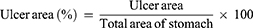Back to Journals » Journal of Inflammation Research » Volume 15
Atractylone Alleviates Ethanol-Induced Gastric Ulcer in Rat with Altered Gut Microbiota and Metabolites
Authors Li L , Du Y, Wang Y, He N, Wang B , Zhang T
Received 5 May 2022
Accepted for publication 3 August 2022
Published 16 August 2022 Volume 2022:15 Pages 4709—4723
DOI https://doi.org/10.2147/JIR.S372389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ling Li,1,2,* Yaoyao Du,1,* Yang Wang,3 Ning He,2 Bing Wang,1,4 Tong Zhang1
1School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2School of Pharmacy, Anhui University of Chinese Medicine, Hefei, People’s Republic of China; 3Metabo-Profile Biotechnology (Shanghai) Co. Ltd, Shanghai, People’s Republic of China; 4Chinese Academy of Sciences, Shanghai Institute of Materia Medica, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bing Wang; Tong Zhang, Email [email protected]; [email protected]
Background: Gastric ulcer (GU) is the most common multifactor gastrointestinal disorder affecting millions of people worldwide. There is evidence that gut microbiota is closely related to the development of GU. Atractylone (ATR) has been reported to possess potential biological activities, but research on ATR alleviating GU injury is unprecedented.
Methods: Helicobacter pylori (H. pylori)-induced GU model in zebrafish and ethanol-induced acute GU model in rat were established to evaluate the anti-inflammatory and ulcer inhibitory effects of ATR. Then, 16S rRNA sequencing and metabolomics analysis were performed to investigate the effect of ATR on the microbiota and metabolites in rat feces and their correlation.
Results: Therapeutically, ATR inhibited H. pylori-induced gastric mucosal injury in zebrafish. In the ulceration model of rat, ATR mitigated the gastric lesions damage caused by ethanol, decreased the ulcer area, and reduced the production of inflammatory factors. Additionally, ATR alleviated the gastric oxidative stress injury by increasing the activity of superoxide dismutase (SOD) and decreasing the level of malondialdehyde (MDA). Furthermore, ATR played a positive role in relieving ulcer through reshaping gut microbiota composition including Parabacteroides and Bacteroides and regulating the levels of metabolites including amino acids, short-chain fatty acids (SCFAs), and bile acids.
Conclusion: Our work sheded light on the mechanism of ATR treating GU from the perspective of the gut microbiota and explored the correlation between gut microbiota, metabolites, and host phenotype.
Keywords: atractylone, gastric ulcer, inflammation, oxidative stress, gut microbiota, metabolomics
Introduction
Gastric ulcer (GU) is a common disease in the upper digestive tract, with a global incidence of 10%.1 Long-term unhealed GU can easily lead to acute and severe diseases and complications such as gastrointestinal perforation, gastrointestinal bleeding, pyloric obstruction, and even cancer.2 Helicobacter pylori (H. pylori) infection, alcohol consumption, stress, and nonsteroidal anti-inflammatory drug intake have been shown to be the main causes of GU.3 H. pylori infection can destroy the gastric mucosal barrier through the production of pathogenic factors and promote the release of inflammatory mediators.4 High concentration of alcohol can directly cause acute inflammation and oxidative stress of the gastric mucosa, as manifested by significantly decreased antioxidant enzyme superoxide dismutase (SOD), increased peroxide product malondialdehyde (MDA), as well as excessive inflammatory cytokines.5–7 Although several medications (eg, proton pump inhibitors, antibiotics, antihistamines, and antacids) have been used in clinic to alleviate the symptoms of GU, their therapeutic efficiencies are unsatisfactory owing to various adverse effects.1,8 Therefore, it is particularly important to use natural medicines with low side effects and good biological activity to prevent and treat gastric injury.
Atractylone (ATR) is a sesquiterpene oxide-derivative in essential oils from Atractylodes macrocephala Koidz., and it has many attractive pharmacological effects, including anti-inflammatory, antibacterial, and antioxidant.9,10 Notably, semi-carbonized nanodots prepared with charred Atractylodes macrocephala Koidz. were found to have a strong inhibitory effect on acute stress ulcer.11 Moreover, other sesquiterpenes in Atractylodes macrocephala Koidz., such as Atractylenolide III, can also play a protective role in acute GU caused by ethanol.12 Thus, ATR is considered to effectively repair gastric mucosal injury. However, the evidence behind these benefits remains limited. Exploring the therapeutic effect and mechanism of ATR on GU will be greatly meaningful and promising in the future medical applications of ATR.
In recent years, comprehensive studies of gut microbiota and metabolomics have been widely used to systematically elucidate the pharmacological mechanisms of Chinese medicine.13–16 Gut microbiota can interfere with gastrointestinal homeostasis to varying degrees, and many metabolites they produce can play a key role in energy supply, inflammation regulation, and immune response, such as short-chain fatty acids (SCFAs), bile acids, and amino acids.17–22 In this work, we firstly adopted H. pylori-induced zebrafish and ethanol-induced rat as two animal models to evaluate the effect of ATR on gastric mucosal injury. The zebrafish can exhibit a high degree of resemblance in their gastrointestinal tract development and physiology to humans and have been shown to be a beneficial complementary model to rodents in many research fields.23,24 Next, we characterized the phenotype of microbiota and metabolome in GU rats by using 16S rRNA gene sequencing and ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) techniques to probe the mechanisms underlying therapeutic effect of ATR on GU.
Materials and Methods
Materials
Atractylone was purchased from Shanghai Xijian Biotechnology Co., Ltd. Clarithromycin was purchased from Aladdin (Shanghai). Ranitidine was purchased from H&S pharmaceutical. Trinitrobenzene sulfonic acid (TNBS, SLCK4178) was purchased from Sigma. Helicobacter pylori culture medium (20200507), Helicobacter pylori additive (20211104), and defibrinated sheep blood (20211026) were from Qingdao Hi-Tech Industrial Park Haibo Biotechnology. CM-DiI (2335589) was from Thermo Fisher. SOD and MDA assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. Tumor necrosis factor (TNF)-α, nitric oxide (NO), and interleukin (IL)-10 ELISA kits were purchased from Proteintech.
Animal Model
Establishment of Gastrointestinal Mucosal Injury Model in Zebrafish
Zebrafish larvae from AB and Tg (mpx: GFP) strains were obtained by natural spawn, collected in 1X E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, pH 7.2) and kept in Petri dishes at 28 °C incubator with 14/10 dark-light cycle with daily medium changes until 3 dpf.25,26 The zebrafish (3 dpf) were placed in a 6-well plate (30 fish per well) and randomly divided into control group, model group, ATR group (7.8 μM) and clarithromycin group (167 μM). Except for the control group, the other groups were first treated with TNBS (125 μg/mL) at 28 °C for 48 h. The total quantity of TNBS to be used was prepared in 1X E3 medium. After that, TNBS was removed, and H. pylori (5 × 107 CFU/mL) was added to all groups to establish a gastrointestinal mucosal injury model in zebrafish, and treated at 28 °C for 24 h.26,27
H. pylori Culture
H. pylori non-resistant strain GDMCC1.1820 was purchased from Guangdong Microbial Culture Collection Center. The strain was stored at −80 °C in brain heart infusion containing 30% glycerol. For solid culture, H. pylori was cultured in Columbia blood agar base supplemented with 5% sheep blood under microaerophilic conditions (5–6% O2, 8–10% CO2, 85% N2) at 37 °C. For liquid proliferation, H. pylori was inoculated in brain heart infusion supplemented with 10% fetal bovine serum and shaken at 150 rpm under the same air environment.28
Establishment of Gastric Ulcer Model in Rat
Sprague Dawley (SD, 6–8 weeks) rats were purchased from B&K Universal Group Limited. All rats were kept in specific pathogen-free (SPF) barrier conditions under 22–24 °C, and were housed in standard rat cages with ad libitum access to water and food for one week before the experimentation. All animal care and experimental protocols were performed under the Institutional Animal Care and Use Committee (IACUC) of Shanghai University of Traditional Chinese Medicine (approval number: PZSHUTCM220307021). The rats were randomly assigned to the following groups (n = 8 each): the control group (0.25% Na-CMC and 0.5% Tween-80), the EtOH group (0.25% Na-CMC and 0.5% Tween-80), ATR (25 mg/kg) group, Ranitidine (40 mg/kg) group. The ATR and Ranitidine groups were orally administered pretreatments from days 1–7. The feces of all group rats were collected every day. On day 7, all rats except those in the control group were orally administered with ethanol (5 mL/kg bw) after 30 minutes of treatment. Two hours after ethanol treatment, all rats were euthanized with carbon dioxide, the organs and serum samples were collected for further studies. For histological examination, the gastric tissue was fixed with 4% paraformaldehyde solution.
Assessment of Gastric Mucosal Injury in Zebrafish
The AB strain zebrafish was used to determine the maximum tolerated concentration (MTC), pathological changes, repair of gastrointestinal mucosal damage, and inhibition of H. pylori. The Tg (mpx: GFP) strain zebrafish was used to count neutrophils. A total of 10 zebrafish were randomly selected from each group to be photographed under a fluorescence microscope and the pictures were saved. The area of zebrafish gastrointestinal tract, the number of neutrophils, and the fluorescence intensity of the gastrointestinal tract were analyzed using NIS-Elements D 3.20 software. The area of gastrointestinal tract was used to evaluate the effect of ATR on the repair of gastrointestinal mucosal damage in zebrafish. The number of neutrophils was used to evaluate the efficacy of ATR on the resolution of gastrointestinal inflammation in zebrafish. The fluorescence intensity was used to evaluate the inhibitory effect of ATR on H. pylori in zebrafish.
Histopathological Evaluation in Zebrafish
The zebrafish in each group were fixed, dehydrated, embedded, sliced, and stained with hematoxylin–eosin (H&E) for histopathological analysis and observation.
Assessment of Gastric Mucosal Injury in Rat
Gastric tissue of rat was photographed, and Image-Pro Plus 6.0 software was used to calculate the area of GU and the total area of the gastric tissue of each rat, and calculate the ratio of ulcer area.
Histopathological Evaluation in Rat
Gastric tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut at a thickness of 5 μm and stained with H&E. Each section was observed by using an Olympus IX83 microscope (Tokyo, Japan). The pathological lesion of each gastric tissue was scored by the method described in Table 1.29
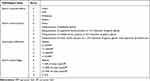 |
Table 1 Histological Scoring Criteria |
Antioxidant Activity Assessment
The levels of SOD and MDA in the serum were assessed by commercial assay kits according to the manufacturer’s protocols. Results of SOD were expressed as U/mL, and results of MDA were expressed as nM.
Determination of TNF-α, IL-10, and NO Levels
The rat cytokines TNF-α, IL-10, and NO were determined in serum using ELISA kits. All procedures were carried out according the manufacture. Results of TNF-α and IL-10 were expressed as pg/mL, and results of NO were expressed as μM.
Gut Microbiota Sequencing and Data Analysis
In the ethanol-induced GU rat model, fecal samples were sequenced for the 16S rRNA gene. Total microbial DNA was isolated by the OMEGA Soil DNA Kit (M5635-02) (Omega Bio-Tek, Norcross, GA, USA). The quantity and quality of DNAs were measured through NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The V3–V4 region of 16S rRNA gene was selected for amplification by an ABI GeneAmp®9700 PCR thermocycler (ABI, CA, United States). The raw 16S rRNA gene sequencing reads were quality-filtered by DADA2 plugin. Sequences were clustered into operational taxonomic units (OTUs) with 97% similarity and analyzed by QIIME2 and R packages (v3.2.0). From phylum to genus, linear discriminant analysis (LDA) and effect size were used to emphasize statistical significance and find biomarkers with significant differences between groups. The set value of the LDA score was >2 though the non-parametric Kruskal–Wallis (KW) test. The student t-test p < 0.05 was considered statistically significant for different bacterial composition.
Metabolomics Analysis
In the ethanol-induced GU rat model, a total of 158 fecal microbial-host cometabolites were quantified by the UPLC-MS/MS system (ACQUITY UPLC-Xevo TQ-S, United States). The solutions of all 158 metabolites standards were prepared in methanol, ultrapure water. The internal standards were used to monitor data quality and compensate for matrix effects. The original data were processed with MassLynx software (v4.1, Waters, MA, USA) to automatically remove baseline values, smooth and pick peak values, and align peak signals. The targeted metabolites were analyzed by the iMAP software (v1.0, Metabo-profile, Shanghai, China). Principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), and student t-test or Mann–Whitney U-test were used for classification and identification of differently altered metabolites. Potential biomarkers of differential metabolites need to meet the following criteria VIP > 1 and p < 0.05.
Statistical Analyses
Data are expressed as mean values ± SD. Statistical significance was evaluated with ANOVA test followed by Tukey’s multiple comparison test (GraphPad Prism 7.0). Statistical significance was expressed by *p < 0.05, **p < 0.01, ***p < 0.001.
Results
ATR Reduced H. pylori-Induced Gastrointestinal Mucosal Damage in Zebrafish
First, MTC of ATR was investigated in zebrafish with H. pylori-induced gastrointestinal mucosal damage. We found that MTC of ATR is 7.8 μM (Table S1). From the pathological changes of gastrointestinal tract, the number of gastrointestinal folds of zebrafish in the model group decreased compared with control group, the intestinal lumen was obviously enlarged and the mucosal tissue was destroyed (Figure 1A). ATR can obviously restore the intestinal dilatation of zebrafish, and the morphology of intestinal tissue cells was similar to that of the control group (Figure 1A). Consistently, ATR has an obvious repair effect on the gastrointestinal mucosal damage caused by H. pylori infection, which is manifested in the significant reduction of the mucosal damage area (p < 0.001, Figure 1B and E). In addition, H. pylori significantly increased neutrophils in the gastrointestinal tract of zebrafish, while ATR showed remarkably reduced the number of neutrophils (p < 0.001), showing a considerable effect of promoting inflammation regression (Figure 1C and F). Also, the enhanced mucosal protection was demonstrated by fluorescence imaging and intensity quantification of H. pylori in gastrointestinal tract (p < 0.001, Figure 1D and G). Taken together, ATR can effectively ameliorate gastrointestinal mucosal damage caused by H. pylori infection in zebrafish.
ATR Mitigated EtOH-Induced Gastric Mucosal Damage in Rat
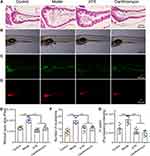
Next, we analyzed the effects of ATR on ethanol-induced GU model by observing the morphological changes in the stomach, calculating the ulcer area ratio, and evaluate pathological changes. Figure 2A shows the photographs of gastric tissue in each group, of which the ulcer area ratio (Figure 2C) was assessed automatically by software. As shown in Figure 2A, the gastric mucosa in the control group was intact without injury and bleeding. In the model group, the gastric body showed obvious erosion and massive bleeding areas around the whole mucosa. Compared with the model group, the gastric mucosal bleeding in each treatment group was significantly reduced. A small amount of obvious punctate and linear bleeding was still observed in the positive drug ranitidine group, while most gastric mucosa tissues in the ATR group had no visible damage and bleeding. As shown in Figure 2C, compared with the control group, the high ulcer area ratio in the model group indicated the significant mucosal damage in rats, while ATR administration alleviated the ulcer injury significantly (p < 0.01). The mucosal protective effects of ATR, with reduced ulcer area ratio, were even stronger compared with that of the ranitidine group. Examination on H&E-stained tissue showed that EtOH-induced ulcer exhibited remarkable damages in gastric mucosal structure with extensive infiltration of inflammatory cells, along with erosion, hemorrhage, edema, and mucosa cells depletion (Figure 2B). While ATR cured the ulcer, it was shown as a nearly normal histological microstructure in ATR group (Figure 2B). Further analysis of histopathological score also indicated that ATR exerts considerable effect on improving mucosal structure (p < 0.001, Figure 2D).
ATR Alleviated EtOH-Induced Oxidative Stress and Inflammation in the Gastric Ulcer
Next, using a rat model, we further evaluated the effects of ATR on the oxidative stress markers and inflammatory factors in serum, including SOD, MDA, TNF-α, NO, and IL-10, which are the primary factors for the function of gastric mucosa. As shown in Figure 3A, the SOD content in the model group (61.10 ± 2.32 U/mL) was significantly lower (p < 0.001) than that of the control group (111.71 ± 1.34 U/mL). Also, the SOD contents of the ranitidine group (79.13 ± 1.52 U/mL) and ATR group (92.98 ± 1.25 U/mL) were significantly higher (p < 0.001) than those of the model group. As shown in Figure 3B, the MDA content in the model group (17.69 ± 1.17 nM) was significantly increased (p < 0.001) compared with the control group (8.74 ± 1.09 nM). Also, the MDA contents of the ranitidine group (13.14 ± 0.90 nM, p < 0.05) and ATR group (12.50 ± 1.03 nM, p < 0.01) were significantly decreased when compared with that of the model group. The inflammatory response of rats was evaluated by determining the levels of inflammatory factors including TNF-α, NO, and IL-10 in serum. In the model group, the levels of proinflammatory factors TNF-α and NO increased significantly compared with that of the control group (p < 0.001, Figure 3C and D). Administration of ATR can significantly reverse the overproduction of TNF-α and NO in the serum (p < 0.001, Figure 3C and D). IL-10 is an anti-inflammatory cytokine which is essential to the function of mucosal barrier. EtOH significantly decreased IL-10 in the serum (p < 0.001, Figure 3E), and ATR effectively restored IL-10 content (p < 0.001, Figure 3E). All these results indicated that ATR can alleviate the gastric mucosal injury in the ethanol-induced ulcer model.
ATR Altered the Structure and Composition of Gut Microbiota
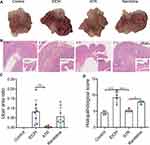
The Chao 1 and Simpson index of the gut microbiota indicated significant difference (p < 0.05, Figure 4A) in α diversity among control group, EtOH group, and ATR group. Compared to the control, community diversity was decreased in the EtOH group and increased in ATR group (Figure 4A), indicating that the intervention of ATR changed the abundance of microbial communities. In the unweighted UniFrac PCoA score plot, the microbial community structure of EtOH group was significantly different from that of the control and ATR group, indicating that the microbial community structure changed after ATR treatment (Figure 4B). Phylum-level analysis revealed a marked shift in the distribution of the gut microbiota from control to ATR with altered abundance of the two dominant phyla Firmicutes and Bacteroidetes (Figure 4C). The ratio of Firmicutes and Bacteroidetes is critical for the stability of gut microbiota, and the ratio in ATR group reduced comparing with EtOH group. Heatmap showed the top 50 differentiated taxa with the highest genus level (Figure 4D). The results showed obvious clustering of the microbial community composition for EtOH group and control group, and the ATR group was closer to the control group. As shown in Figure 4E, the significantly different microbiota information was found at the level of phylum, class, order, family, and genus. Among them, the relative abundance of seven types of bacteria in the ATR group was remarkably higher than that in the other groups, such as Bacteroidaceae family and Bacteroides genus. Genus-level profiling demonstrated that 25 types of bacteria had remarkably changed in ATR group, such as Bacteroides, Ruminococcus, Prevotella, and Mucispirillum. And four types of bacteria had undergone significantly dynamic changes in three groups (Figure 4F−I, p < 0.05). Among them, Bacteroides and Parabacteroides belong to Bacteroidetes and others were Bifidobacterium (Actinobacteria phylum) and Mucispirillum (Deferribacteres phylum). The results showed that ATR notably reversed the microbial dysbiosis in GU rats to control levels. Hence, these findings indicated the changes of ATR-regulated gut microbiota may be involved in the improvement in gastric function.
ATR Modulated the Fecal Metabolites
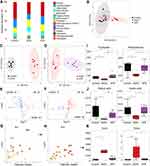
To obtain comprehensive metabolic profiles of ATR, a total of 158 metabolites (including amino acids, bile acids, SCFAs, fatty acids, indoles, organic acids and others) were identified and quantified (Figure 5A). PCA analysis showed the metabolic profiles of the fecal samples in three groups (control group; EtOH group; ATR group) were remarkably separated. The fecal samples of EtOH group were clearly separated from the control group, while the cluster of samples in ATR towards to the control group, suggesting that ethanol caused significant metabolic disturbance and administration of ATR ameliorated this disturbance obviously (Figure 5B). The OPLS analysis between the control and EtOH groups, ATR and EtOH groups, was conducted to investigate the changed metabolites induced by oral administration of ethanol and ATR. Obvious separations between control and EtOH group, as well as ATR and EtOH group, were obtained in the OPLS score plots (control vs EtOH: R2X = 0.431, R2Y = 0.987, Q2 = 0.616; EtOH vs ATR: R2X = 0.465, R2Y = 0.943, Q2 = 0.612, Figure 5C and D). Heatmap of potential biomarkers showed a distinct clustering of metabolites for the EtOH and control groups, and the ATR group was closer to the control group (Figure S1). Volcano plot (Figure 5E and F) showed the differential metabolites screened out by univariate statistical analysis meeting the set criteria (p > 0.05). Compared with the control group, there were 22 up-regulated metabolites and 53 down-regulated metabolites in the EtOH group (Figure 5E, Table S2). And compared with the EtOH group, there were 63 up-regulated metabolites and 15 down-regulated metabolites in ATR group (Figure 5F, Table S3). The significantly differential metabolites were selected as biomarkers (VIP > 1 and p < 0.05) and investigated the most relevant pathways affected by EtOH-induced GU and ATR treatment. As shown in Figure 5G, four main metabolic pathways were affected by EtOH-induced GU, including phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, aminoacyl-tRNA biosynthesis, pantothenate and CoA biosynthesis. However, ATR treatment could affect important metabolic pathways including phenylalanine, tyrosine and tryptophan biosynthesis, glycine, serine and threonine metabolism, phenylalanine metabolism, cyanoamino acid metabolism, aminoacyl-tRNA biosynthesis in the current study (Figure 5H). Ultimately, the levels of three types of metabolites (Figure 5I–K) were found to be shown significant differences in the groups of control, EtOH, and ATR, namely, amino acids (including phenylalanine and tryptophan), SCFAs (including butyric acid and acetic acid), and bile acids (including deoxycholic acid [DCA] and taurodeoxycholic acid [TDCA]).
Correlation Analysis of Gut Microbiota and Fecal Metabolites
As shown in Figure 6, Spearman correlation analysis showed that the functional relationship of the altered gut microbiota and the disrupted metabolites after ATR treatment. On the genus level, the changes in the abundance of Bacteroides were negatively correlated with changes of TDCA (r = 0.783, p = 0.004), whereas the changes in the abundance of Bacteroides were positively correlated with tryptophan (r = 0.727, p = 0.01). The changes in the abundance of Bifidobacterium were negatively correlated with changes of hydroxypropionic acid (r = 0.711, p = 0.009) and glutaric acid (r = 0.824, p = 0.001), whereas the changes in the abundance of Mucispirillum were positively correlated with indole-3-propionic acid (r = 0.702, p = 0.011), isobutyric acid (r = 0.739, p = 0.006). Additionally, the changes in the abundances of Akkermansia were negatively correlated with changes of acetic acid (r = 0.755) and butyric acid (r = 0.713) (all p < 0.05). The changes in the abundances of Ruminococcus were positively correlated with changes of acetic acid (r = 0.818), tryptophan (r = 0.755) (all p < 0.05).
 |
Figure 6 Correlation analysis of microbiota and metabolites. *p < 0.05, **p < 0.01, ***p < 0.001. |
Discussion
GU is a common peptic ulcer disease caused by the destruction of gastric mucosa, which is characterized by easy infection, difficult cure, and easy recurrence.30 The gastric mucosa is a barrier against H. pylori infection, alcohol, stomach acid, and various harmful substances. H. pylori infection can secrete substances such as urease and adhesin, which further mediate the inflammatory response of neutrophils, plasma cells, and macrophages to produce a series of inflammatory cytokines, and eventually lead to gastric epithelial cell lesions.31 ATR derived from the volatile oil of Atractylodes macrocephala Koidz. has been proved to have good anti-inflammatory and antioxidant activities,9,10 but there is a lack of relevant reports on the role of ATR in gastrointestinal tract in vivo. Our work showed that ATR can obviously restore the intestinal dilatation, repair gastrointestinal mucosal damage, and decrease the number of neutrophils in zebrafish (Figure 1), demonstrating that ATR can inhibit H. pylori-induced gastric mucosal injury in zebrafish. Alcohol intake is one of the important causes of GU, gastric mucus and bicarbonate are digested after ethanol enters the digestive tract, and erode the gastric mucosal barrier and intercellular connections, resulting in damage to gastric mucosal epithelial cells.29,32 Therefore, we further evaluated the gastroprotective activity of ATR against EtOH-induced acute gastric lesions in rat model. The results are shown in Figure 2, the gastric mucosa in the control group was intact without injury and bleeding, while in the model group, the gastric body showed obvious linear and massive bleeding areas throughout the whole mucosa. Oral administration of ATR remarkably suppressed ulcers and protected gastric mucosal structure, indicating that ATR is effective in treating acute GU.
Additionally, the gastric mucosa is stimulated and induces the overexpression of reactive oxygen species (ROS) after excessive drinking, which resulting in oxidative damage and peroxidation of biological macromolecules, and leads to the disorder of antioxidant defense system in gastric tissue.33,34 As a substance related to the level of oxidative stress in the body, SOD is an important line of defense against oxidative damage, MDA is the product of lipid peroxidation in the body and can reflect the degree of peroxidation.35 ATR treatment had a potent oxidative stress-inhibition impact in the GU rat, characterized by significant increases in SOD level and decreases in MDA level (Figure 3A and B). Moreover, inflammatory cytokines such as TNF-α and IL-10 associated with the ROS level were regulated by ATR. TNF-α, a proinflammatory cytokine produced from CD4+ cells and M1 macrophages, is known to induce oxidative stress.36 IL-10 acts as an anti-inflammatory cytokine to inhibit oxidative stress and proinflammatory mediator NO production.37 In the presence of ATR, the TNF-α and NO level decreased significantly, and the IL-10 level was highly elevated (Figure 3C–E). Consequently, these findings indicated that ATR can alleviate EtOH-induced GU by increasing anti-inflammatory effects and inhibiting oxidative stress.
We further examined the mechanisms underlying the therapeutic effects of ATR in vivo. Increasing evidence has demonstrated that excessive intake of alcohol can alter the abundance of gut microbiota and affect the metabolic pathways of the microbial community, thereby inducing alcohol-related gastric damage.1,38 Therapy with ATR shifted the microbial community profile from a dysbiotic state toward a normal state with increased abundance of Mucispirillum, Parabacteroides, and Bacteroides, and decreased abundance of Bifidobacterium. Current studies have shown that the abundance of Parabacteroides and Bacteroides in the gut of gastrointestinal ulcer model is significantly increased.39,40 Bacteroides can mediate inflammatory response in the host by stimulating the excitatory process of neutrophils and promoting the secretion of cytokines.41 Moreover, Parabacteroides and Bacteroides are anti-inflammatory bacterium that produces SCFAs.17,40 Therefore, the colonization of Parabacteroides and Bacteroides in the gut has a certain correlation with the occurrence and aggravation of ulcer, which may be potential pathogenic factors in GU. While we speculated that the therapeutic efficacy of ATR was mediated in part by changes in the gut microbiome, further investigation is warranted to understand the role of altered microbiome on GU. In addition, our current studies are limited to the rat model of ethanol-induced acute GU, and validation in other advanced preclinical models of GU is needed.
Gut microbiota can also play a regulatory role in the body through producing a variety of metabolites, so we conducted further metabolomics analysis. We found that ATR can up-regulate the level of phenylalanine and tryptophan. Phenylalanine is an essential aromatic amino acid for human body. It is catalyzed by phenylalanine-4-hydroxylase to generate tyrosine, which is involved in neurotransmitter synthesis, sugar metabolism, and fat metabolism.42,43 Tryptophan metabolites can exert regulatory effect on mucosal barrier immunity. After they bind to aryl hydrocarbon receptor, they can activate the expression of inflammatory factors such as IL-22, IL-17, and IL-10, and can also regulate the expression of tight junction proteins.44,45 Moreover, ATR can up-regulate the level of SCFAs including butyric acid and acetic acid, which is in accordance with the increased abundance of Parabacteroides and Bacteroides after ATR treatment. Commensal bacteria-derived SCFAs are important energy source for intestinal epithelial cells to produce mucins and antimicrobial peptides, they can down-regulate the high levels of proinflammatory factors secreted by macrophages by inhibiting NF-κB, and can also induce the differentiation of regulatory T cells.46–48 The abnormal increase of bile acids will lead to the generation of oxygen radicals and endoplasmic reticulum stress, and finally induce cell apoptosis.49 The results showed that ATR can restore the increased TDCA and DCA to normal level. Overall, our data suggested that gut microbiome remodeling, amino acid metabolism regulation, improved SCFAs, and decreased bile acids collectively contributed to the therapeutic role of ATR in GU rats. Note that, the present results cannot fully reveal the mechanism of ATR against GU. The present work is only to prove that ATR has good biological activity with strong effects against GU in H. pylori-infected zebrafish and ethanol-induced rats. Especially in ethanol-induced GU, the role of ATR is closely related to changes in the structure of gut microbiota and metabolites. From the perspective of multi-target, the present work is only the beginning, and there is still a lot of work to be done to reveal the scientific mechanism of ATR. Also, some efforts are still needed to improve ATR stability in vitro and in vivo.
Conclusion
In summary, our work showed the protective effect of ATR on H. pylori-induced gastrointestinal mucosal damage in zebrafish, and represented a demonstration of ATR capable of alleviating ethanol-induced GU that can be attributed with the regulation of inflammatory factors (eg, TNF-α, NO, and IL-10) and oxidative stress (eg, SOD and MDA), and the protection of the gastric mucosa. In addition, ATR regulated gut microbiota dysbiosis associated with inflammation such as Mucispirillum, Parabacteroides, Bacteroides, and Bifidobacterium. These features may be associated with a series of metabolite changes influenced by gut microbiota, including phenylalanine, tryptophan metabolism, SCFA metabolism, and bile acid metabolism, although further investigation is needed. The correlation between microbiota and metabolites may provide a novel perspective to explore GU-related pathogenic changes. Overall, these results suggested that ATR can inhibit GU induced by H. pylori and ethanol, and protect gastric mucosa against inflammation and oxidative stress while restoring disordered gut microbiome and metabolites.
Abbreviations
GU, gastric ulcer; ATR, atractylone; H. pylori, Helicobacter pylori; SOD, superoxide dismutase; MDA, malondialdehyde; SCFAs, short chain fatty acids; UPLC-MS/MS, ultra-performance liquid chromatography coupled to tandem mass spectrometry; TNBS, trinitrobenzene sulfonic acid; TNF-α, tumor necrosis factor-α; NO, nitric oxide; IL-10, interleukin-10; SD, Sprague Dawley; SPF, specific pathogen-free; H&E, hematoxylin–eosin; HPF, high power field; LPF, low power field; PCA, principal component analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; MTC, maximum tolerated concentration; ROS, reactive oxygen species; DCA, deoxycholic acid; TDCA, taurodeoxycholic acid.
Acknowledgments
We gratefully acknowledge the financial support by programs of the National Scientific and Technological Major Special Project of China [grant number 2019ZX09201004-002, 2018ZX09721005-005]; The program of Shanghai Leading Talents [grant number 2019100]; The Key project of Shanghai 3-year plan [grant number ZY (2018-2020)-CCCX-2001-04]; The budgeted Scientific Research Project of Shanghai University of Traditional Chinese Medicine [grant number 2020LK030].
Disclosure
Author Yang Wang is employed by Metabo-Profile Biotechnology (Shanghai) Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wang GY, Chen SY, Chen YY, et al. Protective effect of rosmarinic acid-rich trichodesma khasianum Clarke leaves against ethanol-induced gastric mucosal injury in vitro and in vivo. Phytomedicine. 2021;80:153382. doi:10.1016/j.phymed.2020.153382
2. Strong VE. Progress in gastric cancer. Updates Surg. 2018;70:157–159. doi:10.1007/s13304-018-0543-3
3. Lanas A, Chan FKL. Chan FKL Peptic ulcer disease. Lancet. 2017;390:613–624. doi:10.1016/s0140-6736(16)32404-7
4. White JR, Winter JA, Robinson K. Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. J Inflamm Res. 2015;8:137–147. doi:10.2147/jir.S64888
5. Zhang Y, Sun L, Lai X, et al. Gastroprotective effects of extract of Jasminum grandiflorum L. flower in HCl/EtOH-induced gastric mucosal ulceration mice. Biomed Pharmacother. 2021;144:112268. doi:10.1016/j.biopha.2021.112268
6. Shirpoor A, Barmaki H, Khadem Ansari M, et al. Protective effect of vitamin E against ethanol-induced small intestine damage in rats. Biomed Pharmacother. 2016;78:150–155. doi:10.1016/j.biopha.2016.01.015
7. Li W, Wang X, Zhang H, et al. Anti-ulcerogenic effect of cavidine against ethanol-induced acute gastric ulcer in mice and possible underlying mechanism. Int Immunopharmacol. 2016;38:450–459. doi:10.1016/j.intimp.2016.06.016
8. Scally B, Emberson JR, Spata E, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. 2018;3:231–241. doi:10.1016/s2468-1253(18)30037-2
9. Kim HY, Nam SY, Hwang SY, et al. Atractylone, an active constituent of KMP6, attenuates allergic inflammation on allergic rhinitis in vitro and in vivo models. Mol Immunol. 2016;78:121–132. doi:10.1016/j.molimm.2016.09.007
10. Wu YX, Lu WW, Geng YC, et al. Antioxidant, antimicrobial and anti-inflammatory activities of essential oil derived from the wild rhizome of atractylodes macrocephala. Chem Biodivers. 2020;17:e2000268. doi:10.1002/cbdv.202000268
11. Lu F, Ma Y, Huang H, et al. Edible and highly biocompatible nanodots from natural plants for the treatment of stress gastric ulcers. Nanoscale. 2021;13:6809–6818. doi:10.1039/d1nr01099a
12. Wang KT, Chen LG, Wu CH, et al. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J Pharm Pharmacol. 2010;62:381–388. doi:10.1211/jpp.62.03.0014
13. Cheng H, Liu J, Tan Y, et al. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J Pharm Anal. 2021. doi:10.1016/j.jpha.2021.10.003
14. Feng W, Ao H, Peng C, et al. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi:10.1016/j.phrs.2019.02.024
15. Feng WW, Liu J, Cheng H, et al. Integration of gut microbiota and metabolomics for Chinese medicines research: opportunities and challenges. Chin J Integr Med. 2021. doi:10.1007/s11655-021-3305-x
16. Liu J, Tan Y, Cheng H, et al. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022;13:1106. doi:10.14336/AD.2022.0104
17. Bi T, Feng R, Zhan L, et al. ZiBuPiYin recipe prevented and treated cognitive decline in ZDF rats with diabetes-associated cognitive decline via microbiota-gut-brain axis dialogue. Front Cell Dev Biol. 2021;9:651517. doi:10.3389/fcell.2021.651517
18. Khadka S, Omura S, Sato F, et al. Curcumin beta-D-glucuronide modulates an autoimmune model of multiple sclerosis with altered gut microbiota in the ileum and feces. Front Cell Infect Microbiol. 2021;11:772962. doi:10.3389/fcimb.2021.772962
19. Wang Y, Zhou W, Lyu C, et al. Metabolomics study on the intervention effect of Radix Salviae Miltiorrhizae extract in exercise-induced exhaustion rat using gas chromatography coupled to mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1178:122805. doi:10.1016/j.jchromb.2021.122805
20. Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Dig Dis Sci. 2013;58:2756–2766. doi:10.1007/s10620-013-2744-4
21. Agus A, Planchais J, Sokol H. microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi:10.1016/j.chom.2018.05.003
22. Jiang T, Xu C, Liu H, et al. Linderae radix ethanol extract alleviates diet-induced hyperlipidemia by regulating bile acid metabolism through gut microbiota. Front Pharmacol. 2021;12:627920. doi:10.3389/fphar.2021.627920
23. Flores EM, Nguyen AT, Odem MA, et al. The zebrafish as a model for gastrointestinal tract-microbe interactions. Cell Microbiol. 2020;22:e13152. doi:10.1111/cmi.13152
24. Cheng D, Shami GJ, Morsch M, et al. Ultrastructural mapping of the zebrafish gastrointestinal system as a basis for experimental drug studies. Biomed Res Int. 2016;2016:8758460. doi:10.1155/2016/8758460
25. Westerfield MM. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio (Brachydanio) Rerio. University of Oregon Press. 1994.
26. Morales Fénero C, Amaral MA, Xavier IK, et al. Short chain fatty acids (SCFAs) improves TNBS-induced colitis in zebrafish. Curr Res Immunol. 2021;2:142–154. doi:10.1016/j.crimmu.2021.08.003
27. Oehlers SH, Flores MV, Okuda KS, et al. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev Dyn. 2011;240:288–298. doi:10.1002/dvdy.22519
28. Ren WK, Xu YF, Wei WH, et al. Effect of patchouli alcohol on Helicobacter pylori-induced neutrophil recruitment and activation. Int Immunopharmacol. 2019;68:7–16. doi:10.1016/j.intimp.2018.12.044
29. Liu J, Wang F, Luo H, et al. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016;30:179–187. doi:10.1016/j.intimp.2015.11.018
30. Xue Z, Shi G, Fang Y, et al. Protective effect of polysaccharides from Radix Hedysari on gastric ulcers induced by acetic acid in rats. Food Funct. 2019;10:3965–3976. doi:10.1039/c9fo00433e
31. Datta De D, Roychoudhury S. To be or not to be: the host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J Gastroenterol. 2015;21:2883–2895. doi:10.3748/wjg.v21.i10.2883
32. Shin MS, Lee J, Lee JW, et al. Anti-inflammatory effect of Artemisia argyi on ethanol-induced gastric ulcer: analytical, in vitro and in vivo studies for the identification of action mechanism and active compounds. Plants. 2021;10:332. doi:10.3390/plants10020332
33. Gomaa AMS, Abd El-Mottaleb NA, Aamer HA. Antioxidant and anti-inflammatory activities of alpha lipoic acid protect against indomethacin-induced gastric ulcer in rats. Biomed Pharmacother. 2018;101:188–194. doi:10.1016/j.biopha.2018.02.070
34. Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–1401. doi:10.1007/bf01296666
35. da Silva LM, Pezzini BC, Somensi LB, et al. Hesperidin, a citrus flavanone glycoside, accelerates the gastric healing process of acetic acid-induced ulcer in rats. Chem Biol Interact. 2019;308:45–50. doi:10.1016/j.cbi.2019.05.011
36. Yan B, Wang H, Rabbani ZN, et al. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi:10.1158/0008-5472.Can-06-2540
37. Li B, Alli R, Vogel P, et al. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869–878. doi:10.1038/mi.2013.103
38. Engen PA, Green SJ, Voigt RM, et al. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37:223–236.
39. Zhao H, Cheng N, Zhou W, et al. Honey polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats. Mol Nutr Food Res. 2019;63:e1900638. doi:10.1002/mnfr.201900638
40. Gou S, Huang Y, Wan Y, et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54. doi:10.1016/j.biomaterials.2019.05.012
41. Wang H, Li Y, Feng X, et al. Dysfunctional gut microbiota and relative co-abundance network in infantile eczema. Gut Pathog. 2016;8:36. doi:10.1186/s13099-016-0118-0
42. Roberts KM, Pavon JA, Fitzpatrick PF. Kinetic mechanism of phenylalanine hydroxylase: intrinsic binding and rate constants from single-turnover experiments. Biochemistry. 2013;52:1062–1073. doi:10.1021/bi301675e
43. Sikalidis AK. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015;21:9–17. doi:10.1007/s12253-014-9860-0
44. Bowerman KL, Rehman SF, Vaughan A, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. 2020;11:5886. doi:10.1038/s41467-020-19701-0
45. Singh R, Chandrashekharappa S, Bodduluri SR, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10:89. doi:10.1038/s41467-018-07859-7
46. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi:10.1038/nature12721
47. Hamer HM, Jonkers D, Venema K, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi:10.1111/j.1365-2036.2007.03562.x
48. Park JS, Lee EJ, Lee JC, et al. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70–77. doi:10.1016/j.intimp.2006.08.015
49. Yang F, Xu Y, Xiong A, et al. Evaluation of the protective effect of Rhei Radix et Rhizoma against α-naphthylisothiocyanate induced liver injury based on metabolic profile of bile acids. J Ethnopharmacol. 2012;144:599–604. doi:10.1016/j.jep.2012.09.049
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.