Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Comprehensive Diagnostics of Diabetic Nephropathy by Transcriptome RNA Sequencing
Authors Lei L , Bai Y, Fan Y, Li Y, Jiang H, Wang J
Received 2 May 2022
Accepted for publication 20 September 2022
Published 10 October 2022 Volume 2022:15 Pages 3069—3080
DOI https://doi.org/10.2147/DMSO.S371026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Lei Lei,1 Yihua Bai,1 Yang Fan,1 Yaling Li,1 Hongying Jiang,1 Jiaping Wang2
1Department of Nephrology, The Second Hospital Affiliated to Kunming Medical University, Kunming, Yunnan, People’s Republic of China; 2Department of Radiology, The Second Hospital Affiliated to Kunming Medical University, Kunming, Yunnan, People’s Republic of China
Correspondence: Yihua Bai, Department of Nephrology, The Second Hospital Affiliated to Kunming Medical University, Kunming, Yunnan, People’s Republic of China, Email [email protected]
Background: Diabetic nephropathy (DN) is a primary driver of end-stage renal disease. Given the heterogeneity of renal lesions and the complex mechanisms of DN, the present-day diagnostic approach remains highly controversial. We aimed to design a diagnostic model by bioinformatics methods for discriminating DN patients from normal subjects.
Methods: In this study, transcriptome sequencing was performed on 6 clinical samples (3 from DN patients and 3 from healthy volunteers) from the Second Affiliated Hospital of Kunming Medical University. Construction of a competing endogenous RNA (ceRNA) network based on differentially expressed (DE)-mRNAs and -long noncoding RNAs (lncRNAs). Subsequently, the CytoHubba plugin was used to identify hub genes from DE-mRNAs in the ceRNA network and to perform functional enrichment analysis on them. The least absolute shrinkage and selection operator (LASSO) regression analysis was responsible for screening the diagnostic biomarkers from hub genes and assessing their diagnostic power using ROC curves. The pathways involved in hub genes were revealed by single-gene Gene Set Enrichment Analysis (GSEA). Moreover, we verified the expression levels of diagnostic biomarkers by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot.
Results: A total of 10 hub genes were screened from the ceRNA network, which appeared to be associated with the viral infection, kidney development, and regulation of immune and inflammatory responses. Subsequently, LASSO regression analysis established a diagnostic model consisting of DDX58, SAMD9L, and TLR6 with a robust diagnostic potency (AUC = 1). Similarly, single-gene GSEA showed a strong association of these diagnostic biomarkers with the viral infection. Furthermore, PCR and Western blot demonstrated showed that DDX58, SAMD9L, and TLR6 were upregulated in DN patients at both transcriptome and protein levels compared to healthy controls.
Conclusion: We confirmed that differentially expressed hub genes may be novel diagnostic biomarkers in DN.
Keywords: diabetic nephropathy, DN, diagnosis, ceRNA, biomarker
Introduction
Diabetes mellitus (DM) is a serious problem affecting human health at present. The International Diabetes Federation estimates that the number of adults with diabetes in the world will rise to 643 million by 2030 and there will be more than 783 million adults living with diabetes in 20451. More than that, the rising number of patients with diabetes has brought a huge economic burden on the medical and health care systems worldwide.2
In addition to having great harm to the endocrine system, vascular-related complications caused by diabetes can also lead to multi-system damage.3 Whereas microangiopathy in the kidney usually causes chronic kidney disease (CKD), which is often called DN4 and is also one of the most important and serious microvascular complications of diabetes.5 DN is the primary cause of end-stage renal disease (ESRD).6 Long-term hyperglycemia could lead to the loss of kidney function, characterized by glomerular hypertrophy, albuminuria, decreased estimated glomerular filtration rate, and renal fibrosis.7 It is generally believed that the pathogenesis of DN may be related to oxidative stress, activation of the RAAS system, inflammation and so on.8,9 The early stage of DN is mainly shown in glomerular hyperfiltration, but with the progression of the disease, microalbuminuria, obvious albuminuria and progressive decrease of eGFR will be found, resulting in ESRD.10 Currently, with evaluating the longtime of diabetes or the presence of diabetic retinopathy exception, the diagnosis of DN is mainly judged by persistent albuminuria and declining eGFR.11,12 Although these biomarkers are widely used clinically, they are inevitably affected by age, gender, heredity, and other factors. In addition, a single biomarker does not have the precise ability to predict disease.13 For example, some studies have shown that microalbuminuria was considered to be the prime indicator of DN though, it was not as accurate as we expected.14–16 Given this, it is very necessary to keep seeking new biomarkers to explore the effective ways of diagnosis and treatment of DN.
In this study, three hub genes (DDX58, SAMD9L, TLR6) related to DN were obtained by bioinformatics analysis based on transcriptome sequencing data. These three genes could distinguish between DN samples and normal samples greatly, suggesting that these genes were expected to be novel DN biomarkers and potential adjuncts for clinical diagnosis.
Materials and Methods
Patient Preparation
In this study, 3 DN patients and 3 healthy volunteers in the Second Hospital Affiliated to Kunming Medical University were selected in 2020 and their blood was taken as the test samples. All the blood samples were obtained separately for the needs of the study. Previously, we ensured that each subject was fully informed the purpose and significance of the study, and the participation was entirely voluntary. All the patients were clinically diagnosed with DN and the inclusion criteria were based on the clinical diagnostic criteria with reference to the expert consensus on clinical diagnosis of DN in Chinese adults:17 the history of diabetes for more than 10 years; persistent albuminuria(>300 mg/day) or a progressive decrease in GFR; simultaneously existing diabetic retinopathy (DR). Furthermore, routine blood-examination results from the 3 healthy volunteers were within the normal range. 2mL Trizol reagent (ThermoFisher Scientific, China) was mixed with per milliliter peripheral blood and then stored at −80°C.
The study was approved by the Medical Ethics Committee of the Second Hospital Affiliated to Kunming Medical University, following the guidelines outlined in the Declaration of Helsinki. Because of the low risk to the subjects, the waiver of informed consent for the study was also agreed by the Second Hospital Affiliated to Kunming Medical University.
RNA Extraction and Qualification
Trizol (Thermo Fisher Scientific) was used to extract RNA from the samples respectively, according to the instructions. The RNA concentration and integrity were evaluated using Nanodrop 2000 spectrophotometer (ThermoFisher Scientific, USA) and Agilent Bioanalyzer 2100 (Agilent Technologies, USA).
Library Construction
Ribo-off rRNA Depletion Kit (Vazyme, China) was used to remove rRNA from each sample, and then VAHTSTM RNA Clean Beads (Vazyme, China) was used to purify rRNA-depleted RNA. According to the manufacturer’s instructions of VAHTS Universal V8 RNA-seq Library Prep Kit for Illumina (Vazyme, China), the first-strand and the second-strand of cDNA were synthesized. Then, the obtained cDNA was end-repaired, 3′adenylated. After that, an optional NEBNext adaptor was used for adaptor ligation and the product was cleaned up next. Subsequently, depending on the reaction conditions of polymerase chain reaction (PCR), the product was amplified by PCR using SYBR Green qPCR Master Mix (Servicebio, China) and purified similarly. Finally, the quality of the library was assessed by Qsep-400 (Bioptic, China) and the qualified library was sequenced on the Illumina novaseq 6000 platforms (Illumina, USA) using NovaSeq 6000 S4 Reagent Kit (Illumina, USA).
Differential Analysis
The limma package was applied to indicate DE-mRNAs and -lncRNAs between normal and DN samples, with screening criteria of P < 0.05 and |log2 fold change (FD)| > 1.
Construction of the ceRNA Network
The ceRNA network was constructed based on DE-mRNAs and -lncRNAs. Human miRNA sequences were obtained by miRbase. In miranda software, the binding score was set to 180 to predict the relationship pairs among miRNAs, DE-lncRNAs, and DE-mRNAs. In this study, we only kept the relationship pairs with consistent expression trends, ie, up-regulated DE-mRNA-miRNA-up-regulated DE-lncRNA and down-regulated DE-mRNA-miRNA-down-regulated DE-lncRNA.
Construction of PPI Networks and Identification of Hub Genes
The Protein-Protein Interaction (PPI) network was created via the STRING website based on the mRNAs in the ceRNA network obtained above and visualized in Cytoscape. For the hub genes, the top10 mRNAs acquired with the CytoHubba plugin of Cytoscape were used to define them.
GO and KEGG Analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed in the clusterProfiler package to reveal the potential functions of hub genes. The GO system consists of three components, biological processes (BP), cellular components (CC), and molecular functions (MF). Terms with P < 0.05 were considered significantly enriched. Unfortunately, hub genes did not appear to be enriched in the CC category.
Construction and Evaluation of Diagnostic Model
We undertook LASSO regression analysis of 10 hub genes using the glmnet software package to derive the optimal variables to construct a diagnostic model for DN. ROC curves were then applied to assess the ability of the diagnostic model and individual variables to distinguish DN from normal samples.
Single-Gene GSEA
Single-gene GSEA was employed to elucidate the signaling pathways involved in diagnostic biomarkers. In this study, we calculated the correlation of each diagnostic biomarker with all other genes separately and ranked all genes from highest to lowest correlation. The ranked genes were used as the set of genes to be tested, the KEGG signaling pathway was used as the pre-defined gene set, and finally, the enrichment of the KEGG signaling pathway in the corresponding set of genes to be tested was examined.
Isolation of Peripheral Blood Mononuclear Cells (PBMC)
We collected peripheral blood samples from 10 DN patients and 10 healthy volunteers (the inclusion criteria of all participants were the same as before) from the Second Hospital Affiliated of Kunming Medical University and anticoagulated them with EDTA to ensure the stability of RNA in the blood samples. Any healthy volunteers had no known disease. Fresh anticoagulated whole blood was added in 3 mL to a 15 mL centrifuge tube containing 3 mL of human peripheral blood lymphocyte isolate (Solarbio, Beijing, China), and the mixed fluid was centrifuged using a high-speed centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) based on 450 g × 30 min at room temperature. After centrifugation, the mixture would be clearly stratified: the top layer is the diluted plasma layer, the middle layer is the clear isolate layer, the white film layer between the plasma and the isolate is the lymphocyte layer, and the bottom of the centrifuge tube is the red blood cells and granulocytes. Subsequently, the cells of the white membrane layer were carefully aspirated into a 15mL clean centrifuge tube and washed with 10mL PBS. The supernatant was discarded and the cells were resuspended in 5 mL of PBS, centrifuged at 250 g for 10 min, and the supernatant was discarded after two repetitions.
RNA Isolation, Reverse Transcription, and Real-Time PCR
Total RNA was isolated from 10 paired samples using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Then the concentration and purity of the RNA solution was quantified using a NanoDrop 2000 nucleic acid protein quantifier (Thermo Fisher Scientific, Waltham, MA, USA). qRT-PCR was performed as described previously.18 Briefly, the extracted RNA was reverse-transcribed to cDNA using the SureScript-First-strand-cDNA-synthesis-kit (Genecopoeia, Guangzhou, China) prior to qRT-PCR. The qRT-PCR reaction consisted of 4 µL of reverse transcription product, 2 µL of 5X BlazeTaq qPCR Mix (Genecopoeia, Guangzhou, China), 0.5 µL each of forward and reverse primer, and 3-µL nuclease-free water. PCR was performed in a CFX96 Touch instrument (Bio-Rad, Hercules, CA) under the following thermal cycle protocol: (1) 95°C for 30s; (2) 95°C for 10s, 60°C for 20s, 40×; (3) 72° for 30s. The GAPDH gene served as an internal control. RNA levels were calculated for DN samples and normal samples using the 2−ΔΔCt method. Primers for amplification were purchased from Tsingke (Beijing, China). Primer sequences used for qRT-PCR were shown in Table 1.
 |
Table 1 Primer sequences |
Protein Extraction and Western Blot
Western blot analysis was conducted with an SDS-PAGE electrophoresis system (Bio-Rad Laboratories, Hercules, CA, USA) to quantify changes in protein levels. Briefly, total protein was extracted from the PBMC using RIPA buffer containing protease inhibitors and phosphatase inhibitors. Protein samples were separated by SDS-PAGE and transferred onto nitrocellulose membranes, which were subsequently blocked for 12h at 4°C with 5% skimmed milk containing TBST solution. Antibodies against DDX58 (107kDa; ab180675; Abcam), SAMD9L (185kDa; PA5-53994; Thermo), TLR6 (92kDa; ab37072; Abcam), and β-actin (42kDa; ab8226; Abcam) were used as primary antibodies. The samples were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature (RT) for 1 h. The membrane was imaged by QuickGel 6100 (Monad, Wuhan, China).
Statistical Analysis
The bioinformatic analysis part of this study was performed in the R software. Statistical plots of PCR and Western blot were completed by GraphPad Prism 8.0, and significant P values were calculated by unpaired t-test, where * indicates P < 0.05, ** indicates P < 0.01, and **** indicates P < 0.0001. P < 0.05 was considered statistically significant if not otherwise stated.
Results
Characterization of DE-mRNAs and -lncRNAs Associated with DN
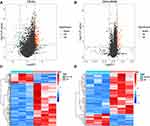
We identified a total of 185 DE-mRNAs between the DN and normal groups, of which 150 were up-regulated genes and the remaining 35 as down-regulated genes (Figure 1A; Supplementary Table 1). Meanwhile, a further 88 DE-lncRNAs were appraised, including 68 up-regulated and 20 down-regulated (Figure 1B; Supplementary Table 2). Additionally, the expression patterns of DE-mRNAs and -lncRNAs in the two groups were graphically visualized with the clustering heatmap (Figures 1C and D). These differentially expressed genes would be available for the subsequent analysis.
Screening and Functional Enrichment Analysis of Hub Genes
With the miRbase database and miranda software, we constructed a DE-mRNA-miRNA-DE-lncRNA network covering 290 nodes and 496 edges (Figure 2A; Supplementary Table 3). Further, we predicted the protein interactions of 93 DE-mRNAs in the above network utilizing the STRING website and performed visualization in Cytoscape, ultimately, we obtained a PPI network consisting of 42 nodes and 84 edges (Figure 2B; Supplementary Table 4). Subsequently, 10 genes defined as hub genes were filtered from the above PPI network according to the CytoHubba plug-in (Supplementary Table 5) and displayed in Figure 2C, which had a total of 37 edges.
To reveal the potential functions of hub genes in ND, GO and KEGG analysis were imperative (Figures 2D and E). We found that hub genes appeared significantly enriched in terms associated with the response to viral infection. For example, the ‘defense response to virus’, ‘response to virus’, “cytoplasmic pattern recognition receptor signaling pathway in response to virus”, “cellular response to virus”, “negative regulation of viral process”, “regulation of defense response to virus by host”, “negative regulation of viral genome replication”, “viral genome replication”, etc. This seemed to coincide with previous reports that viral infections (eg, EBV, HCV, HBV, etc.) induce the development of DN.19–21 Similarly, KEGG also suggested that the hub genes were tightly coupled with “Epstein-Barr virus infection”, “Hepatitis C”, and “Hepatitis B”. Intendedly, hub genes were implicated in a variety of processes related to kidney development, including “metanephric mesenchyme development”, “nephron tubule epithelial cell differentiation”, “negative regulation of kidney development”, “renal vesicle morphogenesis”, and “metanephric nephron tubule development”. Furthermore, the hub gene may also influence the progression of DN by regulating the immune response (“regulation of innate immune response”, “regulation of immune effector process”, “activation of innate immune response”, “positive regulation of innate immune response”, “positive regulation of immune effector process”, etc.) and the status of immune cells (“regulation of dendritic cell cytokine production”, “regulation of granulocyte macrophage colony-stimulating factor production”, “positive regulation of macrophage activation”, etc.). Otherwise, “regulation of inflammatory response”, “NLRP3 inflammasome complex assembly”, “regulation of NLRP3 inflammasome complex assembly”, and “positive regulation of inflammatory response” being also remarkably enriched. The exhaustive results of the GO and KEGG analyses were available in Supplementary Tables 6 and 7, respectively. These results suggested that the hub genes may play a role in the pathogenesis of DN by regulating processes related to viral infection, kidney development, and inflammatory and immune responses.
Diagnostic Biomarkers Filtering for DN from Hub Genes
The minimum lambda value was visible in 3 following the cvfit plot, which indicated that the 3-hub gene-based diagnostic model was optimized in terms of both accuracy and simplicity (Figure 3A). Therefore, only DDX58, SAMD9L, and TLR6 were included (Figure 3B). A ROC curve analysis was conducted to evaluate the sensitivity and specificity of the 3-hub gene-based diagnostic model for differentiating DN from the normal group (Figure 3C). By calculating the AUC, it was revealed that the 3-hub gene signature could accurately classify DN patients from normal individuals (AUC = 1). Also, individual ROC curve analysis of the three hub genes mentioned above indicated that these genes could also be independently distinguished between DN patients and normal subjects (AUC = 1 for all; Figure 3D). Hence, these genes were considered as diagnostic biomarkers for DN and served for subsequent analysis.
Functional Enrichment Analysis of Diagnostic Biomarkers
To further identify the potential pathways involved in diagnostic biomarkers, we performed single-gene GSEA-KEGG on them and the results were displayed in Figures 4A–C. Collectively, all three diagnostic biomarkers were associated with viral infection (“Herpes simplex virus 1 infection”, “Hepatitis C”, “Epstein-Barr virus infection”, etc.). Surprisingly, we found that the “NOD-like receptor signaling pathway”, which is involved in the immune regulation of DN,22,23 was significantly enriched in the above results. Notably, SAMD9L was involved in “Diabetic cardiomyopathy”. Similarly, DDX58 had been linked to cardiac disease pathways (“Dilated cardiomyopathy” and “Arrhythmogenic right ventricular cardiomyopathy”). Detailed enrichment results for each diagnostic biomarker were reported in Supplementary Tables 8–10. In addition, we constructed a ceRNA network encompassing 16 KEGG pathways based on three diagnostic biomarkers, which included three diagnostic biomarkers, three miRNAs, and seven DE-lncRNAs (Figure 4D).
Expression of Diagnostic Markers Was Frequently Elevated in DN Samples
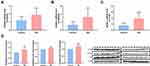
To verify the expression of three diagnostic biomarkers (DDX58, SAMD9L, and TLR6) in DN, qRT-PCR was used to detect the mRNA levels of DDX58, SAMD9L, and TLR6 in PBMC obtained from 10 cases of DN and healthy subjects. The results showed that the mRNA levels of DDX58, SAMD9L, and TLR6 were significantly higher in the DN group compared with healthy subjects (P < 0.05, Figure 5A–C). Moreover, Western blot showed that protein levels of DDX58, SAMD9L, and TLR6 were significantly increased in DN samples compared to healthy subjects (Figure 5D). In summary, our results showed that DDX58, SAMD9L, and TLR6 were upregulated in DN patients at both transcriptome and protein levels compared to healthy controls.
Discussion
As we all know, DN is not only the major cause of ESKD but also a serious complication of diabetes. Early DN may not have any clinical symptoms, but only glomerular hyperfiltration.24 Although renal biopsy is the “gold standard” for the diagnosis of renal disease, glomerular changes in the early stage of diabetes are non-specific and renal biopsy as an invasive operation does have lots of risks, thus it may not be widely accepted in this period.14 In addition, there is no other test to replace renal biopsy at present. However, as for DN, diagnosis and cure earlier are of great significance. Drug intervention in the early stage could delay the progression of renal failure25 and prevent the occurrence of other adverse events such as cardiovascular disease26 (CVD), thereby improving patients’ quality of life. Therefore, it is urgent to find a new biomarker of early DN.
In this study, 185 DE-mRNAs and 88 DE-lncRNAs were obtained by sequencing the samples of DN and healthy volunteers. Because of the large number of differential expression genes, it is very hard to study these genes one by one. We all know that lncRNA is a kind of non-coding RNA.27 Therefore, the study of lncRNA function is usually carried out in lncRNA-RNA, lncRNA-DNA, lncRNA-protein.28 In recent years, many studies have suggested that miRNA could participate in the regulation of many important cellular activities, such as cell proliferation, differentiation, epithelial-mesenchymal transformation and so on,29,30 making miRNA gradually has been a hot area of research. And then, it was found that lncRNA could serve as a miRNA sponge, reducing the degradation or transcriptional inhibition of mRNA by binding to miRNA, which is often referred to as ceRNA.31 Therefore, we hope to further screen the genes which are more relevant to disease among those differential expression genes we got by constructing such a sort of ceRNA network (lncRNA-miRNA-mRNA). Fortunately, we linked the ceRNA network with the PPI network, and 10 hub genes were screened, then the functional enrichment analysis was performed. The diagnostic model constructed by LASSO regression analysis was used to gain the three characteristic genes (DDX58, SAMD9L, and TLR6), which are most related to DN. Finally, the single-gene GSEA analysis of the three genes was carried out, and the gene-miRNA-lncRNA-KEGG pathway was constructed.
We showed great interest in the three genes we finally got, the reason was that these genes showed great sensitivity and specificity to distinguish between DN and non-DN. DDX58, called DExD/H-box helicase 58, is deemed to take part in viral ds RNA recognition and the regulation of the antiviral innate immune response. SAMD9L, called sterile alpha motif domain containing 9 like, is regarded as playing a key role in cell proliferation and the innate immune response to viral infection. TLR6, called toll-like receptor 6, is considered to play a fundamental role in pathogen recognition and activation of innate immunity. In consequence, we speculated that these three genes maybe new biomarkers of DN.
As mentioned before, by observing and comparing the single-gene GSEA results, we found that, without exception, all the genes were associated with “viral infection” and coincide with a “NOD-like receptor signaling pathway”.
It’s well known that viral infection is one of the causes of many kidney diseases. From the latest reports, viruses related to kidney diseases include hepatitis B virus,32 hepatitis C virus,33 human immunodeficiency virus,34 cytomegalovirus, Epstein-Barr virus, parvovirus35 and so on. Covid-19, which is now prevalent all over the world, has also been found to cause kidney damage.36 The pathogenesis of virus infection-associated glomerular disease is still unclear. Some studies suggested that it may be due to virus infection resulting in renal tissue damage, or through viral antigens and corresponding antibodies to form the circulating immune complexes (CIC) deposition, and then activated the specific immunity mechanism.37 Consequently, we will discuss the aspect of immune defense principally.
Some studies have shown that virus infection was the main cause of autoimmunity.38 The innate immune system’s recognition of microbial pathogens was mediated by pattern-recognition receptors (PRR), while PRR detected pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), such as viral nucleic acids.39 At present, the widely accepted PRR mainly includes Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), Nod-like receptors (NLRs), and AIM-2 like receptors.40 It’s also suggested that DDX58, SAMD9L, and TLR6 could indeed participate in innate immune responses through the NOD-like receptor signaling pathway.
NLRs are a cluster of multidomain proteins containing an alterable amino-terminal (N-terminal) domain, a central NOD module, and carboxy-terminal leucine-rich repeats (LRRs).41 There are two prototypic NLRs, NOD1 and NOD2. On the one hand, they could sense the cytosolic presence of the bacterial peptidoglycan fragments which escaped from endosomal compartments, thus driving the activation of nuclear factor kappa B (NF-κB) and interferon regulatory factor (IRF), cytokine production, and apoptosis. On the other hand, they could also induce caspase-1 activation, to regulate the maturation of IL-1B, and IL-18 and drive pyroptosis. Some studies have indicated that the activation of NLRs will lead to the formation of inflammasome.42 However, there’re a large number of studies published regarding the effect and mechanism of NLRP inflammasomes in the pathogenesis and progression of DN,43,44 so it needs no further elaboration here.
The following limitations exist in this study. First, a single source of our research data and the limited sample size could cause some deviations. Secondly, the study needs to be further supplemented to reveal the mechanism of the key genes. Lastly, the diagnostic model remains unproven in more clinical samples.
In general, we obtained 10 hub genes by connecting the ceRNA network with the PPI network. Then the diagnostic model was constructed by LASSO regression analysis to screen three characteristic genes related to the disease. But this was only our guess, so we further verified the genes by regaining other clinical samples of DN, and confirmed that these three genes were indeed highly expressed in the DN, while it was opposite in the control. This obviously differential expression phenomenon of the disease maybe indicate that these three genes are closely related to the occurrence of DN. Through the enrichment analysis of these genes, the key signaling pathway was obtained. We guessed that, on the one hand, these three genes may be able to identify renal damage caused by a viral infection, and the other side may be able to participate in the progression of DN through NOD-like receptor signaling pathway-mediated innate immune responses and inflammatory. However, the more specific mechanism remains to be further studied.
Funding
Natural Science Foundation of China (No. 81860145, 81860144); A special fund for high-level talents cultivation in health and family planning in Yunnan (No. D-2017027); Applied Basic Research Project of Science and Technology Department of Yunnan Province (No. 202001AT070008); Developing yunnan elite plan Youth talent project (NO. YNWR-QNBJ-2020-269) and Famous doctors project (NO. YNWR-MY-2019-075).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7.
2. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021;24:109119.
3. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):117–124. doi:10.2174/1570161117666190502103733
4. Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–377.
5. Yu SM, Bonventre JV. Acute kidney injury and progression of diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25(2):166–180.
6. Li S, Zheng L, Zhang J, Liu X, Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–449.
7. Lu Y, Liu D, Feng Q, Diabetic Nephropathy: LZ. Perspective on Extracellular Vesicles. Front Immunol. 2020;3(11):943.
8. Calle P, Hotter G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int J Mol Sci. 2020;21(8):2806.
9. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;8(2021):1497449.
10. Doshi SM, Friedman AN. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(8):1366–1373. doi:10.2215/CJN.11111016
11. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15. doi:10.1111/dom.14007
12. Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33:2. doi:10.1002/dmrr.2841
13. Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1):36. doi:10.1186/s12882-018-0821-9
14. Qi C, Mao X, Zhang Z, Wu H. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. 2017;2017:8637138. doi:10.1155/2017/8637138
15. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864–2883. doi:10.2337/dc14-1296
16. Van JA, Scholey JW, Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol. 2017;28(4):1050–1061. doi:10.1681/ASN.2016091018
17. Expert consensus on clinical diagnosis of diabetic nephropathy in Chinese adults. [中国成人糖尿病肾脏病临床诊断的专家共识]. 中华内分泌代谢杂志. 2015;31(5):379–385. [Chinese]. doi:10.3760/cma.j.issn.1000-6699.2015.05.001
18. Cai B, Ma W, Ding F, et al. The long noncoding RNA CAREL controls cardiac regeneration. J Am Coll Cardiol. 2018;72(5):534–550. doi:10.1016/j.jacc.2018.04.085
19. Davies JE, Siczkowski M, Sweeney FP, et al. Glucose-induced changes in turnover of Na+/H+ exchanger of immortalized lymphoblasts from type I diabetic patients with nephropathy. Diabetes. 1995;44(4):382–388. doi:10.2337/diab.44.4.382
20. Soma J, Saito T, Taguma Y, et al. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol. 2000;11(4):690–699. doi:10.1681/ASN.V114690
21. Wang XJ, Hu W, Zhang TY, Mao YY, Liu NN, Wang SQ. Irbesartan, an FDA approved drug for hypertension and diabetic nephropathy, is a potent inhibitor for hepatitis B virus entry by disturbing Na(+)-dependent taurocholate cotransporting polypeptide activity. Antiviral Res. 2015;120:140–146. doi:10.1016/j.antiviral.2015.06.007
22. Luan P, Zhuang J, Zou J, et al. NLRC5 deficiency ameliorates diabetic nephropathy through alleviating inflammation. FASEB J. 2018;32(2):1070–1084. doi:10.1096/fj.201700511RR
23. Chen K, Zhang J, Zhang W, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45(5):932–943. doi:10.1016/j.biocel.2013.02.009
24. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. doi:10.1681/ASN.2016060666
25. Khan NU, Lin J, Liu X, et al. Insights into predicting diabetic nephropathy using urinary biomarkers. Biochim Biophys Acta Proteins Proteom. 2020;1868(10):140475. doi:10.1016/j.bbapap.2020.140475
26. Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12(1–2):110–118. doi:10.1900/RDS.2015.12.110
27. Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: an innate immune perspective. Biochim Biophys Acta Gene Regul Mech. 2020;1863(4):194419. doi:10.1016/j.bbagrm.2019.194419
28. Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045. doi:10.1083/jcb.202009045
29. Chan SH, Wang LH. Regulation of cancer metastasis by microRNAs. J Biomed Sci. 2015;22(1):9. doi:10.1186/s12929-015-0113-7
30. Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21(5):1723. doi:10.3390/ijms21051723
31. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol. 2016;1402:271–286.
32. Geng XX, Tian Z, Liu Z, Chen XM, Xu KJ. Associations between hepatitis B infection and chronic kidney disease: 10-Year results from the U.S. National Inpatient Sample. Enferm Infecc Microbiol Clin. 2021;39(1):14–21. [English]. doi:10.1016/j.eimc.2020.02.029
33. Gantumur G, Batsaikhan B, Huang CI, et al. The association between hepatitis C virus infection and renal function. J Chin Med Assoc. 2021;84(8):757–765. doi:10.1097/JCMA.0000000000000561
34. Heron JE, Bagnis CI, Gracey DM. Contemporary issues and new challenges in chronic kidney disease amongst people living with HIV. AIDS Res Ther. 2020;17(1):11. doi:10.1186/s12981-020-00266-3
35. Kupin WL. Viral-associated GN: hepatitis B and other viral infections. Clin J Am Soc Nephrol. 2017;12(9):1529–1533. doi:10.2215/CJN.09180816
36. Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27(5):365–376. doi:10.1053/j.ackd.2020.09.003
37. Kupin WL. Viral-associated GN: hepatitis C and HIV. Clin J Am Soc Nephrol. 2017;12(8):1337–1342. doi:10.2215/CJN.04320416
38. Smatti MK, Cyprian FS, Nasrallah GK, Thani AA A, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi:10.3390/v11080762
39. Velloso FJ, Trombetta-Lima M, Anschau V, Sogayar MC, Correa RG. NOD-like receptors: major players (and targets) in the interface between innate immunity and cancer. Biosci Rep. 2019;39(4):BSR20181709.
40. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353.
41. Saur IML, Panstruga R, Schulze-Lefert P. NOD-like receptor-mediated plant immunity: from structure to cell death. Nat Rev Immunol. 2021;21(5):305–318.
42. Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1(4):226–232.
43. Gu C, Liu S, Wang H, Dou H. Role of the thioredoxin interacting protein in diabetic nephropathy and the mechanism of regulating NOD like receptor protein 3 inflammatory corpuscle. Int J Mol Med. 2019;43(6):2440–2450.
44. Wu M, Yang Z, Zhang C, et al. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism. 2021;118:154748.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.