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 4School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 5Department of Surgery, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 6Department of Internal Medicine, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 7Adera Medical Center PLC, Addis Ababa, Ethiopia; 8Department of Internal Medicine, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 9Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 10Ethiopian National Blood Bank Service, Addis Ababa, Ethiopia
Correspondence: Yayehyirad Tassachew, Department of Microbiology, Immunology & Parasitology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia, Tel +251-916829464, Email [email protected]; [email protected]
Background: The efficient use of antiretroviral drugs has significantly reduced AIDS-related morbidities and mortalities; however, mortality due to non-AIDS-related end-stage liver diseases is escalating in those living with HIV.
Objective: The study was designed to determine the prevalence of HIV and its co-infection with HBV and HCV among chronic liver disease (CLD) patients in Ethiopia.
Methods: Three hundred and forty-five CLD patients were included in this study in two groups: Hepatocellular carcinoma (HCC) (n=128) and non-HCC (n=217) patients. The non-HCC group comprised patients with advanced liver disease (n=98) and chronic hepatitis (n=119). Enzyme immunoassays were used to determine HBV and HCV infection markers. In addition, a serial rapid HIV testing algorithm was employed to screen HIV infection.
Results: Regardless of the stage of liver disease, the overall frequency of HIV was 4.3% (15/345), with a 2% (7/345) and 0.3% (1/345) of HIV/HBV and HIV/HCV co-infection rate. Of all HIV-infected patients (n=15), 46.7% (7/15) and 6.7% (1/15) were co-infected with HBV (HBsAg+HBcAb+) and HCV (anti-HCV+ HCV-RNA+), respectively, and 86.7% (13/15) exhibited a marker of HBV exposure (total HBcAb+). Overall, the frequency of HIV and its co-infection with HBV was more noticeable among HCC than non-HCC patients [8.6% (11/128) vs 1.8 (4/217), p=0.005 and 3.9% (5/128) vs 0.9% (2/217), p=0.1]. The rate of HIV mono-infection was 3.9% (5/128) vs 0.9% (2/217) among HCC and non-HCC patients.
Conclusion: The frequency of HIV and its co-infections with HBV/HCV exhibited an increasing pattern with the severity of the liver disease. Thus, screening all HIV-positive patients for HBV and HCV infection and all CLD patients for HIV infection and taking necessary preventive measures would be an essential strategy to prevent the progression of CLD and death related to liver disease in people living with HIV.
Keywords: HIV, HBV, HCV, HCC, non-AIDS liver diseases
Introduction
Human immunodeficiency virus (HIV), one of the most common blood-borne viruses, has overlapping modes of transmission with the two hepatotropic viruses, hepatitis B virus (HBV) and hepatitis C virus (HCV). These two viruses (HBV & HCV) are the most common viral etiologic agents of chronic liver disease (CLD).1 AIDS-related illnesses have been responsible for the death of 36.3 million people since the HIV epidemic started (https://aidsinfo.unaids.org).2 In comparison, hepatitis B and C infections are accountable for 90% of viral hepatitis-related deaths (1.4 million per annum) globally.3
In 2015, a total of 36.7 million people were infected with HIV, of whom 2.7 and 2.3 million people were co-infected with HBV and HCV, respectively.4 The reason for such a high co-infection rate could be the shared mode of transmission of these viruses, that is, contact with infected blood and body fluid through parenteral and sexual activities. The coexistence of HIV with hepatitis B or C viruses increases the chronicity, early progression, and high fatality of liver diseases compared to HBV or HCV mono-infection.5–9 Although deaths related to HIV infection have considerably dropped after large-scale use of highly active antiretroviral therapy (HAART), mortalities due to non-acquired immunodeficiency syndrome (AIDS) causes, such as viral and non-viral chronic liver diseases, remain prominent and challenging among HIV-infected individuals.10
More than 70% of HIV cases in the world reside in sub-Saharan Africa, where HBV is prevalent and responsible for most viral-related chronic liver disease conditions.4,11 Consequently, about 71% of HIV/HBV co-infected individuals reside in sub-Saharan Africa.4 Generally, HIV/HBV co-infection prevalence is predominant compared to HIV/HCV co-infection in the region.
Chronic hepatitis B (CHB) and C (CHC) infections are the leading factors that complicate and accelerate liver disease progression to advanced stages (Cirrhosis and HCC) in those living with HIV/AIDS.12–14 Equally, HIV infection noticeably affects the natural process of hepatitis B and C infections. In most cases, depleting immune response due to HIV infection favors hepatitis B or C replication in the hepatocytes, subsequently enhancing chronic liver disease sequels, such as liver cirrhosis and fibrosis.13–15
There are several mechanisms for the pathogenesis and injuries to the liver among HIV/AIDS patients, including immune-mediated injury, oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, toxic metabolite accumulation, gut microbial translocation, and systemic inflammation.16
Although chronic hepatitis B, C, and alcoholism-related CLD17,18 and HIV infection19 are common in Ethiopia, no scientific study has been conducted to evaluate the prevalence of HIV and its co-infection with HBV or HCV among CLD patients in Ethiopia. Thus, we aimed to assess the prevalence of HIV and its co-infection with HBV and HCV among CLD patients in Ethiopia.
Materials and Methods
Study Settings and Patients
A cross-sectional study involving 345 CLD patients was conducted between Dec. 2018 and Mar. 2019. The patients were recruited at the gastroenterology (GI) clinics of four selected referral hospitals (Tikur Anbessa Specialized Hospital, St. Paul’s Hospital, Armed Forces Hospital, and MyungSung Christian Medical Center /Korean Hospital/), two specialized private health care institutions (Adera Medical center and Yanet specialized clinic) during routine clinical practices. The four Hospitals and Adera Medical Center are located in Addis Ababa, the capital of Ethiopia, whereas Yanet specialized clinic is found in Hawassa, in southern Ethiopia. The CLD patients were included in the study in two groups: HCC (n=128) and non-HCC (n=217) patients. The non-HCC groups comprised patients with advanced liver disease (AdLD) (n=98) and chronic hepatitis (CH) (n=119).
Patient Selection
Chronic liver disease patients (patients with repeated and prolonged liver inflammation accompanied by gradual deterioration of liver functions) were recruited by the assigned gastroenterologist based on clinical, pathological, and biochemical analysis and imaging modalities during routine clinical practice at the GI unit of each study site. All HCC cases were confirmed with the standard diagnostic tests (Magnetic resonance imaging, MRI/ Computerized tomography, CT), biopsy, or Ultrasound (US) plus elevated serum alpha-fetoprotein (AFP) >400 ng/mL.
Inclusion and Exclusion Criteria
Inclusion Criteria
Chronic liver disease patients aged ≥18 years old, attending the gastroenterology units of all study sites, but mentally competent and willing to participate in the study.
Exclusion Criteria
Chronic liver disease patients with metastasized liver cancer, clinically confirmed chronic hepatic schistosomiasis, and a critical health condition/ hepatic coma were excluded from the study.
Data Collection
At each study site, the pre-assigned trained nurse collected all relevant information related to the sociodemographic data (age, gender, marital status, educational status, occupation, and residence) of the patients and their exposure to risk factors of HIV, HBV, and HCV infection (history of blood transfusion, tattooing, sexual practices, dental extraction, body piercing, abortion, and invasive medical procedures, and habit of smoking and alcoholic beverage drinking) using a pre-structured questionnaire in a face-to-face interview.
Specimen Collection and Processing
Ten milliliters (10 mL) of venous blood were taken from each study participant at each site and was dispensed into an ethylene diamine tetra-acetic acid (EDTA) tube and transported on dry ice to Armauer Hansen research institute (AHRI), where the plasma was separated via centrifugation at 3500 rpm/ 5min, transferred into cryotubes, and stored at - 20 °C until used for HIV, HBV, and HCV serology.
Detection of HIV1/2 Antibody
Plasma samples were screened for antibodies against HIV1/2 following the national serial rapid HIV testing algorithm and the WHO’s guidelines on HIV testing and counseling strategy.20,21 Accordingly, three commercially available rapid tests were used in the following order: Wantai (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., China) (screening test), Uni-GoldTM (Trinity Biotech PLC, Bray, Ireland) (confirmatory test), and Vikia (bioMérieux SA, France) (tiebreaker test). According to the HIV testing algorithm, to declare a person HIV positive, he/she should be positive with at least two tests. The sensitivity and specificity of each rapid test are summarized in the Supplementary Table 1.
Briefly, plasma samples that tested positive with the first test (Wantai) were subjected to the second test (Uni-Gold), and those found positive with the second test too considered positive for HIV1/2 antibodies. Likewise, those samples positive with the first test and negative with the second test (discordant result) were tested with the third test (Vikia), and samples that turned positive were considered positive for HIV1/2 antibodies. Results from each test were interpreted as per the manufacturer’s instruction.
Serodiagnosis of Chronic Hepatitis B and C Infection
The diagnosis of hepatitis B and C virus infection was established through serological assays and additional molecular assay for HCV RNA (RT-PCR). All the assays were employed, adhering to the WHO’s recommendation for diagnosing hepatitis B and C infection in developing and low-income countries.22
Detection of HBsAg and HBcAb
Different 3rd generation enzyme-linked immunosorbent assay (ELISA) kits from Bio-Rad Company were used to screen plasma samples for evidence of HBV infection: HBsAg (MonolisaTM HBs Ag ULTRA) and Anti-HBc (IgM and IgG) (Monolisa Anti-HBc PLUS).23 Patients with positive HBsAg and HBcAb tests (HBsAg+ HBcAb+) were considered for HBV co-infection. All the assays were performed as per the manufacturer’s instruction.
Detection of Anti-HCV
Hepatitis C infection was determined based on anti-HCV and capsid antigens positivity using a 3rd generation enzyme-linked immunosorbent assay (MonolisaTM HCV Ag-Ab ULTRA V2 ELISA kits, Bio-Rad).24 All samples with detectable anti-HCV in the first assay were retested by the ARCHITECT Anti-HCV assay (Abbott).25 Both assays were performed according to the manufacturer’s instructions.
Detection of HCV RNA
The Abbott RealTime HCV assay (Abbott Molecular, Des Plaines, IL, USA) was employed to confirm further anti-HCV positive samples. Steps related to HCV RNA extraction, concentration, amplification, and detection of the target region were performed automatically via the Abbott m2000rt instrument.26 The protocol for the assay was strictly followed as per the manufacturer’s instructions.
Statistical Analysis
Chi-square (X2) or Fisher’s exact tests were used to compare categorical variables, which were summarized as frequencies, based on the Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corporation, Armonk, NY, USA). A p-value ≤0.05 is considered statistically significant.
Results
Sociodemographic and Clinical Characteristics
In this study, 345 CLD patients were enrolled in two groups: HCC (n=128) and non-HCC (n=217) patients. Male participants were dominant, 64.6% (223/345), with a 1.8:1 male to female ratio. The mean ± SD age was 43.6 ± 14.7 ranging from 18–84 years old. Nearly half of the study participants, 49% (169/345), were from Addis Ababa, 78.6% (271/345) were married, and 71% (245/345) were employed. The mean ± SD age of HCC and non-HCC patients was 50.3± 14.5 (range: 20–84 years old) and 39.6± 13.4 (range: 18–77 years old). The study participants’ baseline demographic and clinical characteristics are summarized in Table 1.
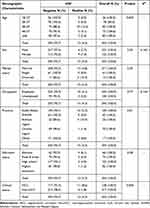 |
Table 1 Frequency of HIV Infection by the Demographic and Clinical Characteristics of CLD Patients |
Serology of HIV ½ and HIV/HBV and HIV/HCV Co-Infection
Plasma samples from all CLD patients were screened for HIV1/2, HBV, and HCV infection markers. The overall frequency of HIV infection was 4.3% (15/345), with a 2% (7/345) and 0.3% (1/345) of HIV/HBV and HIV/HCV co-infection rate. The overall and subgroup prevalence of HIV, HBV, and HCV mono- and HIV/HBV and HIV/HCV co-infection is summarized in Table 2.
 |
Table 2 Frequency of HIV, HBV, and HCV Mono- and HIV/HBV and HIV/HCV Co-Infection Among CLD Patients |
Of all HIV-positive patients (n=15), 46.7%7 and 6.7%1 co-infected with HBV (HBsAg+ HBcAb+) and HCV (anti-HCV+ HCV-RNA+), respectively, and 86.7%13 exhibited a marker of HBV exposure (total hepatitis B core antibody, HBcAb+). Broadly, our results implied a considerably higher distribution of HIV infection among subjects with past or present HBV infection.
Overall, the frequency of HIV and its co-infection with HBV or HCV exhibited an increasing pattern with the severity of liver disease (Figure 1 and Table 1). Hence, the overall frequency of HIV and HIV/HBV co-infection was marked among HCC patients compared to non-HCC patients (8.6% vs 1.8%) and (3.9% vs 0.9%). In addition, the only HIV/HCV co-infection was detected among HCC patients (Tables 1, 2 and Figure 1). Also, the frequency of HIV mono-infection was relatively higher among HCC than non-HCC patients (3.9% Vs 0.9%), but the difference was not significant. Conversely, the frequency of HBV mono-infection was significantly higher in non-HCC patients than in their counterparts (p<0.001) (Table 2); because most of the chronic hepatitis patients in the none-HCC group were referred to the GI clinic from different outpatient clinics based on their HBV status during the routine clinical practice.
 |
Figure 1 Frequency of HIV and co-HIV/HBV and HIV/HCV infection according to the stage of liver disease. |
Factors Associated with HIV and HIV/HBV Co-Infection and HCC
Among the risk factors, age (38–47 years old, p=0.003) (Figure 2), gender (females, p=0.05), tattoo (p=0.004), marital status (divorced, p=0.03), province (Amhara region, p=0.01), clinical status (HCC, p=0.005), and chronic consumption of alcoholic beverages (p=0.01) significantly associated with the rate of HIV infection (Tables 1 and 3). Of the risk factors, only age (38–47 years old) (p=0.006) exhibited a significant association with HIV/HBV co-infection frequency. About 55% (189/345) and 14% (48/345) of chronic liver disease patients were chronic consumers of alcohol and cigarette smokers, respectively. Consequently, chronic alcohol consumption and smoking showed a marginal association with HCC than non-HCC cases (61.7% vs 50.7%, p=0.05 and 18.8% vs 11.1%, p=0.05). Compared with non-HCC, older age (≥ 58 years old) exhibited a significant association with HCC cases (39.1% vs 16.1%, p<0.001).
 |
Table 3 Factors Associated with Human Immunodeficiency Virus Infection |
 |
Figure 2 Trend of HIV and co-HIV/HBV infection across the different age groups of chronic liver disease patients in Ethiopia. |
Discussion
This study has revealed the prevalence of HIV and its co-infection with HBV and HCV and potential associated infection risk factors among hospitalized patients with chronic liver disease in Ethiopia.
Irrespective of the liver disease stage, the overall prevalence of HIV among the study group was 4.3%, slightly higher than rates of community-based prevalence that ranged from 3.0% to 3.7%, reported in Ethiopia.27–30 The prevalence of HIV infection in specific groups and geographic regions is known to vary. For instance, a systematic review indicated a 5.74% pooled prevalence of HIV among pregnant women with some regional variation (4.8% in Addis Ababa, 4.48% in Oromia, and 2.14 in SNNPR).31 Furthermore, others have shown a strikingly high frequency of HIV in patients who took anti-TB treatment in northern Ethiopia and among female sex workers in Addis Ababa (29.3% and 24%).32 The difference in the prevalence of HIV reported in this study from previous reports is due to study population differences (hospitalized CLD patients) and factors including sample size, HIV risk status, and reporting period due to the changing nature of the HIV epidemic.
Due to their shared mode of transmission and risk factors, detecting a considerable amount of HBV and HCV infection among people living with HIV (PLWH) is not unusual. Accordingly, the frequency of HIV/HBV and HIV/HCV co-infection in the present study was 46.7% (7/15) and 6.7% (1/15). The prevalence of HIV/HBV co-infection in this study is higher than the previous reports from Ethiopia, which ranged from 3.0% to 11.7%,23,33–40 and in other African countries, including Nigeria (7.8%), Kenya (5.8%), Uganda (16.9%), and Sudan (11.7%).41–44 Similarly, previous studies from Southern, Central, North-west, and Northeast Ethiopia have reported a higher anti-HCV prevalence than our report, ranging from 1.3% to 5.2% among PLWH.34,35,38–40 Other related studies in Ethiopia, on the other hand, reported a comparable45 and even higher46 frequency of HIV/HCV co-infection with our study. This variation in the frequency of HIV co-infection with HBV/HCV could be explained by a difference in geography, risk factor, transmission mode, and age of infection, plus the possible reasons mentioned above for the difference in HIV prevalence between the present and previous reports. This is also a likely explanation for the differences in HIV prevalence in this study, 4.3%, compared to previous reports among CLD patients from Southern Tamil Nadu, 5.3%; Addis Ababa, 9.8%; and Nigeria, 18.2%.47–49
Chronic liver disease is Ethiopia’s 7th leading cause of death50 and Chronic hepatitis B and C infections and chronic consumption of alcoholic beverages are the principal etiologic agents of CLD.18,51,52 Today, the number of deaths related to AIDS markedly reduced due to the launching of antiretroviral treatment (ART) programs in low and middle-income regions, such as sub-Saharan Africa, where both HBV and HIV are highly prevalent.11 However, viral hepatitis B and C-related liver disease are common among people living with HIV and are responsible for the death of co-infected individuals due to non-AIDS causes.53 In parallel, reports from the above studies and our study evidenced the concurrent existence of the two-hepatotropic viruses (HBV or HCV) among HIV-positive individuals in Ethiopia and their potential to cause CLD and subsequent non-AIDS end-stage liver diseases related death.
The overall frequency of HIV and its co-infection with HBV or HCV showed an increasing pattern with the severity of liver diseases. Notably, the frequency of HIV and HIV/HBV co-infection rate was augmented among HCC than non-HCC patients (8.6% vs 1.8%, p=0.005) and (3.9% vs 0.9%, p=0.1). Besides, the only HIV/HCV co-infection was detected among HCC patients. However, the exact reason for such disproportionate prevalence of HIV and co-HIV/HBV infection among HCC patients is not apparent.
A consistent report by Otedo et al from western Kenya showed a significantly higher frequency of HIV infection among patients with HCC than those without HCC.54 Thus, the present and the Kenyan study findings imply that HIV patients are more prone to HCC than HIV-negative patients. This might be due to the marked decline of most AIDS-related opportunistic infections following extensive and effective use of ARTs; alternatively, infections accompanying HBV or HCV and ART-associated (hepatotoxicity) liver diseases have emerged as fundamental causes of morbidity and mortality among HIV/AIDS patients. Robbins et al showed liver cancer as the second non-AIDS defining cancer with a high incidence rate over time among people living with HIV/AIDS in the United States since the introduction of ART (1996).55 Hence, these might partly be the reason for the high frequency of HIV-Ab among HCC patients in the current study. However, the exact correlation between HIV infection and HCC is unclear and needs further study.
Moreover, 86.7% (13/15) of HIV-positive patients exhibited a marker for HBV exposure (hepatitis B core antibody, HBcAb); the coexistence of these two viruses (HIV and HBV) promotes the faster progression of liver disease to the advanced stage (Cirrhosis and HCC) in those living with HIV/AIDS.56 According to the study of Nina et al among HIV/HBV co-infected individuals, HBV-DNA (>2000IU/mL) or detectable viremia of both HBV and HIV, or undetectable HIV with detectable HBV viremia showed a strong correlation with HCC apart from heavy alcohol drinking and HCV infection.56
In addition to HIV and co-HIV/HBV infections in the present study, older age (≥ 58 years), alcohol consumption, and cigarette smoking showed a significant correlation with HCC.56 In addition, different related studies have shown an increased risk of HCC among chronic alcoholic beverage consumers and cigarette smokers.57,58 Moreover, the incidence of HCC increases with advanced age, which is more pronounced in those who chronically consume alcohol and smoke.56,59,60
We have also demonstrated a significantly higher prevalence of HIV infection in those aged (38–47 years old), women, and divorced, which is in line with the Ethiopian national report. As per the central statistical agency report, the peak prevalence of HIV was between 40–49 years old for men and 40–44 years old for women.29 Likewise, as the 2011 and 2016 DHS national HIV data showed, the second-highest group next to widowed individuals with a high frequency of HIV infection was divorced.29,61 In addition, the high prevalence of HIV was demonstrated among patients from the Amhara region; however, it is difficult to conclude with such a small sample size and study design. Nonetheless, reports from two national surveys have suggested that areas in the Amhara region, bordering the Tigray and Afar regions, are critical hotspot cluster areas for HIV transmission.30,62 Likewise, the association of tattooing and alcohol drinking habits with HIV infection observed in this study was also mentioned previously.49,63
Conclusion and Recommendations
Our study revealed an increasing pattern of HIV and HIV/HBV and HIV/HCV infection rates with the severity of the liver disease. Thus, screening all HIV-positive individuals for HBV and HCV infection and vice versa and taking all the necessary preventive measures, including immunization against HBV, is vital for prevention, early detection, and proper management of CLD and reduce CLD-related death in people living with HIV in Ethiopia.
Limitation of the Study
Since the HBV serology results did not accompanied by data from the HBV DNA test, we might miss occult hepatitis B infections.
Abbreviations
AdLD, advanced liver disease; AHRI, Armauer Hansen Research Institute; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CLD, chronic liver disease; DHS, Demographic & Health Survey; ELISA, Enzyme-linked immunosorbent assay; HAART, Highly Active Antiretroviral Therapy; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; PLWH, People living with HIV; SNNPR, southern nation and nationalities people region.
Data Sharing Statement
All data supporting our report are incorporated in the manuscript.
Ethical Consideration
The Institutional Review Boards (IRBs) of College of Medicine and Health Sciences, Hawassa University (Ref. no: IRB/099/08) and College of Health Sciences, Addis Ababa University (Ref. no: 056/16/DMIP), and Armauer Hansen Research Institute (AHRI)/All Africa Leprosy and Tuberculosis Rehabilitation and Training Center (ALERT) ethics review committee (AAERC) (Ref. no: P025/16) approved the protocol of this study. In addition, all eligible participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to acknowledge all administrative staff and laboratory personnel in AHRI. Our appreciation also extends to all patients, caregivers, and health professionals for their limitless support and active involvement during patient recruitment and data and sample collection in each study hospital/clinic.
Funding
An indirect fund was secured for this study from Addis Ababa University, Hawassa University, and AHRI. The sponsors had no involvement in any of the stages from study design to submission of this manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72 (5):1605–1616. doi:10.1002/hep.31173
2. UNAIDS. Global HIV & AIDS statistics – 2020 fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
3. Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6 (13):589. doi:10.12998/wjcc.v6.i13.589
4. World Health Organization. Global hepatitis report 2017. World Health Organization; 2017.
5. Thio CL, Seaberg EC, Skolasky JR, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). The Lancet. 2002;360 (9349):1921–1926. doi:10.1016/S0140-6736(02)11913-1
6. Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32 (3):492–497. doi:10.1086/318501
7. Martin-Carbonero L, Sanchez-Somolinos M, Garcia-Samaniego J, et al. Reduction in liver-related hospital admissions and deaths in HIV-infected patients since the year 2002. J Viral Hepat. 2006;13 (12):851–857. doi:10.1111/j.1365-2893.2006.00778.x
8. Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modified the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to Cirrhosis. J Hepatol. 1997;26 (1):1–5. doi:10.1016/s0168-8278(97)80001-3
9. Garcia-Samaniego J, Rodriguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96 (1):179–183. doi:10.1111/j.1572-0241.2001.03374.x
10. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384 (9939):241–248. doi:10.1016/S0140-6736(14)60604-8
11. Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34–48. doi:10.2174/1874613601610010034
12. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV co-infection on the risk of Cirrhosis and hepatocellular carcinoma in US veterans with hepatitis C. Am J Gastroenterol. 2005;100 (1):56–63. doi:10.1111/j.1572-0241.2005.40670.x
13. Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356 (14):1445–1454. doi:10.1056/NEJMra065142
14. Hernandez MD, Sherman KE. HIV/hepatitis C co-infection natural history and disease progression. Curr Opin HIV AIDS. 2011;6 (6):478–482. doi:10.1097/COH.0b013e32834bd365
15. Verucchi G, Calza L, Manfredi R, Chiodo F. Human immunodeficiency virus and hepatitis C virus co-infection: epidemiology, natural history, therapeutic options and clinical management. Infection. 2004;32 (1):33–46. doi:10.1007/s15010-004-3063-7
16. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017;4 (1):e000166. doi:10.1136/bmjgast-2017-000166
17. Abdelmenan S, Banes A, Berhane Y, Markos Abebe WJ. Etiology of chronic liver disease in Ethiopia: a case control study with special reference to viral hepatitis and alcohol. EC Gastroenterol digestive sys. 2018;5 (3):120–128. PMC6402780.
18. Tesfaye BT, Feyissa TM, Workneh AB, Gudina EK, Yizengaw MA. Chronic liver disease in Ethiopia with a particular focus on the etiological spectrums: a systematic review and meta-analysis of Observational Studies. Can J Gastroenterol Hepatol. 2021;2021:8740157. doi:10.1155/2021/8740157
19. Lulseged S, Belete W, Ahmed J, et al. Factors associated with unawareness of HIV-positive status in urban Ethiopia: evidence from the Ethiopia population-based HIV impact assessment 2017–2018. PLoS One. 2021;16 (8):e0255163. doi:10.1371/journal.pone.0255163
20. World Health Organization. Service delivery approaches to HIV testing and counseling (HTC): a strategic HTC programme framework; 2012.
21. Belete W, Deressa T, Feleke A, et al. Evaluation of diagnostic performance of non-invasive HIV self-testing kit using oral fluid in Addis Ababa, Ethiopia: a facility-based cross-sectional study. PLoS One. 2019;14 (1):e0210866. doi:10.1371/journal.pone.0210866
22. WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization Copyright (c) World Health Organization; 2017.
23. Ayana DA, Mulu A, Mihret A, Seyoum B, Aseffa A, Howe R. Hepatitis B virus seromarkers among HIV infected adults on ART: an unmet need for HBV screening in eastern Ethiopia. PLoS One. 2019;14 (12):e0226922. doi:10.1371/journal.pone.0226922
24. Abebe F, Seyoum B, Teklemariam Z, et al. Seroprevalence of Hepatitis B And Hepatitis C virus and their associated factors among mothers living in Harar Town, Eastern Ethiopia. Ethiop Med J. 2019;57:119–127.
25. Vermehren J, Schlosser B, Domke D, et al. High prevalence of anti-HCV antibodies in two metropolitan emergency departments in Germany: a prospective screening analysis of 28,809 patients. PLoS One. 2012;7 (7):e41206. doi:10.1371/journal.pone.0041206
26. Abreha T, Woldeamanuel Y, Pietsch C, et al. Genotypes and viral load of hepatitis C virus among persons attending a voluntary counseling and testing center in Ethiopia. J Med Virol. 2011;83 (5):776–782. doi:10.1002/jmv.21788
27. Abera B, Adem Y, Yimer M, Mulu W, Zenebe Y, Mekonnen Z. Community seroprevalence of hepatitis B, C and human immunodeficiency virus in adult population in gojjam zones, northwest Ethiopia. Virol J. 2017;14 (1):21. doi:10.1186/s12985-017-0696-6
28. Ameha Z, Tadesse S, Assefa A, Tessema B. Prevalence and associated factors of Hepatitis C virus and human immunodeficiency virus infections among voluntary counseling and testing clients attending private health facilities in Bahir Dar city, North West Ethiopia 2014. BMC Res Notes. 2019;12 (1):693. doi:10.1186/s13104-019-4727-9
29. Central Statistical Agency - CSA/Ethiopia, ICF. Ethiopia Demographic and Health Survey 2016.. Addis Ababa, Ethiopia: CSA and ICF; 2017.
30. Ephi P, Cdc W, ICAP. Ethiopia Population-based HIV impact assessment EPHIA 2017–2018. Summary sheet: Preliminary findings; 2020.
31. Geremew D, Tajebe F, Ambachew S, Endalamaw A, Eshetie S. Seroprevalence of HIV among pregnant women in Ethiopia: a systematic review and meta-analysis. BMC Res Notes. 2018;11 (1):908. doi:10.1186/s13104-018-4022-1
32. Gezahegn LK, Argaw E, Assefa B, Geberesilassie A, Hagazi M. Magnitude, outcome, and associated factors of anti-tuberculosis drug-induced hepatitis among tuberculosis patients in a tertiary hospital in North Ethiopia: a cross-sectional study. PLoS One. 2020;15 (11):e0241346. doi:10.1371/journal.pone.0241346
33. Weldemhret L, Asmelash T, Belodu R, Gebreegziabiher D. Sero-prevalence of HBV and associated risk factors among HIV positive individuals attending ART clinic at Mekelle hospital, Tigray, Northern Ethiopia. AIDS Res Ther. 2016;13 (1):6. doi:10.1186/s12981-016-0090-2
34. Shimelis T, Tassachew Y, Tadewos A, et al. Co-infections with hepatitis B and C virus and syphilis among HIV-infected clients in Southern Ethiopia: a cross-sectional study. HIV AIDS. 2017;9:203–210. doi:10.2147/HIV.S150795
35. Manyazewal T, Sisay Z, Biadgilign S, Abegaz WE. Hepatitis B and hepatitis C virus infections among antiretroviral-naive and -experienced HIV co-infected adults. J Med Microbiol. 2014;63 (Pt 5):742–747. doi:10.1099/jmm.0.063321-0
36. Belayneh F. Prevalence of Hepatitis B virus infection and associated factors among HIV positive adults attending ART clinic at hawassa referral hospital, SNNPR, Ethiopia. OALib. 2015;02 (05):1–7. doi:10.4236/oalib.1101490
37. Deressa T, Damtie D, Fonseca K, et al. The burden of hepatitis B virus (HBV) infection, genotypes and drug resistance mutations in human immunodeficiency virus-positive patients in Northwest Ethiopia. PLoS One. 2017;12 (12):e0190149. doi:10.1371/journal.pone.0190149
38. Wondimeneh Y, Alem M, Asfaw F, Belyhun Y. HBV and HCV seroprevalence and their correlation with CD4 cells and liver enzymes among HIV positive individuals at University of Gondar Teaching Hospital, Northwest Ethiopia. Virol J. 2013;10 (1):171. doi:10.1186/1743-422X-10-171
39. Gedefie A, Adamu A, Alemayehu E, Kassa Y, Belete MA. Hepatitis C Virus Infection among HIV-infected patients attending Dessie Referral Hospital, Northeastern Ethiopia. Int J Microbiol. 2021;2021:6675851. doi:10.1155/2021/6675851
40. Balew M, Moges F, Yismaw G, Unakal C. Assessment of hepatitis B virus and hepatitis C virus infections and associated risk factors in HIV infected patients at Debretabor hospital, South Gondar, Northwest Ethiopia. Asian Pac j Trop Med. 2014;4 (1):1–7. doi:10.1016/s2222-1808(14)60304-2
41. Maina DN, Nyerere AK, Gicho RW, Mwangi JM, Lihana RW. Prevalence and factors associated with Hepatitis B and C co-infection among HIV-1-infected patients in Kenya. East Afr Health Res J. 2017;1 (2):73–79. doi:10.24248/EAHRJ-D-16-00334
42. Nnakenyi ID, Uchechukwu C, Nto-Ezimah U. Prevalence of hepatitis B and C virus co-infection in HIV positive patients attending a health institution in southeast Nigeria. Afr Health Sci. 2020;20 (2):579–586. doi:10.4314/ahs.v20i2.5
43. Baseke J, Musenero M, Mayanja-Kizza H. Prevalence of hepatitis B and C and relationship to liver damage in HIV infected patients attending Joint Clinical Research Centre Clinic (JCRC), Kampala, Uganda. Afr Health Sci. 2015;15 (2):322–327. doi:10.4314/ahs.v15i2.3
44. Mudawi H, Hussein W, Mukhtar M, et al. Overt and occult hepatitis B virus infection in adult Sudanese HIV patients. Int J Infect Dis. 2014;29:65–70. doi:10.1016/j.ijid.2014.07.004
45. Taye S, Lakew M. Impact of hepatitis C virus co-infection on HIV patients before and after highly active antiretroviral therapy: an immunological and clinical chemistry observation, Addis Ababa, Ethiopia. BMC Immunol. 2013;14 (1):23. doi:10.1186/1471-2172-14-23
46. Alemayehu A, Tassachew Y, Sisay Z, Shimelis T. Prevalence and risk factors of Hepatitis C among individuals presenting to HIV testing centers, Hawassa city, Southern Ethiopia. BMC Res Notes. 2011;4 (1):1–5. doi:10.1186/1756-0500-4-193
47. Anbazhagan GK, Krishnamoorthy S, Thiyagarajan T. Seroprevalence of HCV and its co-infection with HBV and HIV among liver disease patients of South Tamil Nadu. World J Hepatol. 2010;2 (1):42–48. doi:10.4254/wjh.v2.i1.42
48. Mohammed Y Prevalence of hepatitis B, hepatitis C and HIB among chronic liver disease patients in selected hospitals [MSc thesis]. Addis Ababa, Ethiopia Addis Ababa University; 2014.
49. Oluremi ASOO, Ogbolu DO, Alli OT, et al. Serological evidence of HIV, Hepatitis B, C, and E viruses among liver disease patients attending tertiary hospitals in Osun State, Nigeria. J Immunoassay Immunochem. 2021;1 (42):69–81. doi:10.1080/15321819.2020.1821214
50. World Health Organization. Global health estimates: leading causes of death; 2019. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
51. Mekonnen HD, Sharma S, Shewaye A, Feld J, Lulu E. Major risk factors, clinical and laboratory characteristics of patients with hepatocellular carcinoma; a retrospective study at Tikur Anbassa Hospital, Addis Ababa University, Addis Ababa, Ethiopia. Ethiop Med J. 2015;53 (3):127–132. PMC26677522.
52. Erkabu S, Demeke B, Desallegn H, Getachew S. Liver disease: a Retrospective hospital based study in Addis Ababa-Ethiopia. J Spleen and Liver Res. 2021. doi:10.14302/issn.2578-2371.jslr-21-3912
53. Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Human immunodeficiency virus and hepatotropic viruses co-morbidities as the inducers of liver injury progression. World J Gastroenterol. 2019;25 (4):398–410. doi:10.3748/wjg.v25.i4.398
54. Otedo ASK, Were V, Ongati O, Estambale BA. Risk factors for liver cancer in HIV endemic areas of Western Kenya. Infect Agent Cancer. 2018;13 (1):1–9. doi:10.1186/s13027-018-0214-5
55. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28 (6):881–890. doi:10.1097/QAD.0000000000000163
56. Kim HN, Newcomb CW, Carbonari DM, et al. Risk of HCC with Hepatitis B Viremia among HIV/HBV-coinfected persons in North America. Hepatology. 2021;74 (3):1190–1202. doi:10.1002/hep.31839
57. Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004;96 (24):1851–1856. doi:10.1093/jnci/djh334
58. Hamed MA, Ali SA. Non-viral factors contributing to hepatocellular carcinoma. World J Hepatol. 2013;5 (6):311–322. doi:10.4254/wjh.v5.i6.311
59. Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52 (2):518–527. doi:10.1002/hep.23691
60. Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer. 2018;124 (13):2748–2757. doi:10.1002/cncr.31406
61. Central Statistical Agency and ICF International. Ethiopia Demographic and Health Survey 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International; 2012.
62. Kibret GDFA, Leshargie CT, Wagnew F, Ketema DB, Alebel A. Trends and spatial distributions of HIV prevalence in Ethiopia. Infect Dis Poverty. 2019;8 (1):1–9. doi:10.1186/s40249-019-0594-9
63. Adal M. Systematic review on HIV situation in Addis Ababa, Ethiopia. BMC Public Health. 2019;19 (1):1. doi:10.1186/s12889-019-7885-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
