Back to Journals » Journal of Pain Research » Volume 16
Effectiveness of Infliximab in Patients with Complex Regional Pain Syndrome: A Case Series
Authors van den Berg C , Dirckx M, Huygen FJPM, Tiemensma J
Received 16 February 2023
Accepted for publication 3 May 2023
Published 6 June 2023 Volume 2023:16 Pages 1915—1926
DOI https://doi.org/10.2147/JPR.S408858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Corinne van den Berg, Maaike Dirckx, Frank JPM Huygen, Jitske Tiemensma
Department of Anesthesiology, Center for Pain Medicine, Erasmus University Medical Center, Rotterdam, the Netherlands
Correspondence: Corinne van den Berg, Department of Anesthesiology, Center for Pain Medicine, Erasmus University Medical Center, Postbus 2040, Rotterdam, 3000CA, the Netherlands, Tel +31 107040140, Fax +31 107035184, Email [email protected]
Purpose: Complex regional pain syndrome (CRPS) is a multi-mechanism disease, with an exaggerated inflammatory response as an important underlying mechanism. Auto-inflammation can theoretically be combated by anti-inflammatories, such as TNF-α inhibitors. This study’s aim was to assess the effectiveness of intravenous infliximab, a TNF-α inhibitor, in patients with CRPS.
Patients and Methods: CRPS patients treated with infliximab between January 2015 and January 2022 were approached to participate in this retrospective study. Medical records were screened for age, gender, medical history, CRPS duration, and CRPS severity score. Additionally, treatment effect, dose and duration, and side effects were extracted from medical records. Patients who still receive infliximab completed a short global perceived effect survey.
Results: Eighteen patients received infliximab, and all but two gave consent. Trial treatment with three sessions of 5 mg/kg intravenous infliximab was completed in 15 patients (93.7%). Eleven patients (73.3%) were categorized as responders with a positive treatment effect. Treatment was continued in nine patients, and seven patients are currently treated. Infliximab dose is 5 mg/kg, and frequency is every four to six weeks. Seven patients completed a global perceived effect survey. All patients reported improvement (median 2, IQR 1– 2) and treatment satisfaction (median 1, IQR 1– 2). One patient described side effects such as itching and rash.
Conclusion: Infliximab proved effective in 11 out of 15 CRPS patients. Seven patients are still being treated. Further research is needed on the role of infliximab in the treatment of CRPS and possible predictors of response to treatment.
Keywords: complex regional pain syndrome, CRPS, TNF-α inhibitors, treatment, inflammation, anti-inflammatories
Introduction
Complex regional pain syndrome (CRPS) is a clinical disorder characterized by severe, continuous regional pain that is disproportionate to the initial trauma. The pain is often accompanied by sensory, vasomotor, sudomotor, motor, and trophic symptoms.1 CRPS is preceded by tissue or nerve damage that can occur after trauma or surgery. Incidence of CRPS has been reported ranging from 5.5 to 26.2 per 100,000 and is more common in women (ratio 3.4).2,3 The clinical diagnosis is based on signs and symptoms. The new International Association for the Study of Pain (IASP) clinical diagnostic criteria are currently used to diagnose CRPS.4
Various mechanisms play a role in the pathophysiology of CRPS, with inflammation being most important. Previous studies reported higher concentrations of pro-inflammatory cytokines (ie, IL1, IL6, IL8, and TNF-α) and reduced levels of anti-inflammatory cytokines (ie, IL4, IL10, and transforming growth factor beta-1).5–10 Additionally, increased T-cell activity is suggested with higher median soluble IL-2 receptor (sIL-2R) plasma levels in CRPS patients compared to healthy blood donors.11 The inflammatory appearance in CRPS ensures a role for anti-inflammatory medication. A recent review exhibited corticoid treatment to be effective in CRPS, especially concerning pain relief and improved movement.12 However, longer duration of corticoid treatment is associated with a high risk of side effects.13 Alternative treatment options are therefore necessary.
Previous research suggested CRPS to be more similar to an auto-inflammatory disease such as rheumatoid arthritis (RA). CRPS patients showed a significantly higher prevalence of antinuclear antibodies (ANAs) compared to the healthy population. This ANA prevalence in CRPS was much lower than in patients with classic systemic autoimmune diseases (eg, systemic lupus erythematosus (SLE)) and appeared to be more in line with the prevalence of RA.14 As a result, comparable treatments for RA could be a potential option in the treatment of CRPS. In RA, TNF-α inhibitor drugs are successfully used, with the treatment being given increasingly earlier in the disease.15 TNF-α inhibitors suppress the physiological response to TNF-α, thereby providing a rapid and prolonged suppression of inflammation.16 In total, five TNF-α inhibitors are known, including infliximab, which is a chimeric monoclonal antibody.
In our clinic, two CRPS cases demonstrated a successful effect of treatment with infliximab.17 As follow-up to these cases, a randomized controlled trial (RCT) was performed to compare infliximab with a placebo. However, the study stopped prematurely due to recruitment problems. Nonetheless, a trend toward a larger reduction in TNF-α in artificial skin blisters was described that compared infliximab infusion with saline solution 0.9% infusion.18 Despite this limited evidence, we continued to clinically treat CRPS patients with signs and symptoms of inflammation (eg, an elevated sIL-2R level) with anti-inflammatory drugs. We first treated these patients with short-term corticoid therapy. If inflammation continues to play a prominent role and no alternative treatment is available, these patients may be eligible for off-label treatment with infliximab.
In our opinion, infliximab is a promising therapy in treating CRPS. The aim of the current study was to assess the efficacy of infliximab in patients with CRPS treated in our clinic. Therefore, we conducted a retrospective study and described an additional case series.
Materials and Methods
Ethical Approval
This study was conducted according to the principles of the Declaration of Helsinki. The Medical Ethics Committee of Erasmus University Medical Center Rotterdam (MEC-2022-0133) approved this study.
Patient Selection
All electronic medical records were searched for patients with a CRPS diagnosis in combination with infliximab treatment. Diagnoses are encoded in the International Classification of Diseases, 10th edition (ICD-10) with a specific code for each diagnosis. Three codes were available for CRPS: G90.5, G90.6, and G60.7. Additionally, a so-called diagnosis–treatment combination (in Dutch: Diagnose-behandelcombinatie) code was used for CRPS diagnosis (ie, 150). A combination of both the ICD-10 and diagnosis-treatment code was used to identify all patients with a diagnosis of CRPS at Erasmus University Medical Center between January 2015 and January 2022. Furthermore, this group of patients was checked for infliximab treatment. In accordance with the General Data Protection Regulation (in Dutch: AVG), all CRPS patients treated with infliximab were approached to participate in the study.19 They were asked for permission to allow the research team to view their medical records. Only patients who gave permission were included in this study.
Treatment with Infliximab
Infliximab treatment is administered intravenously. Opportunistic infections, abscesses, sepsis, and tuberculosis are contraindications to treatment with infliximab. Therefore, before initial treatment, a screening consisting of hepatitis B, hepatitis C, quantiferon, and chest X-ray is performed. The dose is weight-based, and we opted for a 5 mg/kg dose in our clinic. This dose is the same as used in a previous study in our clinic, which was determined in consultation with an immunologist.18 The dose of 5mg/kg is also commonly used in treating Crohn’s disease, psoriasis, and RA.20
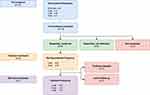
In our clinic, each patient starts with a trial treatment of three sessions infliximab 5 mg/kg, diluted to 250 mL with sodium chloride 0.9%. This solution is administered with an infusion duration of two hours, and treatment is given in a four-to-six-week interval. After trial treatment, the effect is evaluated and once the patient notices effect, infliximab is often continued (see Figure 1). An immunologist is readily available for consultation.
Today, in our clinic, CRPS patients with both signs and symptoms of inflammation and an elevated sIL-2R level are primarily treated with short-term corticoid therapy. This therapy contains 30 mg of oral prednisolone per day for ten days. If the inflammation continues to play a prominent role, despite a short-term effect or no effect of corticoid therapy, patients are eligible for treatment with infliximab. Thus, all patients in this study underwent a brief corticoid treatment before infliximab was started.
Data Collection
Data was extracted from medical records. Medical records of all included patients were viewed by one investigator (CvdB). Patients whose treatment started before the age of 18 remained in the study. The main study parameter was treatment effect. Based on treatment effect, it was assessed whether a patient was a responder or a non-responder. Responders reported a positive treatment effect (ie, improvement of symptoms), and non-responders reported no effect. Information on age, gender, medical history, duration of CRPS, and type of CRPS (distinguishing between both type 1/2 and warm or cold subtype) at the start of trial treatment was also extracted from the medical records. Additionally, the dose and frequency, duration, and side effects were noted if they had been recorded in the medical record. Finally, adverse effects were also reported.
In addition, between November and December 2022, all included patients who were still being treated with infliximab visited the outpatient clinic prior to new infliximab treatment. During this visit, both CRPS severity score21 (CSS, see Table 1) and global perceived effect (GPE) were taken by one investigator (CvdB).
 |
Table 1 CRPS Severity Score by Harden et al21 |
Global Perceived Effect
A GPE questionnaire is a widely used tool in both research and practice to obtain an assessment from patients about the effect of their treatment.22,23 The GPE asks the patient to rate on a numerical scale whether their condition has improved or deteriorated since a predefined time point. Satisfaction with the treatment is also rated (see Supplemental 1).
Statistical Analysis
Statistical analysis was conducted with IBM SPSS software, version 28 (IBM Corp., Armonk, NY). Descriptive statistics were used to calculate frequencies of categorical variables and to calculate measures of central tendency and variability of continuous variables. The Shapiro–Wilk test was used to analyze whether continuous variables were normally distributed. Variables with a skewed distribution are reported in median and 25th and 75th percentiles; otherwise, means and standard deviations are used. Due to the small sample size, no additional statistical analysis could be reliably performed. The data is therefore described narratively.
Results
Patients
Between January 2015 and January 2022, 18 CRPS patients received infliximab in our expert center. All patients were approached to obtain consent to access their medical records. All but two gave consent, resulting in inclusion of 16 patients (ie, 14 women and two men). Figure 2 shows the flowchart of included patients in the three different phases: trial treatment (blue), the continuation of treatment (orange), and still active treatment (purple). We refollowed the same phases in the further description of the patients in this study.
Trial Treatment
All patients started with a trial treatment of three sessions. Baseline characteristics are presented in Table 2. Initially, there was a search for a starting schedule for the therapy. In RA, it is common to start with an accelerated induction schedule. Four schedules were used during the trial period. Most patients (n = 9) received three administrations per four weeks. One patient received infliximab every three weeks and another every five weeks. In five patients, treatment consisted of an induction regimen, in which infliximab was given with increasing duration between treatments. After receiving the first administration, the patients received the next administration after two and four weeks, respectively.
 |
Table 2 Baseline Characteristics at Start of Trial Treatment |
Trial treatment was completed in all but one patient. Of the 15 patients, 11 (73.3%) were responders (ie, they showed positive treatment effect on trial treatment). The mean age at start of trial treatment was 37.2 years (range 16–56).
In the one patient who did not complete trial treatment (patient 16 in Table 2; a woman with lower extremity CRPS), trial treatment was stopped after the second administration due to the development of a chronic inflammatory demyelinating polyneuropathy (CIDP). The patient received intravenous immune globulin (IVIG) for CIDP. This was successful for her CIDP, and she also indicated a temporary positive effect on CRPS of the leg. In addition to CIDP, seven patients described various other mild side effects to trial treatment: headache, myalgia, erysipelas, urticaria, pruritus, rash, nausea, fatigue, and hair loss. Of the seven patients reporting side effects, two were non-responders and five were responders to infliximab treatment.
Continuation of Treatment
Infliximab was continued in nine out of 11 patients who reported a positive treatment. In one patient, treatment continuation was withheld due to a short-term effect of infliximab treatment. In the other patient, trial treatment was completed and continued treatment is pending. The final repeat frequency was partly determined by the duration of the effect experienced by the patient and therefore varies between four and six weeks. In four patients, treatment frequency was continued as started, and in the other five patients, frequency was adapted. Treatment frequency increased in three patients: one from a six-week to a five-week interval and from six to four weeks for two patients. Treatment was given less frequently in two patients; both went from a four- to a six-week interval. This frequency reduction also reduced the burden on patients since they came to the hospital less often.
Still Active Treatment
Today, of the nine patients in whom treatment was continued, seven are still receiving infliximab. In one patient, treatment was stopped because this patient also had scleroderma and dermatomyositis, and these complaints increased due to the infliximab treatments. This patient still receives weekly methotrexate injections. Another patient could not be reached for additional measurements due to other medical problems and is therefore described as lost to follow-up, resulting in a case series of the remaining seven patients (see Table 3). All patients have CRPS of the lower extremity: unilateral in five cases and both lower extremities affected in two cases. At the start of infliximab treatment, the patients had CRPS for 10 to 224 months. All patients received a body-weight-based dose of 5 mg/kg (range 255–525 mg), and treatment frequency ranged from four to six weeks. All cases are treated for three years or more (range 36–92 months). One patient is treated in combination with oral tavegyl 2 mg because of an infliximab infusion reaction (ie, itching and rash). With additional tavegyl, these symptoms are well controlled.
 |
Table 3 Characteristics of Still Actively Treated Patients |
Global Perceived Effect
The seven described cases visited the outpatient clinic prior to new infliximab treatment between November and December 2022 and completed the GPE. All patients reported to be extremely satisfied (n = 2, 28.6%) or quite satisfied (n = 5, 71.4%), and recovery was scored as very much improved (n = 4, 57.1%) or much improved (n = 3, 42.9%). The median score for improvement was 2 (IQR 1–2), and the median for satisfaction was 1 (IQR 1–2), with lower scores indicating greater improvement and satisfaction.
CRPS Severity Score
In addition to GPE, CSS was completed in these seven patients. Table 4 displays the proportion of symptoms and signs according to the CSS. Six of seven patients still meet the new IASP criteria. Motor abnormalities in both signs and symptoms could not be assessed due to upper leg amputation of both legs in patient 3. The mean pain score at time of the visit was 4 ± 2.2, and the pain score 24 hours before the visit was 4.6 ± 2.4. Five symptoms (ie, subjective symptoms reported by patients) were reported to be present in six of seven patients: allodynia and/or hyperalgesia, temperature asymmetry, color asymmetry, dystrophic changes, and motor abnormalities. Three signs (ie, objective signs observed by the physician) were also present in six out of seven cases: hyperalgesia to pinprick, temperature asymmetry, and dystrophic changes. The affected side was colder in both symptoms and signs in six patients, and color asymmetry was present in five of these patients. Three patients had blue or pale color asymmetry, and the affected limb was mottled in two patients. The median CRPS severity score was 13 (IQR 10.5–14).
 |
Table 4 CRPS Severity Score: Presence of Symptoms and Signs in Patients |
Discussion
In our expert clinic, 18 patients were treated off-label with infliximab between January 2015 and January 2022. Sixteen of these patients gave consent for inclusion in the present study. The trial treatment, consisting of three intravenous infliximab administrations (5 mg/kg), was completed in all but one patient. The trial proved effective in 11 out of 15 patients. Currently, seven patients are still being treated. These patients are described in our case series. Infliximab appears to be a promising therapy for at least some of the patients with CRPS.
In accordance with our results, previous studies on TNF-α inhibitors also showed a promising effect. Huygen et al described two CRPS cases with successful infliximab treatment.17 In addition, a consecutive RCT by Dirckx et al showed a trend toward a larger reduction in TNF-α in artificial skin blisters when comparing infliximab infusion with saline solution 0.9% infusion.18 Furthermore, Eisenberg et al investigated another TNF-α inhibitor (ie, adalimumab) in 10 patients. Three of the treated patients experienced at least a two-point reduction in pain score at the six-month follow-up.24 Remarkably, all previously described successful cases had the warm CRPS subtype.17,25 In addition, the two patients who did not improve in pain scores from baseline until completion of the adalimumab treatment typically had cold CRPS.24 TNF-α inhibitors were therefore thought to be especially effective in the warm CRPS subtype. However, six of our cases had the cold subtype. The subtypes thus appear to be less associated with treatment effect than previously thought. This may be explained by the fact that inflammation may continue to play a role in cold CRPS. Previous studies have compared proinflammatory cytokines levels (ie, interleukin-6 and TNF-α) in artificial skin blisters on the affected and contralateral extremity in patients with cold, intermediate, and warm CRPS. No significant differences were shown among these patient subgroups.26
An exaggerated inflammatory response is described especially in the acute stage (ie, the early months) of CRPS.5,27 This evidence of inflammation with proven increased concentrations of pro-inflammatory cytokines in CRPS, including TNF- α, provides a role for anti-inflammatory medication such as TNF-α inhibitors.5–10 In addition to their effect on inflammation, TNF-α inhibitors may also play a role in preventing central changes and residual damage. The ongoing inflammatory response in CRPS itself can cause damage. For example, small fiber neuropathy, endothelial dysfunction, central sensitization, and neuronal plasticity can result from the ongoing inflammatory response.28–32 An early initiation of infliximab in CRPS could possibly limit the damage caused by the inflammation. In Crohn’s disease, early initiation of infliximab is associated with more endoscopic remission and less development of stricturing disease.33 In our patients, CRPS duration at infliximab initiation varied between 10 and 224 months. Despite the longer duration of CRPS at the start of the therapy, infliximab treatment proved effective.
All patients treated today have CRPS of one or both lower extremities. Although CRPS is known to affect both upper and lower extremities, the upper extremity is more prone to being affected.3,34
For CRPS, optimal dosage and treatment duration are currently unknown. All of our patients received a body-weight-based infliximab dose (ie, 5 mg/kg intravenous). This dose is commonly used to treat Crohn’s disease, psoriasis, and RA.20 However, both lower (3 mg/kg) and higher doses (10 mg/kg) are described in RA treatment and in treating hidradenitis suppurativa.20,35 Due to the heterogeneity in RA, individual dose optimization is often used.36 In our case series, all patients received an equal treatment dose based on their weight. However, treatment frequency was individually considered. For example, the frequency was shortened in patients who developed more complaints before they received a new dose of infliximab. Additionally, treatment frequency was lengthened in certain patients whose CRPS complaints were under control, and the frequency was set to the maximum possible.
The desired duration of treatment with infliximab is also unknown. It would be logical to stop infliximab treatment at some point, expecting that the therapy may no longer be necessary given the natural course of CRPS. The idea is that the inflammation in CRPS eventually dies out, and only residual damage remains, on which infliximab will have no effect. However, based on this study, we cannot make a statement on this. For RA, treatment with TNF-α inhibitors is evaluated based on both effect and side effects. A Belgian study described that 31% of 511 patients with RA were still successfully using infliximab after seven years. However, more than half of the patients discontinued therapy due to ineffectiveness and safety issues.37 In addition, other reasons, such as the possibility of switching to a subcutaneous TNF-α inhibitor (adalimumab), played a role in treatment discontinuation.
Like any medication, TNF-α inhibitors may cause side effects. The most common side effect is a local or systemic infusion reaction (eg, redness, itching, fever, chills, or muscle aches). In addition, there is an increased risk for infections (especially respiratory). Less frequent side effects are malignancies, worsening of heart failure, and demyelinating disorders.20,34,35 In these case studies, one patient who did not complete trial treatment developed CIDP, a demyelinating neurological adverse event. Several reports are known in which the central nervous system demyelination is reported with TNF-α inhibitors.38–40 However, it is still unclear whether the demyelinating events are causally associated with the use of TNF-α inhibitors or whether it is a coincidence.40 Our patient was treated with IVIG for CIDP, and she indicated that IVIG had a positive effect on her CRPS leg. This is supported by Goebel et al, who report the positive effects of IVIG in CRPS.41 In addition to the CIDP, we mainly observed mild side effects in our patients.
Several limitations of the present study should be mentioned. First, the retrospective design, which bounds that not all information is available. For example, used cotreatments on initiation of infliximab treatment, such as NSAIDS or opioids, were unknown. Additionally, outcome assessment was not standardized. For example, standardized sIL-2R determinations before and after treatment with TNF-α inhibitors would be of interest. However, sIL-2R values were variably determined with a range of 224 to 0 days prior to the start of treatment and 35–759 days afterwards. This variation prevents reliable conclusions from being drawn. Today, CSS is the only measure to describe the severity of CRPS. However, a retrograde CSS does not provide clarity because all types of therapy in the interim can have an influence. Furthermore, the included patients suffer from persistent CRPS and are treated in a tertiary referral hospital, which limits the generalizability of our results to widespread pain practice. Additionally, in our case series, we only narratively described patients who still receive infliximab, which makes our results prone to bias. However, it is important to emphasize the promising role of TNF-α inhibitors. Because CRPS is a rare disease, patient numbers are limited. These limited patient numbers caused the well-designed RCT of Dirckx et al to be terminated early.18 In addition, TNF-α inhibitors for CRPS are only given in expert centers such as ours. We postulate that more people may be eligible for treatment. The expertise should preferably be centralized so that each CRPS patient has an equal chance of receiving TNF-α inhibitors if necessary. It is also important that patients visit an expert center as early as possible to start therapy early and thus limit the damage caused by the exaggerated inflammatory response.
In conclusion, intravenous infliximab appears to be effective, as described in our case series, in some CRPS patients. To further examine infliximab’s place in treating patients with CRPS, a randomized controlled trial should be conducted. Attention should be paid to which CRPS patients are responders and which are not. Based on our current experience, it is preferable to standardize the treatment protocol, whereby CSS and sIL-2R levels are determined at fixed time points before and after treatment and GPE is requested. Such a standardization ensures the broadest possible assessment of the effectiveness of TNF-α inhibitors in treating CRPS.
Disclosure
Frank J.P.M. Huygen reports personal fees from Abbott; grants and personal fees from Saluda; and personal fees from Boston Scientific, Grunenthal, and Pfizer outside the submitted work. Corinne van den Berg, Maaike Dirckx and Jitske Tiemensma report no conflicts of interest in this work.
References
1. Bruehl S. Complex regional pain syndrome. BMJ. 2015;351:h2730. doi:10.1136/bmj.h2730
2. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103(1–2):199–207. doi:10.1016/s0304-3959(03)00065-4
3. de Mos M, de Bruijn AGJ, Huygen FJ, Dieleman JP, Stricker CBH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1):12–20. doi:10.1016/j.pain.2006.09.008
4. Harden NR, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150(2):268–274. doi:10.1016/j.pain.2010.04.030
5. Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11(1):47–51. doi:10.1080/09629350210307
6. Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. PAIN. 2005;116(3):213–219. doi:10.1016/j.pain.2005.04.013
7. Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22(3):235–239. doi:10.1097/01.ajp.0000169669.70523.f0
8. Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain. 2012;13(1):10–20. doi:10.1016/j.jpain.2011.10.003
9. Bruehl S, Warner DS. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113(3):713–725. doi:10.1097/aln.0b013e3181e3db38
10. Parkitny L, McAuley JH, Di Pietro F, et al. Inflammation in complex regional pain syndrome. A Systematic Review and Meta-Analysis. Neurology. 2013;80(1):106–117. doi:10.1212/wnl.0b013e31827b1aa1
11. Bharwani KD, Dirckx M, Stronks DL, Dik WA, Schreurs MWJ, Huygen F. Elevated plasma levels of sIL-2R in complex regional pain syndrome: a pathogenic role for T-lymphocytes? Mediators Inflamm. 2017;2017:2764261. doi:10.1155/2017/2764261
12. van den Berg C, de Bree PN, Huygen F, Tiemensma J. Glucocorticoid treatment in patients with complex regional pain syndrome: a systematic review. Eur J Pain. 2022;26:2009–2035. doi:10.1002/ejp.2025
13. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–426. doi:10.1002/art.21984
14. Dirckx M, Schreurs MW, de Mos M, Stronks DL, Huygen FJ. The prevalence of autoantibodies in complex regional pain syndrome type I. Mediators Inflamm. 2015;2015:718201. doi:10.1155/2015/718201
15. Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr. 2015;165(1–2):3–9. doi:10.1007/s10354-015-0344-y
16. Markham A, Lamb HM. Infliximab: a review of its use in the management of rheumatoid arthritis. Drugs. 2000;59(6):1341–1359. doi:10.2165/00003495-200059060-00010
17. Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. J Pain Symptom Manage. 2004;27(2):101–103. doi:10.1016/j.jpainsymman.2003.12.006
18. Dirckx M, Groeneweg G, Wesseldijk F, Stronks DL, Huygen FJ. Report of a preliminary discontinued double-blind, randomized, placebo-controlled trial of the anti-TNF-α chimeric monoclonal antibody infliximab in complex regional pain syndrome. Pain Pract. 2013;13(8):633–640. doi:10.1111/papr.12078
19. EU General Data Protection Regulation (GDPR). Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), OJ 2016 L 119/1. 2016.
20. Summary of product characteristics European Medicines Agency; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf.
21. Harden NR, Bruehl S, Perez R, et al. Development of a severity score for CRPS. Pain. 2010;151(3):870–876. doi:10.1016/j.pain.2010.09.031
22. Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;282(12):1157–1162. doi:10.1001/jama.282.12.1157
23. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN. 2005;113(1):9–19. doi:10.1016/j.pain.2004.09.012
24. Eisenberg E, Sandler I, Treister R, Suzan E, Haddad M. Anti tumor necrosis factor - alpha adalimumab for complex regional pain syndrome type 1 (CRPS-I): a case series. Pain Pract. 2013;13(8):649–656. doi:10.1111/papr.12070
25. Bernateck M, Rolke R, Birklein F, Treede RD, Fink M, Karst M. Successful intravenous regional block with low-dose tumor necrosis factor-alpha antibody infliximab for treatment of complex regional pain syndrome 1. Anesth Analg. 2007;105(4):1148–51, table of contents. doi:10.1213/01.ane.0000278867.24601.a0
26. Dirckx M, Stronks DL, van Bodegraven-Hof EA, Wesseldijk F, Groeneweg JG, Huygen FJ. Inflammation in cold complex regional pain syndrome. Acta Anaesthesiol Scand. 2015;59(6):733–739. doi:10.1111/aas.12465
27. Lenz M, Üçeyler N, Frettlöh J, et al. Local cytokine changes in complex regional pain syndrome type I (CRPS I) resolve after 6 months. PAIN. 2013;154(10):2142–2149. doi:10.1016/j.pain.2013.06.039
28. Birklein F, Ajit SK, Goebel A, Perez R, Sommer C. Complex regional pain syndrome - phenotypic characteristics and potential biomarkers. Nat Rev Neurol. 2018;14(5):272–284. doi:10.1038/nrneurol.2018.20
29. Bank PJ, van Rooijen DE, Marinus J, Reilmann R, van Hilten JJ. Force modulation deficits in complex regional pain syndrome: a potential role for impaired sense of force production. Eur J Pain. 2014;18(7):1013–1023. doi:10.1002/j.1532-2149.2013.00446.x
30. Schattschneider J, Hartung K, Stengel M, et al. Endothelial dysfunction in cold type complex regional pain syndrome. Neurology. 2006;67(4):673–675. doi:10.1212/01.wnl.0000229931.40631.31
31. Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707. doi:10.1212/01.wnl.0000098939.02752.8e
32. Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10(7):637–648. doi:10.1016/s1474-4422(11)70106-5
33. Schnitzler F, Seitz T, Tillack-Schreiber C, Lange S, Waggershauser C, Ochsenkühn T. Early start of infliximab in Crohn’s disease increases rates of endoscopic remission and decreases stenosis formation: experiences from a single center cohort. Crohns Colitis. 2021;3(3). doi:10.1093/crocol/otab060
34. Ott S, Maihöfner C. Signs and symptoms in 1043 patients with complex regional pain syndrome. J Pain. 2018;19(6):599–611. doi:10.1016/j.jpain.2018.01.004
35. Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81(3):702–708. doi:10.1016/j.jaad.2019.05.022
36. Alten R, van den Bosch F. Dose optimization of infliximab in patients with rheumatoid arthritis. Int J Rheum Dis. 2014;17(1):5–18. doi:10.1111/1756-185X.12202
37. Vander Cruyssen B, Durez P, Westhovens R, De Keyser F. Seven-year follow-up of infliximab therapy in rheumatoid arthritis patients with severe long-standing refractory disease: attrition rate and evolution of disease activity. Arthritis Res Ther. 2010;12(3):R77. doi:10.1186/ar2997
38. Kopp TI, Delcoigne B, Arkema EV, et al. Risk of neuroinflammatory events in arthritis patients treated with tumour necrosis factor alpha inhibitors: a collaborative population-based cohort study from Denmark and Sweden. Ann Rheum Dis. 2020;79(5):566. doi:10.1136/annrheumdis-2019-216693
39. Gharib MH, AlKahlout MA, Garcia Canibano B, Theophiel Deleu D, Malallah AlEssa H, AlEmadi S. Demyelinating neurological adverse events following the use of anti-TNF-α agents: a double-edged sword. Case Rep Neurol Med. 2022;2022:3784938. doi:10.1155/2022/3784938
40. Andreadou E, Kemanetzoglou E, Brokalaki C, et al. Demyelinating disease following Anti-TNFa treatment: a causal or coincidental association? Report of four cases and review of the literature. Case Rep Neurol Med. 2013;2013:671935. doi:10.1155/2013/671935
41. Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152(3):152–158. doi:10.7326/0003-4819-152-3-201002020-00006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.