Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease
Received 20 December 2022
Accepted for publication 27 March 2023
Published 6 April 2023 Volume 2023:18 Pages 493—506
DOI https://doi.org/10.2147/COPD.S402122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Qi Wang, Sha Liu
Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, People’s Republic of China
Correspondence: Sha Liu, Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital, Hengyang Medical School, University of South China, 35 Jiefang Avenue, Zhengxiang District, Hengyang, Hunan, 421001, People’s Republic of China, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD), a heterogeneous disease, is the leading cause of death worldwide. In recent years, air pollution, especially particulate matter (PM), has been widely studied as a contributing factor to COPD. As an essential component of PM, PM2.5 is associated with COPD prevalence, morbidity, and acute exacerbations. However, the specific pathogenic mechanisms were still unclear and deserve further research. The diversity and complexity of PM2.5 components make it challenging to get its accurate effects and mechanisms for COPD. It has been determined that the most toxic PM2.5 components are metals, polycyclic aromatic hydrocarbons (PAHs), carbonaceous particles (CPs), and other organic compounds. PM2.5-induced cytokine release and oxidative stress are the main mechanisms reported leading to COPD. Nonnegligibly, the microorganism in PM 2.5 may directly cause mononuclear inflammation or break the microorganism balance contributing to the development and exacerbation of COPD. This review focuses on the pathophysiology and consequences of PM2.5 and its components on COPD.
Keywords: particulate matter 2.5, chronic obstructive lung disease, oxidative stress, inflammation, DNA methylation, microorganisms
Graphical Abstract:
Introduction
The detrimental consequences of air pollution on human health have long been a worry for industrial development’s impact on worldwide public health. According to The World Health Organization, approximately 7 million people die yearly from air pollution.1 Around 9 million premature deaths in 2015 were attributed to diseases associated with pollution.2 Significant pollutants include particulate matter (PM), carbon compounds, ozone, nitrogen compounds, and sulfur compounds.3 The main environmental factor influencing the worldwide disease burden is exposure to ambient PM2.5 (particulate matter with an aerodynamic diameter of fewer than 2.5μm).4,5 The impacts of ambient PM on mortality and Disability-adjusted life years (DALYs) in China were among the leading four risk factors.6
In vitro studies have indicated that exposure to confident PM may increase the susceptibility of pulmonary cells,7 which could cause lung, trachea, or bronchus damage.8 The exposure route and alveolar deposition capacity of PM vary according to its size, with the coarse fraction (PM10μm −2.5μm) having the ability to enter the upper bronchi. In contrast, the fine fraction (PM≤2.5μm) can directly access the respiratory system’s gas exchange area and deposit in the airway and lung tissue, leading to an aberrant immunological inflammatory response and impaired epithelial cell function.9,10 In addition, exposure to PM2.5 early in lung growth and development will result in permanent irreversible damage.11 Through the blood-air barrier and circulatory system, ultrafine particles (PM≤1μm) can accumulate in various body organs, causing a range of health problems.12,13 Therefore, it is essential to recognize the effects of air pollution on the respiratory system.
In 2017, chronic respiratory conditions ranked third among causes of death. COPD accounted for most chronic respiratory disease-attributable deaths and DALYs.14 The burden of COPD in China estimates for 2019 disclosed that COPD remains a significant public health problem in China.15 COPD is a diverse illness that causes persistent inflammation, alveolar damage, and irreversible airway obstruction. It has long been believed that cigarette smoke (CS) is the main factor contributing to the onset of COPD.16 However, there is growing evidence that non-smokers are developing COPD at a higher rate. According to estimates, 25–45% of patients with COPD are nonsmokers.17,18 Studies have pointed out that PM2.5 can trigger COPD and reduce lung function.19 Researchers are interested in discovering if these COPD patients who do not smoke are affected by PM2.5 pollution. Determining the precise mechanism via which COPD manifestation caused by PM2.5 occurs is therefore necessary.
As this review aims to provide the foundation for preventing and managing COPD, it will explore the relationship between PM2.5 constituents and COPD as well as PM2.5 on the development, exacerbations, and pathogenesis of COPD.
Effects of PM2.5 on COPD: Epidemiological Evidence
PM2.5 is a high risk of COPD.20 Recent epidemiological studies have revealed that PM2.5 increased the incidence and prevalence of COPD during long-term exposure.21,22 Ambient concentrations of PM2.5 was strongly correlated with lower pulmonary function and increasing emphysema, even at relatively low concentrations.23,24 Research found that a rise in PM2.5 (2.4μg/m3) was associated with a drop in FEV1 of 101.7 mL,25 which aided in the progression of COPD.
Epidemiological research revealed a link between atmospheric pollution and COPD exacerbation.26 Air pollution was significantly correlated to the hospitalization rate of COPD.27 What is noteworthy is that among COVID-19 patients with COPD, hospitalization rates were more significant when exposed to PM2.5.28 Numerous studies have discovered a connection between PM2.5 concentrations and AECOPD episodes.29,30 According to a meta-analysis, with every 10μg/m3 elevation in PM2.5, the probability of COPD-related emergency room visits and hospital admissions rose by 1.4–2.5%.31 More PM exposure causes individuals with chronic obstructive pulmonary disease to have worse lung function,32,33 lower FEV1 and oxygen saturation, more severe emphysema,34 higher Blood Pressure, and cardiovascular mortality.35,36 Furthermore, Patients with COPD are more vulnerable to pulmonary inflammation after exposure to PM2.5, especially those with poorer lung function.19,37,38 These might be to blame for the disease’s deterioration.
The adverse effects of particulate matter can affect the elderly more severely.39 Despite recent improvements in general air quality, the disease burden from air pollution is still relatively high as the ageing process speeds up. The second most frequent reason contributing to the DALYs rates for COPD still was particulate matter.40 Air pollution is thought to be a contributing factor in roughly 1.6 million deaths from COPD.41 Especially in a region with a low Socio-demographic index, particulate matter was the main cause of COPD fatalities.42 Especially particles <0.5μm in diameter might be responsible for the adverse effects of particulate air pollution on COPD mortality.43
Moreover, A study indicates that the risk of acquiring COPD was reduced by 12% for each 5 µg/m3 decrease in PM2.5.44 A different study also supports this claim. It shows that in comparison to the estimated figures based on the natural trends in exposure to PM2.5, 0.27 million deaths could be avoided if the global particulate matter concentration achieves its control targets by 2030. Early mortality would be reduced to 0.52%,45 suggesting that interventions to lessen patients’ exposure to PM2.5 may benefit for those with COPD.
The aforementioned epidemiological evidence taken together strongly suggests that exposure to PM2.5 is significantly linked with the occurrence, death, and progression of COPD. Air pollution reduction may be an effective strategy for lowering the chances of developing COPD.
PM2.5 Components and COPD
Physicochemical characteristics (size, water solubility, and chemical composition) of PM affect its mechanism. Research shows that airborne oil mist particulate matter (OMPM) of 1.0–10μm induced oxidative stress and increased the production of pro-inflammatory cytokines. However, only OMPM<1.0 induced disruption of the pulmonary epithelial barrier.46 Thus, the magnitude of PM should be considered when analyzing its adverse effects. We will not go into great detail about this in this article. We concentrate on the mechanisms of the various PM2.5 components.
PM2.5 can be classified as aqueous and organic extracts, depending on how soluble it is. Interestingly, the variations in respiratory and immunological effects can also be attributed to the differences in PM2.5’s constituents. A study from Japan found that organic extracts of PM2.5 generated IL-6 rather than aqueous extracts, promoting inflammation. Additionally, compared to extracts from the urban region, those from the industrial sector appeared to have more potent effects.47 A Brazilian study found that PM2.5 aqueous extracts reduced the release of IL-6 and IL-8.48 This phenomenon might be because the study’s water-soluble extracts of PM2.5 samples had metal components and polar organic constituents. It is still unknown how specific PM2.5 components contribute to the decline in cytokines. According to a similar study, metal-rich water-soluble extracts impact metabolism and the respiratory system.49 In general, water-soluble extracts significantly contribute to DNA oxidative damage and plasma-membrane peroxidation, while organic extracts are primarily linked to genotoxicity and mutagenicity.50,51 Furthermore, the cell toxicity of PM2.5 water-soluble and organic extracts was not consistently demonstrated.52,53 Therefore, more investigation is required to completely comprehend the mechanisms and immediate effects of the diverse PM2.5 components.
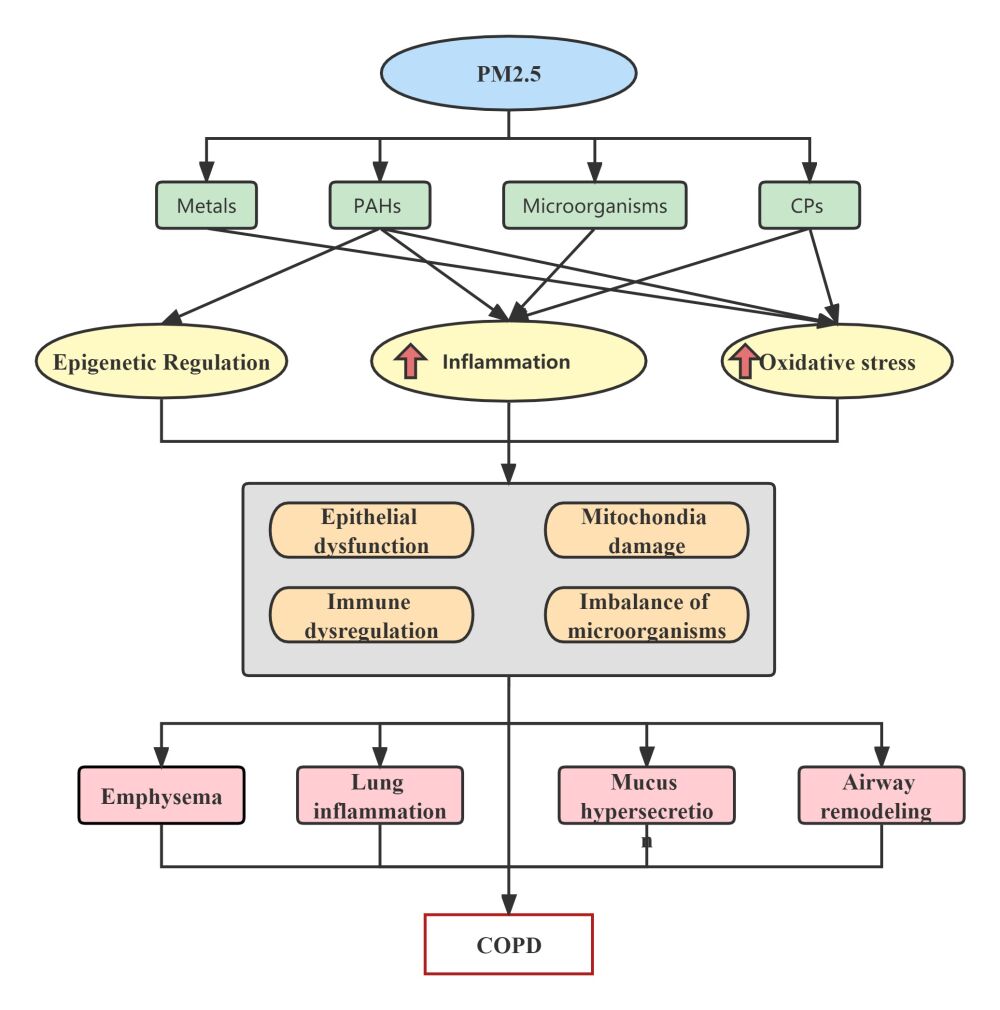
Significant components of PM2.5, such as organic compounds (organic carbon, polycyclic aromatic hydrocarbons, and benzene), inorganic compounds (sulfate, nitrate, ammonia, quartz, silica, mineral oxides), and biological components (bacteria, fungi, and virus), are typically similar. However, The ratios of the various components vary greatly depending on the local sources and emissions.54 PM’s composition, concentration, and specific surface area influence its pathophysiological effects.55–57 It has been determined that the most toxic PM components are metals, polycyclic aromatic hydrocarbons (PAHs), carbonaceous particles(CPs), and other organic compounds, they have different toxicity to human health.58
Metals
Interestingly, the different metal composition of PM2.5 affects different organs. For instance, As or Cr harms the lungs typically. However, Pb2+ predominantly affects the kidneys.59 Research reported that several metal components in PM2.5 were directly related to lower LVEF, FEV1, FVC, and PEF.60 Some redox-active metals (As, Zn, and Fe) in them play a role that can promote the production of reactive oxygen species (ROS) and thereby decrease antioxidant enzyme activity, causing cells to experience oxidative stress.61,62 According to the biological solubility of metals, they can be divided into water-insoluble and water-soluble metals. Zhao et al found that water-insoluble metals of PM2.5 inhibits Nrf2, thus reducing the body’s antioxidant capacity, resulting in excessive lung oxidation.63 Another investigation on soluble metals present in PM2.5 revealed that they could activate both the pro-oxidative and anti-oxidative systems in these pulmonary cells.64 In conclusion, the metals in PM2.5 can cause an imbalance between oxidative and antioxidant effects in the body, leading to lung damage.
Polycyclic Aromatic Hydrocarbons (PAHs)
Particles’ carbon surfaces can absorb harmful organic compounds. Polycyclic Aromatic Hydrocarbons (PAHs) are the leading organic compounds of PM2.5. For elderly patients with COPD, exposure to PAHs-rich PM2.5 could impair their small airway functions.65 FEV1/FVC is also decreased by exposure to PAH-enriched PM2.5.66 Nevertheless, the exact mechanism is still unknown. The well-known PAH can result in lung cancer by elevating the expression of the CYP1A1 gene and promoting DNA damage.67 Recent research has revealed that OGG1 expression and methylation are responsible for PAH’s ability to produce oxidative DNA damage.68 Furthermore, it was also discovered that PAHs could induce the expression of oxidative stress genes (HMOX-1) and inflammatory cytokine genes (IL-6 and IL-8).69 Many studies have shown that PAHs can induce ROS formation and inflammatory response.70,71 Additionally, PAHs may cause the mitochondrial ROS to produce to activate the NLRP3 inflammasome, further inducing lung damage.72 The inflammatory signaling pathways nuclear factor kappa B (NF-κB), Akt phosphorylation, and MAPK pathway can all be activated by acute 1-NP, a type of nitrated polycyclic aromatic hydrocarbons (NPAHs).73 Through the AhR/ROS axis, PAHs cause pro-inflammatory mediators to enter the bloodstream, which ultimately causes damage to the lung epithelium or tissue.70
Microorganisms
It is reported that household dust’s microbial makeup may impact adults’ allergy reactions.74 Bacteria, eukaryotes, and viruses are among the microorganisms found in PM2.5.75 A component of gram-negative bacteria’s cell walls called LPS is an endotoxin linked to a chronic inflammatory pulmonary illness.76 It has been proved that indoor and outdoor PM2.5 contain endotoxins.77 Recent research has discovered that most outdoor airborne LPS stems from Artemisia pollen.78 According to earlier research, the concentration of LPS determines how it affects the lungs. High LPS exposure triggers a Th17 cell response that increases the synthesis of IL-17 and leads to neutrophilic inflammation, which aids in the emergence of severe pulmonary illnesses.79 On the other hand, exposure to low concentrations of LPS in the airways is associated with a Th2 immune response, promoting asthma onset.80 Extracellular vesicles (EVs) produced by bacteria are another critical element of biological UPF. Th1 cell induction and Th17 cell induction are two ways whereby bacteria-derived EVs cause inflammation in the body’s immune system.81 Because LPS is a vital ligand for TLR4, Chen et al revealed that the dust fall-activated TLR4/NF-κB signaling pathway might be mediated by LPS present in PM2.5.82 Pretreating PM2.5 with an antibiotic that selectively suppresses endotoxin could effectively inhibit the inflammatory reaction.83 Antibiotics may be a selection for reducing the inflammatory response brought on by PM2.5.
Carbonaceous Particles
A significant fraction of PM is CPs. Elemental carbon (EC) and organic carbon (OC) are makeup CPs.84 EC consists of black carbon (BC) and carbon black (CB).85 By causing oxidative stress and inflammation, CPs seriously impair lung function and result in COPD.86 Some works demonstrated that CB drove pulmonary fibrosis through an activated NLRP3 inflammasome pathway87 and mediated autophagy.88,89 One study found that when exposed to both CB and metals, CB can increase the permeability of cells membrane and ultimately result in increased cytotoxicity caused by Cadmium (Cd).90 Pan et al made a PM2.5 Models Base that contains all possible combinations of four types of harmful contaminants, where Cr/Pb pairings result in cell cycle arrest.91 So, evaluating the health impacts of PM2.5, we should also take into account how its various complex components work together.
PM2.5 Induced Pathogenesis in COPD
Epithelial Alterations
The first line of defense against inhaled PM2.5 is the lining of the airways. Studies have shown that exposure to PM2.5 can alter cilia movement and increase mucus formation, which reduces their capacity to perform airway clearing and delays the prompt removal of PM2.5 from the lungs and airways.92 PM2.5 can impair the expression of 8-oxoguanine DNA glycosylase 1 (OGG1) in alveolar epithelial cells,93 which prevents Type 2 alveolar epithelial cells damaged by PM2.5 from proliferating and renewing themselves.94 After being exposed to PM2.5, it is observed that there are fewer multilamellar bodies in the Alveolar epithelial type II; cell (AT2) increases mitochondrial swelling, collagen deposition, and pulmonary inflammation.95 In addition, it can be observed that the dysregulated AT2 cells into AT1 cells in the PM2.5-induced COPD mouse model96 damage cellular repair mechanisms. Even worse, it has been shown that inflamed bronchial epithelial cells caused by air pollution particles trigger apoptosis,97 particularly when PM2.5 is injected into those inflamed by cigarettes.98 Another study has found that PM2.5-induced apoptosis of alveolar epithelial cells through the PI3K/AKT/mTOR pathway regulates autophagy.99 By turning on the AMPK-Beclin1 pathway, PM2.5 can also cause ferroptosis.100 PM2.5-induced cell death encourages the evacuation of cell contents, aberrant pro-inflammatory mediator hyperplasia, and inadequate macrophage clearance of dead cells, all of which contribute to small-airway illness and emphysema in COPD.101 Therefore, it is crucial to understand the effects of cells induced by PM2.5 exposure to find the appropriate reversal methods.
Imbalance of Microorganisms
Humans’ airway micro-environment includes the microbiome as a constitutive component. The severity of COPD has been reported to correlate with microorganisms’ components and functions.102 Exposure to PM2.5 significantly impacts the airway microbiota.103 A study on animals found that exposed mice to diesel exhaust particles had an expansion in Proteobacteria in their BALF.104 The altered microbiota composition can promote and affect pulmonary inflammation and oxidative stress during PM2.5 exposure.105 In a recent study, Jia et al found that PM2.5-induced differential expression of miRNAs was enriched in microbial signaling pathways, including HIF-1 signaling, IL-17 signaling, and Th17 cell differentiation pathways. MiR-149-5p might connect to how PM2.5 leads to dysbiosis in the lung’s microbiome.106 This might be an effective way to prevent the PM2.5-induced imbalance of the lungs’ microbiota. Moreover, a rat model of chronic obstructive pulmonary disease is induced by chronic exposure to ambient particulate matter, which leads to gut microbial dysbiosis.107 The intestinal microbiome encourages PM-induced neutrophilia in the lung, which may be related to Tγδ17 cells.108 However, there are still a lot of unanswered concerns about how the microbiome and PM2.5-related COPD interact.
Potential Mechanisms
The development of COPD is assumed to involve a number of mechanisms, including proteolytic–anti-proteolytic imbalance, oxidative stress, inflammatory response, epigenetic alterations, and more. The lung tissue proteomics analysis showed that the proteins involved in oxidative stress, cellular metabolism, inflammatory responses, and actin dynamics are dysregulated under exposure to traffic-related air pollution.109 Chronic exposure to PM2.5 causes significant impairment in lung function, emphysematous lesions, inflammation in the lungs, and remodeling of the airway walls.110 PM2.5 could harm COPD development and progression. In order to better understand how PM2.5 causes COPD, it is necessary to better understand its pathophysiology and probable mechanisms. Inflammatory reactions and oxidative stress were once thought to be the two main factors in COPD (Figure 1).111
Oxidative Stress
Cellular damage results from an imbalance between the endogenous antioxidant defense system and the generation of ROS production, known as oxidative stress.112 Studies have shown that PM2.5 contributes to an imbalance between oxidants and antioxidants. For example, there was a trend toward reduced antioxidant production (SOD1 and SOD2) in bronchial epithelial cells after exposure to diesel emissions.113 In COPD rats, PM2.5 increased malondialdehyde (MDA) levels while decreasing total superoxide dismutase (T-SOD).114 Besides, in the lungs of COPD rats exposed to PM2.5, there was a decreasing trend in the expression of the Nrf2 protein and its downstream component heme oxygenase-1 (HO-1).115 The transcription factor Nrf2, which is inversely correlated with the severity of COPD, controls the majority of antioxidants.116 The primary source of endogenous ROS in mitochondria.117 Damage to redox equilibrium and structural abnormalities in the mitochondria will result from exposure to PM2.5, which leads to a persistent rise in ROS.10,118 Meanwhile, ROS function as intermediary signaling molecules and can activate the NF-κB, TLR, and MAPKs.119 These signaling pathways affect cell-signaling proteins, which contribute to the beginning of inflammatory responses.120 BEAS-2B cells, normal HBECs, and in particular sensible COPD HBECs exposed to PM2.5 caused ROS overproduction and activated oxygen-sensitive NRF2 and NF-kB signaling pathways.10 By activating the IL-6/AKT/STAT3/NF-κB signaling pathways in the epithelial cells of the lungs, PM2.5-induced ROS also can contribute to intercellular adhesion molecule-1 (ICAM-1) expression.53 ICAM-1 facilitates the adhesion and infiltration of inflammatory cells, such as monocytes and macrophages, into inflammatory sites that result in lung lesions.121,122
Inflammation
Inflammation and oxidative stress are two biological processes that are connected and appear to occur together to increase harm.123 Previous research has revealed that biomarkers of inflammation were increased by brief PM2.5 exposure.124,125 In COPD patients, short-term exposure to PM2.5 elevates Th1 and Th17 cytokines while decreasing Th2 cytokines. This improves circulation levels of IL-2, IL-12, IL-17A, IFN-γ, MCP-1 and sCD40L, which exacerbated systemic inflammation.126 Urban particulate matter may worsen inflammation by inducing epithelial remodeling and malfunctioning dendritic cells (DCs) in COPD patients.127 An in vivo experiment found that the activation of lung DCs by biomass smoke caused Th17 responses and emphysema in rats.128 Another study found that early-life exposure to PM2.5 caused young adult mice to develop COPD-like phenotypes, which caused inflammation and had long-term negative effects on lung development.11
The activation of toll-like receptors (TLRs) is one of the suggested pathways for the inflammatory response in PM2.5-induced COPD. The most important receptors in the COPD’s clinical manifestation development are TLR-2 and TLR-4.129 Myeloid differentiation marker 88 (MyD88), one of the downstream adaptor proteins that TLRs recruit, is recruited by activated TLRs, starting the TLR-2/MyD88 and TLR-4/MyD88 signaling cascades. PM2.5 Causes TRAF6 accumulation, thereby promoting the synthesis of inflammatory chemokines and the activation of the NF-κB pathway.130 Following exposure to PM2.5, TLRs and MAPKs can also trigger NF-κB signaling.111,131
Moreover, epidermal growth factor receptor (EGFR) signaling is a paramount regulator of inflammation associated with COPD. A pro-inflammatory response to PM2.5 exposure has been reported in BEAS-2B cells by triggering EGFR signaling,132 which also caused MUC5AC overexpression.133 Similar findings were made by Val et al, who discovered that PM2.5 exposure activates the EGFR pathway and results in MUC5AC overproduction in rat airways and primary epithelial cells.134 Amphiregulin (AREG) is an essential ligand for EGFR. AREG promotes the production of IL-1α, IL-1β, and Muc5AC to increase PM2.5-induced inflammation and mucus hypersecretion via activating the EGFR-PI3K-AKT/ERK pathway.135 Subsequently, chronic effects such as reduced expiratory flow and permanent remodeling may occur.136
The NLRP3 inflammasome, a crucial part of the inflammatory response, is linked to COPD exacerbations.137 ROS could activation of Transient Receptor Potential Melastatin2 (TRPM2), inducing the influx of Ca2+ intracellular, with subsequent activation of NLRP3.138 Particulate matter in the environment reduces Sirtuin1 and increases the SREBP1-PIR/NLRP3 axis to cause inflammation in human lung fibroblasts.139 As a result of PP2A dephosphorylating IRE1α,140 PM2.5 activates the NLRP3/Caspase-1 mediated macrophages pyroptosis, causing inflammation and oxidative stress to damage the lungs.141 Accordingly, NLRP3-mediated macrophage pyroptosis may present an attractive therapeutic target for PM-induced COPD. By preventing the production of NLRP3 inflammasomes and apoptosis through the Nrf2-dependent pathway, the gaseous signaling molecule hydrogen sulfide (H2S) protected mice from developing emphysema and airway inflammation brought on by PM2.5.142
In COPD patients, exposure to ambient ultrafine particles was also linked to higher levels of IL-8, MCP-1, MIP-1α, MIP-1β, TNF-α, and IL-1β.143 There is also evidence that PM2.5 can release pro-inflammatory compounds into the blood circulation, resulting in systemic inflammation, which is indicated by white blood cells (WBC), C-reactive protein (CRP) and serum cytokine levels.144 This further contributes to the progression of COPD and its incidence.145 Hence, it is crucial to prevent and treat the inflammatory reaction caused by PM2.5 effectively.
Epigenetic Regulation
DNA methylation is impacted by long-term exposure to ambient air pollution,146 which is considered a vital regulator when PM2.5 induces lung injury. When PM2.5 dust levels were high enough, the PI3K/Akt/DNMT3b pathway was stimulated, increasing the hypermethylation of the interferon-gamma IFN-γ gene promoter.147 Rats exposed to traffic-related PM2.5 experienced an aggravation of inflammation because the exposure altered the methylation state of the IFN-γ and interleukin 4 (IL-4) genes and the levels of their associated cytokines.148 A study revealed that DNA hypomethylation, a P16 gene promoter hypermethylation, and a decreasing DNA methyltransferase activity in HBE after repeated exposure to PM2.5.149 PM2.5 exposure may influence TNF-α through a reduction in methylation.150 Exposure to PM2.5, CO, and O3 also alters the methylation patterns of numerous CpG sites of the immunoregulatory genes, including Foxp3, IL-4, IL-10, and IFN-γ, are also altered by exposure to, which alters the immune response.151 The DNA methylation patterns of several genes, including p53, p15, p16, APC, RASSF1A, HIC1, iNOS, hTERT, and IL-6, are altered by PM exposures, and these alterations have an impact on the development of respiratory illnesses.152 Ji et al found that lung injury and recovery are impacted by changes in H3K27ac brought on by PM2.5 exposure.153 To better understand how histone alterations affect PM2.5-related disease, the connection between PM2.5 and histone modification requires further study.
MiRNAs are non-coding, 20–25 nucleotide length, short RNA molecules. MiRNAs have drawn interest because they are important pathogenic pathway regulators.154 After exposure to PM2.5, miR-194-3p is downregulated and positively correlates with FVC and FEV1.155 A low expression of miR-140-5P induced by PM2.5 can trigger a strong inflammatory response by over-activating TLR4.82 The ability of the lungs of rats to eliminate 8-OHdG, which is linked to DNA damage, is further impacted by high miR485/miR-145 levels and suppressed mRNA expression of MTH1 in rat lung tissues.156 Interestingly, lncRNAs are a viable diagnostic and therapeutic tool since they have amazing tissue selectivity that mRNA lacks.157 TRAPM2.5 can up-regulated lncRNA RP11-86H7.1, which acts as a competing endogenous RNA of miR-9-5p to promote airway inflammation.158 Air pollution PM regulates the immune activities of DCs via the GATA3/lncRNA MHC-R nexus, which leads to the immune dysregulation of COPD patients.159 lncRNA NONMMUT065867, lncRNA NONMMUT064312, lncRNA NONMMUT018123 up-regulated following exposure to PM2.5. These lncRNAs might be linked to lung inflammation.160 It is poorly understood how air pollution affects lncRNAs and eventually leads to illness.
Conclusion
In this article, we have reviewed how PM2.5 and its components affect COPD and its possible mechanisms. However, only few reports demonstrated the potential effects of PM2.5 compositions on COPD and the specific mechanisms. Another problem is that the toxicity of specific PM2.5 components needs to be accurately determined, as well as the exact proportion of these components in the PM mix. Therefore, it is of great importance to comprehensively characterize the composition of environmental PM2.5 and identify the significant pathogenic components, and make it possible to investigate the underlying mechanisms by which PM2.5 components contribute to COPD. This is essential to treat health problems caused by PM2.5.
Abbreviations
AT1, Alveolar epithelial type I cell; AT2, Alveolar epithelial type II cell; BC, black carbon; CB, carbon black; CPs, carbonaceous particles; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; CS, cigarette smoke; DALYs, Disability-adjusted life years; DCs, dendritic cells; EC, Elemental carbon; EGFR, epidermal growth factor receptor; EVs, Extracellular vesicles; IFN-γ, interferon-gamma; MAD, malondialdehyde; NF-κB, nuclear factor kappa B; OC, Organic carbon; OGG1, 8-oxoguanine DNA glycosylase 1; PAHs, polycyclic aromatic hydrocarbons; PM, particulate matter; ROS, reactive oxygen species; TLRS, toll-like receptors; TRPM2, Transient Receptor Potential Melastatin2 WBC, white blood cells.
Acknowledgments
We appreciate Prof. Tan Xiaowu’s work on the article revision.
Funding
The study was funded by National Natural Science Foundation of China (81900044, to Dr. S. Liu), Natural Science Foundation of Hunan Province (2021JJ40484, to Dr. S. Liu), The Key project of Science and Technology Plan of Health Commission of Hunan Province (20201922, to Prof. X. Tan).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Shaddick G, Thomas ML, Amini H, et al. Data integration for the assessment of population exposure to ambient air pollution for global burden of disease assessment. Environ Sci Technol. 2018;52(16):9069–9078.
2. Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656–664.
3. World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Geneva: World Health Organization; 2021.
4. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994.
5. Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52(3):311–317.
6. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158.
7. Pfeffer PE, Donaldson GC, Mackay AJ, Wedzicha JA. Increased chronic obstructive pulmonary disease exacerbations of likely viral etiology follow elevated ambient nitrogen oxides. Am J Respir Crit Care Med. 2019;199(5):581–591.
8. Wang G, Zhao J, Jiang R, Song W. Rat lung response to ozone and fine particulate matter (PM2.5) exposures. Environ Toxicol. 2015;30(3):343–356.
9. Mukherjee A, Agrawal M. A global perspective of fine particulate matter pollution and its health effects. Rev Environ Contam Toxicol. 2018;244:5–51.
10. Leclercq B, Kluza J, Antherieu S, et al. Air pollution-derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ Pollut. 2018;243(PtB):1434–1449.
11. de Souza Xavier Costa N, Mirtes Teles A, de Brito JM, et al. Allergic sensitization and exposure to ambient air pollution beginning early in life lead to a COPD-like phenotype in young adult mice. Ecotoxicol Environ Saf. 2022;241:113821.
12. Kwon HS, Ryu MH, Carlsten C. Ultrafine particles: unique physicochemical properties relevant to health and disease. Exp Mol Med. 2020;52(3):318–328.
13. Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, Segura-Medina P. Aeroparticles, Composition, and Lung Diseases. Front Immunol. 2016;7:3.
14. Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596.
15. Yin P, Wu J, Wang L, et al. The burden of COPD in China and its provinces: findings from the global burden of disease study 2019. Front Public Health. 2022;10:859499.
16. Global strategy for prevention, diagnosis and management of COPD: 2023 report; 2022. Available from: https://goldcopd.org/2023-gold-report-2/.
17. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743.
18. Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792.
19. Yao Y, Chen X, Chen W, et al. Susceptibility of individuals with chronic obstructive pulmonary disease to respiratory inflammation associated with short-term exposure to ambient air pollution: a panel study in Beijing. Sci Total Environ. 2021;766:142639.
20. Sarkar C, Zhang B, Ni M, et al. Environmental correlates of chronic obstructive pulmonary disease in 96 779 participants from the UK Biobank: a cross-sectional, observational study. Lancet Planet Health. 2019;3(11):e478–e490.
21. Park J, Kim HJ, Lee CH, Lee CH, Lee HW. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ Res. 2021;194:110703.
22. Hsu HT, Wu CD, Chung MC, et al. The effects of traffic-related air pollutants on chronic obstructive pulmonary disease in the community-based general population. Respir Res. 2021;22(1):217.
23. Doiron D, de Hoogh K, Probst-Hensch N, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54(1):1802140.
24. Wang M, Aaron CP, Madrigano J, et al. Association Between Long-term Exposure to Ambient Air Pollution and Change in Quantitatively Assessed Emphysema and Lung Function. JAMA. 2019;322(6):546–556.
25. Bourbeau J, Doiron D, Biswas S, et al. Ambient air pollution and dysanapsis: associations with lung function and chronic obstructive pulmonary disease in the Canadian cohort obstructive lung disease study. Am J Respir Crit Care Med. 2022;206(1):44–55.
26. Huh JY, Hong J, Han DW, Park YJ, Jung J, Lee SW. The impact of air pollutants and meteorological factors on chronic obstructive pulmonary disease exacerbations: a nationwide study. Ann Am Thorac Soc. 2022;19(2):214–226.
27. Liang L, Cai Y, Barratt B, et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013–17: an ecological analysis. Lancet Planetary Health. 2019;3(6):e270–e279.
28. Mendy A, Wu X, Keller JL, et al. Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir Med. 2021;178:106313.
29. Sun XW, Chen PL, Ren L, et al. The cumulative effect of air pollutants on the acute exacerbation of COPD in Shanghai, China. Sci Total Environ. 2018;622–623:875–881.
30. Sun Q, Liu C, Chen R, et al. Association of fine particulate matter on acute exacerbation of chronic obstructive pulmonary disease in Yancheng, China. Sci Total Environ. 2019;650(Pt 2):1665–1670.
31. DeVries R, Kriebel D, Sama S. Outdoor air pollution and COPD-related emergency department visits, hospital admissions, and mortality: a meta-analysis. COPD. 2017;14(1):113–121.
32. Hart JE, Grady ST, Laden F, et al. Effects of indoor and ambient black carbon and PM2.5 on pulmonary function among individuals with COPD. Environ Health Perspect. 2018;126(12):127008.
33. Duan R, Niu H, Yu T, et al. Adverse effects of short-term personal exposure to fine particulate matter on the lung function of patients with chronic obstructive pulmonary disease and asthma: a longitudinal panel study in Beijing, China. Environ Sci Pollut Res Int. 2021;28(34):47463–47473.
34. Tran HM, Chen TT, Lu YH, et al. Climate-mediated air pollution associated with COPD severity. Sci Total Environ. 2022;843:156969.
35. Alexeeff SE, Deosaransingh K, Liao NS, Van Den Eeden SK, Schwartz J, Sidney S. Particulate matter and cardiovascular risk in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204(2):159–167.
36. Zhang W, Li H, Pan L, et al. Chemical constituents and sources of indoor PM2.5 and cardiopulmonary function in patients with chronic obstructive pulmonary disease: estimation of individual and joint effects. Environ Res. 2021;197:111191.
37. Chen X, Que C, Yao Y, et al. Susceptibility of individuals with lung dysfunction to systemic inflammation associated with ambient fine particle exposure: a panel study in Beijing. Sci Total Environ. 2021;788:147760.
38. Ho SC, Chuang KJ, Lee KY, et al. Chronic obstructive pulmonary disease patients have a higher risk of occurrence of pneumonia by air pollution. Sci Total Environ. 2019;677:524–529.
39. Dyer C, Pugh L. Lung health in older adults. Age Ageing. 2019;48(3):319–322.
40. Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679.
41. Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and non communicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: air pollution and organ systems. Chest. 2019;155(2):417–426.
42. Li X, Cao X, Guo M, Xie M, Liu X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020;368:m234.
43. Peng L, Xiao S, Gao W, et al. Short-term associations between size-fractionated particulate air pollution and COPD mortality in Shanghai, China. Environ Pollut. 2020;257:113483.
44. Bo Y, Chang LY, Guo C, et al. Reduced ambient PM2.5, better lung function, and decreased risk of chronic obstructive pulmonary disease. Environ Int. 2021;156:106706.
45. Li RT, Rao ZZ, Fu YH, et al. 2030年中国慢性阻塞性肺疾病的疾病负担预测与危险因素控制效果模拟. [Prediction on the burden of disease of chronic obstructive pulmonary disease and simulation of the effectiveness of controlling risk factors in China by 2030]. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(2):201–206. Chinese.
46. Lai CH, Chen YC, Lin KA, Lin YX, Lee TH, Lin CH. Adverse pulmonary impacts of environmental concentrations of oil mist particulate matter in normal human bronchial epithelial cell. Sci Total Environ. 2022;809:151119.
47. Honda A, Fukushima W, Oishi M, et al. Effects of components of PM(2.5) collected in Japan on the respiratory and immune systems. Int J Toxicol. 2017;36(2):153–164.
48. Rodriguez-Cotto RI, Ortiz-Martinez MG, Rivera-Ramirez E, et al. Particle pollution in Rio de Janeiro, Brazil: increase and decrease of pro-inflammatory cytokines IL-6 and IL-8 in human lung cells. Environ Pollut. 2014;194:112–120.
49. Zhao C, Niu M, Song S, et al. Serum metabolomics analysis of mice that received repeated airway exposure to a water-soluble PM2.5 extract. Ecotoxicol Environ Saf. 2019;168:102–109.
50. Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–362.
51. Xue Z, Gao X, Yu W, et al. Biochanin A alleviates oxidative damage caused by the urban particulate matter. Food Funct. 2021;12(5):1958–1972.
52. Yan J, Lai CH, Lung SC, et al. Industrial PM(2.5) cause pulmonary adverse effect through RhoA/ROCK pathway. Sci Total Environ. 2017;599–600:1658–1666.
53. Liu CW, Lee TL, Chen YC, et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-kappaB-dependent pathway. Part Fibre Toxicol. 2018;15(1):4.
54. Perrone MG, Gualtieri M, Consonni V, et al. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ Pollut. 2013;176:215–227.
55. Maheswaran D, Zeng Y, Chan-Yeung M, et al. Exposure to Beta-(1,3)-D-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLoS One. 2014;9(6):e98878.
56. Wang H, Shen X, Liu J, et al. The effect of exposure time and concentration of airborne PM2.5 on lung injury in mice: a transcriptome analysis. Redox Biol. 2019;26:101264.
57. Di Ianni E, Moller P, Cholakova T, Wolff H, Jacobsen NR, Vogel U. Assessment of primary and inflammation-driven genotoxicity of carbon black nanoparticles in vitro and in vivo. Nanotoxicology. 2022;16(4):526–546.
58. DeMarini DM, Warren SH, Brooks LR. Mutagenicity of the organic fraction of world trade center dust. Environ Mol Mutagen. 2022;64(1):16–25.
59. Bai X, Liu Y, Wang S, et al. Ultrafine particle libraries for exploring mechanisms of PM2.5-induced toxicity in human cells. Ecotoxicol Environ Saf. 2018;157:380–387.
60. Zhou L, Tao Y, Li H, et al. Acute effects of fine particulate matter constituents on cardiopulmonary function in a panel of COPD patients. Sci Total Environ. 2021;770:144753.
61. Heo J, Antkiewicz DS, Shafer MM, Perkins DA, Sioutas C, Schauer JJ. Assessing the role of chemical components in cellular responses to atmospheric particle matter (PM) through chemical fractionation of PM extracts. Anal Bioanal Chem. 2015;407(20):5953–5963.
62. Xu F, Qiu X, Hu X, et al. Effects on IL-1beta signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ Pollut. 2018;237:592–600.
63. Zhao C, Pu W, Wazir J, et al. Long-term exposure to PM2.5 aggravates pulmonary fibrosis and acute lung injury by disrupting Nrf2-mediated antioxidant function. Environ Pollut. 2022;313:120017.
64. Shuster-Meiseles T, Shafer MM, Heo J, et al. ROS-generating/ARE-activating capacity of metals in roadway particulate matter deposited in urban environment. Environ Res. 2016;146:252–262.
65. Wang T, Song X, Xu H, et al. Combustion-derived particulate pahs associated with small airway dysfunction in elderly patients with COPD. Environ Sci Technol. 2022;56(15):10868–10878.
66. Guo L, Wang Y, Yang X, et al. Aberrant mitochondrial DNA methylation and declined pulmonary function in a population with polycyclic aromatic hydrocarbon composition in particulate matter. Environ Res. 2022;214(Pt 1):113797.
67. Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12(8):689–698.
68. Fu Y, Niu Y, Pan B, et al. OGG1 methylation mediated the effects of cell cycle and oxidative DNA damage related to PAHs exposure in Chinese coke oven workers. Chemosphere. 2019;224:48–57.
69. Lauer FT, Mitchell LA, Bedrick E, et al. Temporal-spatial analysis of U.S.-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2009;238(1):1–10.
70. Wang E, Liu X, Tu W, et al. Benzo(a)pyrene facilitates dermatophagoides group 1 (Der f 1)-induced epithelial cytokine release through aryl hydrocarbon receptor in asthma. Allergy. 2019;74(9):1675–1690.
71. Choi H, Kim CS. Polycyclic aromatic hydrocarbons from fine particulate matter induce oxidative stress and the inflammatory response in human vocal fold fibroblast cells. Oxid Med Cell Longev. 2021;2021:5530390.
72. Zheng R, Song P, Wu Y, et al. Property-activity relationship between physicochemical properties of PM2.5 and their activation of NLRP3 inflammasome. NanoImpact. 2022;25:100380.
73. Hu B, Tong B, Xiang Y, et al. Acute 1-NP exposure induces inflammatory responses through activating various inflammatory signaling pathways in mouse lungs and human A549 cells. Ecotoxicol Environ Saf. 2020;189:109977.
74. Lee MK, Wyss AB, Carnes MU, et al. House dust microbiota in relation to adult asthma and atopy in a US farming population. J Allergy Clin Immunol. 2021;147(3):910–920.
75. Jeong YJ, Kim CU, Lee KS, et al. Pseudomonas stutzeri PM101005 inhaled with atmospheric particulate matter induces lung damage through inflammatory responses. Environ Pollut. 2022;317:120741.
76. Yang J, Kim YK, Kang TS, Jee YK, Kim YY. Importance of indoor dust biological ultrafine particles in the pathogenesis of chronic inflammatory lung diseases. Environ Health Toxicol. 2017;32:e2017021.
77. Barraza F, Jorquera H, Heyer J, et al. Short-term dynamics of indoor and outdoor endotoxin exposure: case of Santiago, Chile, 2012. Environ Int. 2016;92–93:97–105.
78. Oteros J, Bartusel E, Alessandrini F, et al. Artemisia pollen is the main vector for airborne endotoxin. J Allergy Clin Immunol. 2019;143(1):369–377 e365.
79. Yang J, Kim EK, Park HJ, McDowell A, Kim YK. The impact of bacteria-derived ultrafine dust particles on pulmonary diseases. Exp Mol Med. 2020;52(3):338–347.
80. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680.
81. Kim YS, Lee WH, Choi EJ, et al. Extracellular vesicles derived from Gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol. 2015;194(7):3361–3368.
82. Chen X, Deng T, Huo T, Dong F, Deng J. MiR-140-5p/TLR4 /NF-kappaB signaling pathway: crucial role in inflammatory response in 16HBE cells induced by dust fall PM2.5. Ecotoxicol Environ Saf. 2021;208:111414.
83. Tang Q, Huang K, Liu J, et al. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-kappaB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere. 2019;236:124373.
84. Poschl U. Aerosol particle analysis: challenges and progress. Anal Bioanal Chem. 2003;375(1):30–32.
85. Lyu R, Zhang J, Wu J, Feng Y. Primary carbonaceous particle emission from four power plants with ultralow emission in China. ACS Omega. 2021;6(2):1309–1315.
86. Niranjan R, Thakur AK. The toxicological mechanisms of environmental soot (Black Carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front Immunol. 2017;8:763.
87. Zhou L, Li P, Zhang M, et al. Carbon black nanoparticles induce pulmonary fibrosis through NLRP3 inflammasome pathway modulated by miR-96 targeted FOXO3a. Chemosphere. 2020;241:125075.
88. Han B, Chu C, Su X, et al. N(6)-methyladenosine-dependent primary microRNA-126 processing activated PI3K-AKT-mTOR pathway drove the development of pulmonary fibrosis induced by nanoscale carbon black particles in rats. Nanotoxicology. 2020;14(1):1–20.
89. Zhang L, Cheng S, Jiang X, et al. Pregnancy exposure to carbon black nanoparticles exacerbates bleomycin-induced lung fibrosis in offspring via disrupting LKB1-AMPK-ULK1 axis-mediated autophagy. Toxicology. 2019;425:152244.
90. Wang L, Bao S, Liu X, et al. Low-dose exposure to black carbon significantly increase lung injury of cadmium by promoting cellular apoptosis. Ecotoxicol Environ Saf. 2021;224:112703.
91. Pan X, Wu J, Jiang C, Yu Q, Yan B. Synergistic effects of carbon nanoparticle-Cr-Pb in PM2.5 cause cell cycle arrest via upregulating a novel lncRNA NONHSAT074301.2 in human bronchial epithelial cells. J Hazard Mater. 2021;411:125070.
92. He F, Liao B, Pu J, et al. Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Sci Rep. 2017;7:45666.
93. Yang L, Wang Y, Lin Z, et al. Mitochondrial OGG1 protects against PM2.5-induced oxidative DNA damage in BEAS-2B cells. Exp Mol Pathol. 2015;99(2):365–373.
94. Yang L, Liu G, Fu L, Zhong W, Li X, Pan Q. DNA repair enzyme OGG1 promotes alveolar progenitor cell renewal and relieves PM2.5-induced lung injury and fibrosis. Ecotoxicol Environ Saf. 2020;205:111283.
95. Niu R, Cheng J, Sun J, et al. Alveolar type II cell damage and Nrf2-SOD1 pathway downregulation are involved in PM2.5-induced lung injury in rats. Int J Environ Res Public Health. 2022;19(19):12893.
96. Yu H, Lin Y, Zhong Y, et al. Impaired AT2 to AT1 cell transition in PM2.5-induced mouse model of chronic obstructive pulmonary disease. Respir Res. 2022;23(1):70.
97. Yan P, Liu P, Lin R, et al. Effect of ambient air quality on exacerbation of COPD in patients and its potential mechanism. Int J Chron Obstruct Pulmon Dis. 2019;14:1517–1526.
98. Zhou T, Hu Y, Wang Y, et al. Fine particulate matter (PM2.5) aggravates apoptosis of cigarette-inflamed bronchial epithelium in vivo and vitro. Environ Pollut. 2019;248:1–9.
99. Zhang F, Ma H, Wang ZL, Li WH, Liu H, Zhao YX. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J Int Med Res. 2020;48(7):300060520927919.
100. Yan K, Hou T, Zhu L, Ci X, Peng L. PM2.5 inhibits system Xc- activity to induce ferroptosis by activating the AMPK-Beclin1 pathway in acute lung injury. Ecotoxicol Environ Saf. 2022;245:114083.
101. Liu C, Li P, Zheng J, Wang Y, Wu W, Liu X. Role of necroptosis in airflow limitation in chronic obstructive pulmonary disease: focus on small-airway disease and emphysema. Cell Death Discov. 2022;8(1):363.
102. Cameron SJ, Lewis KE, Huws SA, et al. Metagenomic sequencing of the chronic obstructive pulmonary disease upper bronchial tract microbiome reveals functional changes associated with disease severity. PLoS One. 2016;11(2):e0149095.
103. Wang L, Cheng H, Wang D, et al. Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol Environ Saf. 2019;167:269–277.
104. Daniel S, Phillippi D, Schneider LJ, Nguyen KN, Mirpuri J, Lund AK. Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet. Part Fibre Toxicol. 2021;18(1):3.
105. Wang S, Zhou Q, Tian Y, Hu X. The lung microbiota affects pulmonary inflammation and oxidative stress induced by PM2.5 exposure. Environ Sci Technol. 2022;56(17):12368–12379.
106. Jia Q, Li Q, Wang Y, et al. Lung microbiome and transcriptome reveal mechanisms underlying PM2.5 induced pulmonary fibrosis. Sci Total Environ. 2022;831:154974.
107. Li N, Yang Z, Liao B, et al. Chronic exposure to ambient particulate matter induces gut microbial dysbiosis in a rat COPD model. Respir Res. 2020;21(1):271.
108. Yang C, Kwon DI, Kim M, Im SH, Lee YJ. Commensal microbiome expands tgammadelta17 cells in the lung and promotes particulate matter-induced acute neutrophilia. Front Immunol. 2021;12:645741.
109. Jheng YT, Putri DU, Chuang HC, et al. Prolonged exposure to traffic-related particulate matter and gaseous pollutants implicate distinct molecular mechanisms of lung injury in rats. Part Fibre Toxicol. 2021;18(1):24.
110. Zhao J, Li M, Wang Z, et al. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir Res. 2019;20(1):120.
111. Kaur M, Chandel J, Malik J, Naura AS. Particulate matter in COPD pathogenesis: an overview. Inflamm Res. 2022;71(7–8):797–815.
112. Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32(4–6):234–246.
113. Vaughan A, Stevanovic S, Jafari M, et al. The effect of diesel emission exposure on primary human bronchial epithelial cells from a COPD cohort: n-acetylcysteine as a potential protective intervention. Environ Res. 2019;170:194–202.
114. Ren H, Lu J, Ning J, et al. Exposure to fine particulate matter induces self-recovery and susceptibility of oxidative stress and inflammation in rat lungs. Environ Sci Pollut Res Int. 2020;27(32):40262–40276.
115. Wang J, Li Y, Zhao P, et al. Exposure to air pollution exacerbates inflammation in rats with preexisting COPD. Mediators Inflamm. 2020;2020:4260204.
116. Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95.
117. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950.
118. Jin X, Xue B, Zhou Q, Su R, Li Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM(2.5) exposure. J Toxicol Sci. 2018;43(2):101–111.
119. Liu K, Hua S, Song L. PM2.5 exposure and asthma development: the key role of oxidative stress. Oxid Med Cell Longev. 2022;2022:3618806.
120. Grady ST, Koutrakis P, Hart JE, et al. Indoor black carbon of outdoor origin and oxidative stress biomarkers in patients with chronic obstructive pulmonary disease. Environ Int. 2018;115:188–195.
121. Lin CM, Huang TH, Chi MC, et al. N-acetylcysteine alleviates fine particulate matter (PM2.5)-induced lung injury by attenuation of ROS-mediated recruitment of neutrophils and Ly6C(high) monocytes and lung inflammation. Ecotoxicol Environ Saf. 2022;239:113632.
122. Vezina FA, Cantin AM. Antioxidants and chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2018;5(4):277–288.
123. Marzec JM, Nadadur SS. Inflammation resolution in environmental pulmonary health and morbidity. Toxicol Appl Pharmacol. 2022;449:116070.
124. Li W, Dorans KS, Wilker EH, et al. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: the Framingham heart study. Arterioscler Thromb Vasc Biol. 2017;37(9):1793–1800.
125. Grilli A, Bengalli R, Longhin E, et al. Transcriptional profiling of human bronchial epithelial cell BEAS-2B exposed to diesel and biomass ultrafine particles. BMC Genom. 2018;19(1):302.
126. Gao N, Xu W, Ji J, et al. Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ Health. 2020;19(1):12.
127. Paplinska-Goryca M, Misiukiewicz-Stepien P, Proboszcz M, et al. Interactions of nasal epithelium with macrophages and dendritic cells variously alter urban PM-induced inflammation in healthy, asthma and COPD. Sci Rep. 2021;11(1):13259.
128. Pu J, Xu J, Chen L, et al. Exposure to biomass smoke induces pulmonary Th17 cell differentiation by activating TLR2 on dendritic cells in a COPD rat model. Toxicol Lett. 2021;348:28–39.
129. Budulac SE, Boezen HM, Hiemstra PS, et al. Toll-like receptor (TLR2 and TLR4) polymorphisms and chronic obstructive pulmonary disease. PLoS One. 2012;7(8):e43124.
130. Liu J, Li S, Fei X, et al. Increased alveolar epithelial TRAF6 via autophagy-dependent TRIM37 degradation mediates particulate matter-induced lung metastasis. Autophagy. 2022;18(5):971–989.
131. Fernando IPS, Jayawardena TU, Kim HS, et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ Res. 2019;172:150–158.
132. Wang G, Zhang G, Gao X, et al. Oxidative stress-mediated epidermal growth factor receptor activation regulates PM2.5-induced over-secretion of pro-inflammatory mediators from human bronchial epithelial cells. Biochim Biophys Acta Gen Subj. 2020;1864(10):129672.
133. Memon TA, Nguyen ND, Burrell KL, et al. Wood smoke particles stimulate MUC5AC overproduction by human bronchial epithelial cells through TRPA1 and EGFR signaling. Toxicol Sci. 2020;174(2):278–290.
134. Val S, Belade E, George I, Boczkowski J, Baeza-Squiban A. Fine PM induce airway MUC5AC expression through the autocrine effect of amphiregulin. Arch Toxicol. 2012;86(12):1851–1859.
135. Wang J, Zhu M, Wang L, Chen C, Song Y. Amphiregulin potentiates airway inflammation and mucus hypersecretion induced by urban particulate matter via the EGFR-PI3Kalpha-AKT/ERK pathway. Cell Signal. 2019;53:122–131.
136. Haider SH, Oskuei A, Crowley G, et al. Receptor for advanced glycation end-products and environmental exposure related obstructive airways disease: a systematic review. Eur Respir Rev. 2019;28(151):180096.
137. Nachmias N, Langier S, Brzezinski RY, et al. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS One. 2019;14(5):e0214622.
138. Wang C, Meng X, Meng M, et al. Oxidative stress activates the TRPM2-Ca(2+)-NLRP3 axis to promote PM2.5-induced lung injury of mice. Biomed Pharmacother. 2020;130:110481.
139. Tien CP, Chen CH, Lin WY, et al. Ambient particulate matter attenuates Sirtuin1 and augments SREBP1-PIR axis to induce human pulmonary fibroblast inflammation: molecular mechanism of microenvironment associated with COPD. Aging. 2019;11(13):4654–4671.
140. Han B, Liu Q, Su X, et al. The role of PP2A /NLRP3 signaling pathway in ambient particulate matter 2.5 induced lung injury. Chemosphere. 2022;307(Pt 2):135794.
141. Xiong R, Jiang W, Li N, et al. PM2.5-induced lung injury is attenuated in macrophage-specific NLRP3 deficient mice. Ecotoxicol Environ Saf. 2021;221:112433.
142. Jia G, Yu S, Sun W, et al. Hydrogen sulfide attenuates particulate matter-induced emphysema and airway inflammation through Nrf2-dependent manner. Front Pharmacol. 2020;11:29.
143. Wang T, Chen X, Li H, et al. Pro-thrombotic changes associated with exposure to ambient ultrafine particles in patients with chronic obstructive pulmonary disease: roles of lipid peroxidation and systemic inflammation. Part Fibre Toxicol. 2022;19(1):65.
144. Pope CA, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204–1214.
145. van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):61–67.
146. Lee MK, Xu CJ, Carnes MU, et al. Genome-wide DNA methylation and long-term ambient air pollution exposure in Korean adults. Clin Epigenetics. 2019;11(1):37.
147. Bai J, Wang H, Yang S, et al. Dust fall PM2.5-induced lung inflammation in rats is associated with hypermethylation of the IFN-gamma gene promoter via the PI3K-Akt-DNMT3b pathway. Environ Toxicol Pharmacol. 2022;95:103942.
148. Wang C, Wang J, Zheng X, et al. Epigenetic regulation is involved in traffic-related PM2.5 aggravating allergic airway inflammation in rats. Clin Immunol. 2022;234:108914.
149. Leclercq B, Platel A, Antherieu S, et al. Genetic and epigenetic alterations in normal and sensitive COPD-diseased human bronchial epithelial cells repeatedly exposed to air pollution-derived PM2.5. Environ Pollut. 2017;230:163–177.
150. Wang C, O’Brien KM, Xu Z, Sandler DP, Taylor JA, Weinberg CR. Long-term ambient fine particulate matter and DNA methylation in inflammation pathways: results from the Sister Study. Epigenetics. 2020;15(5):524–535.
151. Mukherjee S, Dasgupta S, Mishra PK, Chaudhury K. Air pollution-induced epigenetic changes: disease development and a possible link with hypersensitivity pneumonitis. Environ Sci Pollut Res Int. 2021;28(40):55981–56002.
152. Pacchierotti F, Spano M. Environmental impact on DNA methylation in the germline: state of the art and gaps of knowledge. Biomed Res Int. 2015;2015:123484.
153. Ji X, Yue H, Ku T, et al. Histone modification in the lung injury and recovery of mice in response to PM2.5 exposure. Chemosphere. 2019;220:127–136.
154. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
155. Zhou T, Yu Q, Sun C, Wang Y, Zhong Y, Wang G. A pilot study of blood microRNAs and lung function in young healthy adults with fine particulate matter exposure. J Thorac Dis. 2018;10(12):7073–7080.
156. Zhao L, Zhang M, Bai L, et al. Real-world PM2.5 exposure induces pathological injury and DNA damage associated with miRNAs and DNA methylation alteration in rat lungs. Environ Sci Pollut Res Int. 2022;29(19):28788–28803.
157. De Smet EG, Mestdagh P, Vandesompele J, Brusselle GG, Bracke KR. Non-coding RNAs in the pathogenesis of COPD. Thorax. 2015;70(8):782–791.
158. Zhao J, Pu J, Hao B, et al. LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Sci Rep. 2020;10(1):11587.
159. He F, Wang N, Yu X, et al. GATA3/long noncoding RNA MHC-R regulates the immune activity of dendritic cells in chronic obstructive pulmonary disease induced by air pollution particulate matter. J Hazard Mater. 2022;438:129459.
160. Zhong Y, Wang Y, Zhang C, et al. Identification of long non-coding RNA and circular RNA in mice after intra-tracheal instillation with fine particulate matter. Chemosphere. 2019;235:519–526.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.