Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy
Authors Chen H , Huang X, Dong M , Wen S , Zhou L, Yuan X
Received 3 March 2023
Accepted for publication 19 May 2023
Published 30 May 2023 Volume 2023:16 Pages 1541—1554
DOI https://doi.org/10.2147/DMSO.S410834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Huiling Chen,1,2 Xiaojing Huang,2 Meiyuan Dong,2 Song Wen,2 Ligang Zhou,2 Xinlu Yuan2
1Graduate School of Fudan University, Shanghai, People’s Republic of China; 2Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, People’s Republic of China
Correspondence: Xinlu Yuan, Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China, Tel +8613370211617, Email [email protected]
Abstract: Diabetes and sarcopenia are emerging as serious public health issues. Sarcopenia, an age-related disorder characterized by loss of skeletal muscle mass and function, is recognized as a new complication in elderly patients with type 2 diabetes mellitus (T2DM). Type 2 diabetes is characterized by insulin resistance, chronic inflammation, accumulation of advanced glycation products and increased oxidative stress, which can negatively affect skeletal muscle mass, strength and function leading to sarcopenia. There is a mutual interrelationship between T2DM and sarcopenia in light of pathophysiology mechanism and long-term outcome. T2DM will accelerate the decline of muscle mass and function, which will in turn lead to glucose metabolism disorders, reduced physical activity and the risk of diabetes. However, the specific mechanism involved has not been thoroughly studied. Therefore, this review aims to explore the pathophysiology and therapeutic strategy related to sarcopenia and diabetes and provide insight for future investigations, which is of great significance for improving the quality of life in the elderly with diabetes and concurrently reducing the incidence of related complications.
Keywords: type 2 diabetes mellitus, sarcopenia, insulin resistance, inflammation, gut microbiota, therapeutic strategy
Introduction
Type 2 Diabetes mellitus (T2DM) is a metabolic disease characterized by chronic hyperglycemia due to insulin secretion and (or) utilization disorders. Epidemiological evidence shows that about 387 million adults are suffering from DM worldwide,1 and it is estimated that it will increase to 693 million by 2045,2 which has greatly burdened patients and society. Studies have found that both men and women with diabetes have lower bone mass index values3 and the risk of sarcopenia in patients with T2DM is 1.5–2 times higher than that of non-diabetic people.4 Currently, sarcopenia has been regarded as a new complication of T2DM,5 which not only leads to poor quality of life but also increases the risk of physical disability and even death.6–8
The Asian Working Group for Sarcopenia (AWGS) 2014 consensus defined sarcopenia as “age-related decline of skeletal muscle plus low muscle strength and/or physical performance”,9 which refers to age-related syndrome of progressive skeletal muscle loss with decrease in muscle strength and/or muscle function. It is a progressive and systemic skeletal muscle disorder that usually occurs as an age-related process in older adults10 and is associated with serious consequences in the elderly such as frailty, falls, fractures, physical disability and death.11,12 According to the latest diagnostic criteria of the Asian Working Group on Sarcopenia (AWGS) in 2019, the prevalence rates of suspected sarcopenia, sarcopenia, and severe sarcopenia in China are 38.5%, 18.6%, and 8.0%, respectively.13 It is estimated that the prevalence of sarcopenia will increase significantly within the next 30 years,14 becoming a major public issue. Sarcopenia and T2DM have a bidirectional relationship,15 increase the risk of each other16 and lead to functional decline and disability.17 Recent studies have shown that patients with T2DM and sarcopenia have a higher mortality rate than those without sarcopenia.18 However, little attention has been paid to elderly T2DM patients with sarcopenia. Hence, it is important to study the pathogenesis of both for a proper management of this clinical complexity.
The Converging Risk Factors for Sarcopenia and Diabetes
Aging is one of the most important risks of diabetes and sarcopenia.19 One remarkable physiological sign of the aging process is the gradual loss of skeletal muscle mass and function,20 which latently onset around the age of 30,21 and will accumulate to 30–50% extremely loss in 80 years old.22 The partial mechanism for this phenomenon may lie in the skeletal muscle, the major organ that possesses glucose transporter 4 (GLUT4) responsible for uptake of glucose, accounting for approximately 80% of glucose clearance,23,24 and the utility of glucose also latently declines, increasing susceptibility to T2D.25
Unlike muscle tissue, the transformation of body fat occurs at a certain age.26 The significant re-distribution of the adipose tissue is pivot to this change.12 It is found that persistent localization switches from subcutaneous to visceral may lead to the ectopic accumulation of adipocytes and lipids in, such as bone marrow, liver, and especially skeletal muscle.12,27 Compared with healthy elderly individuals, diabetic patients acquire up to 3-folds of the intramuscular fat stores,28 a trend for the increased risk of sarcopenia and insulin resistance (IR).
In addition, the imbalance between muscle protein anabolic and catabolic pathways paves way for the overall loss of skeletal muscle in aging. Microstructure alterations include the shrinkage and reduction of myofibers, particularly type II myofibers.25 Aging also promotes the satellite cells decrease, as well as the shift from type II to type I myofibers.29 This is more overt in the functional change of muscle fibers, especially type IIb, which release certain proteins and myokines that influence glucose metabolism in a more direct manner. Thus, loss of skeletal muscle mass due to aging or underlying diseases can exacerbate disarrangement of glucose metabolism.30
Due to the decreased protection mechanism or the other agents, aging is also accompanied by an increase in inflammatory markers and oxidative stress, both of which have been implicated as significant pathophysiological changes in DM and sarcopenia.31,32 Specifically, mitochondrial dysfunction and advanced glycation products associated with aging may adversely affect muscle quality and glycemic levels.17 Concurrently, changes in hormone levels also contribute to chronic inflammation.33 For instance, reduced testosterone levels impaired self-renewal of satellite cell in muscle.34 It is noteworthy that decline in serum testosterone levels in males with diabetes may be more significant.35 Therefore, testosterone deficiency is closely associated with sarcopenia in aged diabetic patients.
Besides, both energy requirements and intake decrease with aging. Between the ages of 40 and 70, energy intake decreases by an average of 25%. This reduction inevitably leads to weight and muscle loss, which adversely affects strength and physical performance,36 which in turn has an effect upon glucose metabolism. In addition, a sedentary lifestyle during aging is also a major contributor to the decline of skeletal muscle mass and insulin sensitivity.37
Vitamin D, a dietary precursor of 1,25 (OH)2D3, is often considered an important nutrient for maintaining proper bodily function.38 Vitamin D may affect metabolism and muscle health. Low vitamin D levels are associated with poorer physical function,39 worse glycemic control40 and an increased risk of developing diabetes in elderly adults.41 Inversely, vitamin D deficiency is common in patients with T2DM.42 Vitamin D plays many critical roles in the function of skeletal muscle, such as maintaining muscle excitability through intracellular calcium, the proliferation and differentiation of skeletal muscle stem cells, and so on.43,44 Multiple studies have also demonstrated that vitamin D supplementation can improve muscle strength and prevent atrophy of type II fibers where vitamin D is deficient.45 These evidences suggest that vitamin D emerges as a critical risk factor mediating the development of IR and sarcopenia, but the conclusion remains to be determined.
According to the 2014 Surgeon General’s Report, active smokers are at higher risk of developing T2D by 30–40% when compared to non-smokers.46 Cigarette smoking impacts body weight and composition, peripheral insulin sensitivity, and pancreatic β cell function.47 Numerous studies have described the vicious effects of smoking on skeletal muscle function and morphology, especially, the thigh muscles.48 The partial consequence is degeneration in muscle fatigue resistance49 associated with compromised muscle oxidative capacity,50 and a transition from slow twitch to fast twitch fiber type.51 Furthermore, smoking could promote skeletal muscle wasting via smoking-induced inflammation that facilitate protein breakdown and suppress protein synthesis52 (Figure 1).
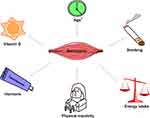 |
Figure 1 The converging risk factors between sarcopenia and diabetes. Sarcopenia was variably associated with some risk factors, notably age (marked with*). |
The Metabolic Pathophysiology for Sarcopenia in T2DM
Insulin Resistance (IR)
The main mechanism for T2DM is insulin resistance (IR). Skeletal muscle is one of the target organs for insulin action and insulin resistance.53 Impaired insulin action may promote protein degradation and hamper protein synthesis, resulting in both mass and strength drops of muscle.4 IR in skeletal muscle is the most important factor exacerbating sarcopenia in T2DM patients.54
The main proteolytic pathways in muscle involve the ATP-dependent ubiquitin proteasome pathway (UPP), lysosomal autophagy pathway, caspase hydrolysis pathway and calcium-dependent calpain pathway, of which were mainly mediated by IL6/STAT, TNF&IL6/NFκB, myostatin/Smad2/3 and FoxO1/3 signaling pathways.55 Insulin itself reduces proteasome catalytic activity in muscles.56 In the case of IR, high blood glucose levels may cause muscle atrophy via the proteolytic pathway of the ubiquitin-proteasome.57 Metabolic dysregulation due to insulin resistance58 leads to hyperglycemia and muscle atrophy via the WWP1/KLF15 pathway.59 WWP1 is a member of the ubiquitin ligase protein family. Studies have shown that hyperglycemia upregulates KLF15 protein in skeletal muscle of diabetes models of animals by downregulating the E3 ubiquitin ligase WWP1, followed by inhibition of ubiquitin-dependent degradation of KLF15. KLF15 is a protein associated with muscle wasting. The abundance of the transcription factor KLF15 and the expression of genes associated with muscle atrophy were increased in skeletal muscle of diabetic mice, and mice with muscle-specific KLF15 deficiency were protected from diabetes-induced muscle atrophy.60 This pathway may serve as a therapeutic target for insulin resistance-induced sarcopenia.
Muscle protein synthesis is mainly regulated by two factors: insulin-like growth factor 1 (IGF1) and myostatin.61,62 Protein synthesis inhibition is mediated by inhibition of the IGF1-PI3K-Akt-mTOR and SC-Gαi2 pathways.55 The signaling system of IGF1 and a series of intracellular components plays a crucial role in the regulation of skeletal muscle growth. Akt kinase, also known as protein kinase B (PKB), is a central component of this cascade, controlling protein synthesis through mammalian target of rapamycin (mTOR) and glycogen synthase kinase 3 (GSK3), and through FoxO family of transcription factors for protein degradation.63 IR inhibits mammalian targets of rapamycin pathway,64 while simultaneously stimulating the ubiquitin-proteasome system to upregulate protein catabolism through proteins belonging to FoxO family, and their downstream E3 ubiquitin ligases, resulting in decreased muscle mass.65 As skeletal muscle mass decreases, IR increases lipolysis, releases free fatty acids (FFA) from adipose tissue, and inhibits the growth hormone (GH)-insulin-like growth factor (IGF1) axis that promotes protein synthesis in skeletal muscle.66
Myostatin, a member of the transforming growth factor β (TGF-β) superfamily, downregulates mTORC1 signaling and increases AMPKα2 phosphorylation, both processes could lead to dysregulation of protein synthesis and enhance autophagic proteolysis.67 Myostatin binds to its cognate receptor, the activin II receptor type B (ActRIIB), and thereby exerts multiple effects. After activation of Smad2/Smad3 and dephosphorylation of Akt, muscle protein ubiquitination and proteasomal degradation as well as autophagy will be induced by Atrogin-1 and MuRF1, resulting in process favors for protein degradation.68,69 Additionally, myostatin also inhibits the mammalian target of rapamycin complex 1 (mTORC1).68,69 Besides, caspase, an apoptotic enzyme specific for caspase-3, is overexpressed in T2DM. Myostatin-induced apoptosis was shown to be elicited via activation of the p38-caspase pathway, leading to increased protein degradation.69,70 Hyperinsulinemia caused by IR increases the content of myostatin,71 thereby reducing skeletal muscle through the above-mentioned pathway.
Increased IR increases ectopic fat accumulation and promotes the production of pro-inflammatory factors that inhibit myogenesis and increase muscle catabolism.72 In skeletal muscle, insulin binds to the insulin receptor (GLUT4), allowing glucose uptake by the muscle cells. In the case of IR, its uptake is reduced, leading to hyperglycemia.57
Inflammation
Type 2 Diabetes Mellitus (T2DM) is characterized by chronic low-grade inflammation73 which disrupts glucose and muscle homeostasis.57 This inflammation leads to a downregulation of protein anabolism via the PI3K-Akt pathway, while simultaneously upregulating protein catabolism through the stimulation of the ubiquitin-proteasome system by FoxO family proteins and their downstream E3 ubiquitin ligases.74 The main inflammatory molecules involved in inflammation are TNF-α, IL-6, IL-1 and chemokines, which promote inflammatory cell infiltration and muscle degeneration through NF-κB.75 Inflammatory markers, including interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), are often elevated in individuals with T2DM76,77 and associated with IR.78 Increases in pro-inflammatory factors such as IL-6, TNF-α, and CRP have been shown to adversely affect muscle mass and function.79 IL-6 can increase muscle catabolism.80 In an animal experiment, low concentrations of IL-6 were injected into the muscles of mice. The muscles exposed to IL-6 subsequently atrophied due to protein breakdown.81 In addition, clinical studies have also shown that compared with non-diabetic controls, older adults with T2DM have greater loss of leg muscle and strength over 3 years. After adjusting for cytokines including IL-6 and TNF-α, these associations were only partially attenuated.82 IL-6 is associated with increased loss of muscle and strength in T2DM, probably due to direct effects on myocytes, as well as indirect effects on neurons and vasculature. Furthermore, IL-6 in chronically low levels controls signal transducer and activator of transcription 3 (STAT3) phosphorylation through a negative feedback mechanism leading to protein catabolism in muscle via JAK/STAT81 and NF-κB-dependent pathways.83 The inflammatory cascade begins with the activation of other interleukins, such as TNF and IL1, which act through NFκB, FOXO4 and other signaling pathways, resulting in muscle atrophy.55 Although IL-10 inhibits mTOR activity, it induces mitophagy, which leads to muscle atrophy.84 The specific molecular mechanisms underlying the adverse effects of CRP on muscle require further investigation.
Oxidative Stress
Oxidative stress, caused by increased reactive oxygen species (ROS) and decreased antioxidant effects,85 is considered an important contributor to aging and diseases. Muscle cells produce ROS (hydroxyl radicals, peroxides, and superoxides) as a by-product of normal metabolism and are more susceptible to oxidative stress.54 While T2DM is associated with increased oxidative stress,57 hyperglycemia in T2DM triggers increased production of ROS.86 ROS activates the ubiquitin-proteasome system and accelerates the degradation of muscle proteins,87 which leads to sarcopenia. It has been shown that increased oxidative stress impairs muscle repair,88 satellite cell differentiation and DNA in diabetic rat.89 Furthermore, oxidative stress inhibits the Akt/mTOR pathway and its downstream targets, subsequently inhibiting protein synthesis and promoting muscle atrophy.90
Mitochondrial dysfunction is another age- and T2DM-related factor associated with oxidative stress leading to deterioration of both metabolic and muscle health.91,92 Mitochondria play an important role in muscle function and metabolism. Compared with younger generation, the oxidative capacity per muscle unit is reduced by 50% in the elderly,93 and accompanied by increased incident of mitochondrial DNA mutations.94 Diabetes-induced mitochondrial dysfunction leads to myocyte apoptosis,95 thereby increasing the risk of sarcopenia. Furthermore, loss of Ca2+ homeostasis can activate non-lysosomal Ca2+-regulated calpains due to increased oxidative stress (another marker of T2DM).96 In diabetic patients, abnormally activated calpain leads to muscle atrophy through its activation of the ubiquitin-proteasome pathway (UPP) and inhibition of the Akt pathway.97
Advanced Glycation End-Products (AGEs)
AGEs, a few of heterogeneous molecules, which are derived from non-enzymatic products of the reaction of glucose or other sugar derivatives with proteins or lipids, are biomarkers of aging, and utilized as assessment of disease status.98 Hyperglycemia in T2DM patients develops with chronic accumulation of AGEs, which are associated with IR and aging.99,100 Accumulation of AGEs has been shown to lead to skeletal muscle atrophy and dysfunction in both basic experiments101 and human studies.102 The formation of AGEs is irreversible, and accumulates in various neuromusculoskeletal tissues, such as bone, cartilage, muscle, tendons, ligaments, and nerves, where they significantly affect biomechanical properties of those tissues.98 The accumulation of AGEs in diabetes affects skeletal muscle health through pathways such as mitochondrial dysfunction and induction of cell death.17 In addition, AGEs also interfere with muscle contractility by causing charge changes and increasing intramuscular protein cross-linking. By binding to AGEs receptors (RAGE) on skeletal muscle cell membranes, AGEs induce inflammation and activate NADPH oxidase through intracellular signal transduction, which increases the amount of circulating reactive oxygen species (ROS) that promotes oxidative stress.98 Recently, a study shows that higher skin AGEs are associated with a higher prevalence of sarcopenia.103 Whether AGEs measurement can be used to detect pathological changes in skeletal muscle caused by hyperglycemia and aging remains to be further investigated.
Intramuscular Adipose Tissue (IMAT)
IMAT is an ectopic fat deposit, which has been associated with poor outcome of metabolism104 or health of muscle.105 A hallmark pathophysiology of diabetes is obesity and ectopic deposition of fat in many insulin target tissues including skeletal muscle.106 Accumulation of ectopic lipids in skeletal muscle increases the risk of skeletal muscle IR.107 Obese adults with T2DM and peripheral neuropathy had higher calf muscle IMAT content compared with age-matched obese controls.108 Fat accumulation increases adipokine secretion and low-grade inflammation, which leads to both insulin signaling and mitochondrial dysfunction in skeletal muscle, which in turn leads to muscle atrophy.109 Abnormal mitochondrial morphology caused by impaired lipid metabolism leads to membrane stiffness and increased ROSs production in the process of muscle degeneration.110 In addition, IMAT is composed of non-contractile tissue and infiltration of fat into muscle affects the elasticity of skeletal muscle.111 The role of IMAT in sarcopenia and T2DM among the elderly is significant and should be prioritized as a crucial outcome measure in upcoming intervention studies.
Gut Microbiota
Human gut microbiota is able to influence host physiology by regulating multiple processes, including nutrient absorption, inflammation, oxidative stress, immune function, and anabolic balance.112 Dysbiosis of the gut microbiota plays an important role in the pathogenesis of IR and T2D through multiple mechanisms,113 which involve increased intestinal permeability, low-grade endotoxemia, short-chain fatty acids (SCFAs) or changes in the production of branched-chain amino acids (BCAAs), bile acid metabolism, and/or effects on gut hormone secretion.114 Compositional and functional changes in the gut microbiota have been observed in patients with T2DM and prediabetes.115,116 Additionally, fecal microbiota transplantation from healthy donors to patients with metabolic syndrome was associated with increased microbial diversity, improved glycemic control, and insulin sensitivity.117 Recent studies have shown that the gut microbiota also influences the onset and progression of sarcopenia.118,119 An in vitro study showed that phenolic conjugates produced by the microbiota increase glucose uptake in muscle fibers by upregulating GLUT4 and PI3K and induce an anabolic response that increases muscle mass.120 Intake of probiotics and prebiotics modulates skeletal muscle mass and function by modulating gut permeability and the gut-muscle axis.121 Thus, the gut microbiota may be an essential contributor involved in the pathogenesis of sarcopenia and diabetes (Figure 2).
The Therapeutic Strategy for Sarcopenia in T2DM
Non-Pharmacological Approaches
Physical Exercise
Exercise is the most cost-effective lifestyle intervention for the prevention and treatment paradigm of diabetes.122 Numerous studies have shown that exercise can also retard muscle loss by attenuating the activation of NF-κB.123,124 The 2018 clinical practice guidelines strongly recommend physical exercise as the primary treatment for sarcopenia.125 Both aerobic and resistance exercise can prevent and treat the decline in muscle mass and strength that occurs with aging.126 Aerobic exercise decreases the level of HbA1c, fasting blood glucose, and IR, as well improves skeletal muscle mass, especially leg strength. Resistance exercise (RE) can effectively enhance muscle strength while ameliorating IR, fat accumulation, and maintaining mitochondrial function in patients with T2DM.127,128 Current clinical experiments have proven short-term moderate-intensity resistance exercise (Each exercise consisted of (i) 5 min of warm-up; (ii) 30 min of RE: biceps (left hand 12 times × 3 groups; right hand 12 times × 3 groups; 1–2 min rest between each group); posterior neck arm extension (12 times × 3 groups, 1–2 min rest between each group); static squat (30s × 3 groups, 1–2 min rest between each group); standing upright against the wall for 10 min; and (iii) 5 min of stretching) is an effective and safe form of exercise. This type of exercise can reduce glycemic levels and its fluctuations, as well as hypoglycemia risks in elderly patients with T2DM and sarcopenia, improving the time in target range (TIR).129 It is noteworthy that for the elderly or (and) patients with neurological diseases, high-intensity exercise is difficult to implement; however, blood flow restriction (BFR) training can be used as an alternative to traditional exercise, and even low strength training also significantly increases muscle strength.130
The Nutrition Supplements
Although the exact mechanism is not yet clear, adequate energy and protein intake can help prevent loss of muscle mass. Animal protein intake is associated with an increased overall risk of T2DM compared with plant protein intake.131 Therefore, protein derived from plant may be the most appropriate source to ensure protein needs in older adults with or at risk of T2DM. Notably, leucine exhibits strong insulinotropic properties that increase amino acid availability for muscle protein synthesis, reduce muscle protein breakdown, and enhance glucose disposal to help maintain glycemic homeostasis.132 A recent study showed that the branched-chain amino acids valine, leucine, and isoleucine longitudinally reduce the odds of muscle mass loss.133 Therefore, supplementation with branched-chain amino acids, leucine, or leucine-rich proteins is one of the most common interventions for the treatment of sarcopenia in older adults.134
In addition, intake of other specific nutrients, including Omega-3 fatty acids, nicotinamide adenine dinucleotide (NAD+) precursor, vitamin D, foods containing anti-inflammatory and antioxidants and dietary fiber can modify insulin sensitivity, oxidative stress and inflammation to maintain muscle mass and improve glycemic level.37 Omega-3s are polyunsaturated fatty acids, mainly derived from marine fish, that increase muscle mass and function in healthy older adults.135 Omega-3 supplementation alone and in combination with exercise can substantially improve metabolic and muscle health. Omega-3 supplementation affects muscle directly by increasing muscle protein synthesis136 and indirectly by reducing systemic inflammation.137 Opacity, type and dose of Omega-3 supplements are most effective in the prevention and treatment of sarcopenia, thus, more prospective cohort studies are needed.
Vitamin D can improve muscle mass and function and is beneficial for patients with sarcopenia.138 Therefore, adequate vitamin D intake is recommended for elderly diabetic patients, especially those with sarcopenia.139 However, the optimal dose and frequency of administration, as well as the duration of treatment, remain unclear.4 In a study conducted on older adults with sarcopenia, it was found that combining exercise with daily intake of whey protein (22 g), essential amino acids (11 g, including 4 g leucine) and vitamin D (100 IU) resulted in a gain of almost 2 kg greater in lean mass compared to exercise alone. Additionally, the group that received the combination treatment showed significant improvements in hand grip strength and a reduction in CRP levels. In addition, it was reported that low intake of vitamins B1 and B12 increased the chance of sarcopenia in elderly patients with T2D.8 Further research is needed on whether vitamin B1 and B12 supplementation improves metabolic and muscle health issues. Inversely, the results of vitamin A and E in different studies are still controversial, where the sample size needs to be further expanded. Interestingly, reduced speed of eating was associated with an increased risk of sarcopenia in aged T2D patients.140 Recent studies have shown potential benefits of low-dose lithium (Lithium) in T2D and sarcopenia,141 while specific mechanisms need further study.
Although sarcopenia and T2DM are prevalent in the modern population, the current evidence for nutritional intervention is not consistent. The complement of evidences in the future research and practice will assist successful management of sarcopenia and diabetes.
Pharmacotherapy
There are currently no approved specific drugs for the therapy of sarcopenia. Possible therapeutic targets include hormonal interventions such as transdermal testosterone gels142 and selective androgen receptor modulators (SARMs).143 Transdermal testosterone gel increases the blood concentration of androgens, which has a favorable anabolic effect on skeletal muscle.144 However, adverse side effects exist, such as dyslipidemia, benign prostatic hypertrophy and uterine hyper-proliferation.145 Androgens are important for building and maintaining skeletal muscle, and due to their anabolic effects on muscle, they are considered superior in the potential treatment of sarcopenia.146 SARMs have an acceptable safety profile and positive effects on body composition and function in clinical trials.143,146 Hepatotoxicity and off-target effects on genitalia were major treatment-limiting side effects.147 Nonetheless, even if drugs are approved for the treatment of sarcopenia in the future, lifestyle modification will likely remain the mainstay for T2DM and sarcopenia.
Antidiabetic drugs may affect muscle mass; however, the mechanism for most of them on sarcopenia is still unknown. Metformin is a drug widely used in the treatment of T2DM, and different studies have also shown it has positive effects on muscle mass and muscle strength.148,149 However, in the elderly, inconveniences such as digestive intolerance, dysgeusia, hyperoxia, and vitamin b12 deficiency may hamper their prospect widely utilization in these populations.150 Sulfonylureas predispose to muscle atrophy and should be avoided in patients with sarcopenia or in patients with diabetes at risk of developing sarcopenia.151,152 Glinides have a similar mechanism of action to sulfonylureas153 and should also be avoided. Thiazolidinediones act as insulin sensitizers, and some studies have shown that thiazolidinediones are beneficial for muscle performance in diabetics. Pioglitazone improves skeletal muscle energy expenditure by reducing intramuscular lipid content and improving fatty acid metabolism,154 but increases risk of fractures and decompensated heart failure.155 Dipeptidyl peptidase-4 inhibitors (DPP-4) have positive effects on muscle mass and glycemic control,156 which may be related to the ability to enhance the action of GLP-1 or inhibit DPP-4 activity itself, or both.149 DPP-4 inhibitors have few side effects and exhibit minimal risk of hypoglycemia.157 Thus, such drugs may serve as a promising approach for T2DM with sarcopenia. Glucagon-like peptide 1 agonists (GLP-1RA) have anti-inflammatory and antioxidant properties, and the mechanism of their effects on skeletal muscle is still a matter of debate.153 Sodium-Glucose Cotransporter 2 Inhibitors (SGLT-2i) have been shown to be good therapeutic options for the treatment of aged T2D patients. In animal experiments, SGLT2i not only improved hyperglycemia but also improved fatty acid metabolism in muscle, thereby preventing muscle atrophy.158 Regardless of this proposed preclinical studied merit, the effects of SGLT-2i on muscle are still unclear in limited follow-up duration of clinical trials.159 Insulin is actually the most powerful hypoglycemic drug known so far. In limited studies, it claimed that long-term application of insulin therapy may negatively affect muscles encompassing mechanism such as inflammation, oxidative stress and increased ACEs, while its effect on bodyweight may be favored for low BMI patients. Therefore, there is currently no homogenous evidence that insulin therapy is beneficial for sarcopenia in older adults153 (Table 1).
 |
Table 1 Effects of Drug Therapy on Muscle and Adverse Side Effects in Diabetics |
Future Directions
Mitochondrial-targeted antioxidants are another potential therapy to reverse oxidative dysfunction, lower ATP levels and restore protein synthesis and muscle mass.160 In addition, myostatin (MYO) plays a powerful role as a negative muscle growth regulator. This is supported by studies showing that MYO gene knockout mice exhibit abnormal muscle hypertrophy and MYO overexpression can lead to severe muscle atrophy.161 In preclinical models, follistatin, a myostatin antagonist, was associated with reduced myostatin expression and increased muscle mass and protein synthesis.162 Endothelin-1 (ET-1) may play a critical role in mediating myopathy and adipose inflammation through the release of IL-6, TNF-α, or adipokines, visfatin, which involve PI3K/Akt/miR-let-7g-5p pathways in elderly individuals with diabetes.163 These findings can promote conceptualization and development of novel approaches or strategies for sarcopenia and with its related diseases in the future.
Conclusion
T2DM and sarcopenia are interrelated and entangled each other. Aging, insulin resistance, inflammation, oxidative stress, accumulation of AGEs, ectopic fat, vitamins and other factors could obviously affect muscle health. Impaired muscle health may also contribute to the development and progression of T2DM. Therefore, specific exercise methods and protein-rich nutrition combination-specific plans should be adopted, and appropriate hypoglycemic drugs selected to be individualized. The evidences backup the most effective and feasible interventions for patients complicated with both diseases are relatively sparse, and important directions in the future needed including intensive exploring underlying comprehensive cellular and molecular pathways and their interrelationships for two diseases, the advancement in developing biomarkers, the accuracy of diagnosis throughout whole life-span. Only by solving these techniques and knowledge limits, could prevention and treatment of sarcopenia and T2DM pierce the chaos of cognitive and practice cave.
Funding
This work was supported by Talents Training Program of Pudong Hospital affiliated to Fudan University (PJ202001), National Natural Science Foundation of China (82100850), the Project of Key Medical Specialty of Pudong Hospital of Fudan University (No Tszb2023-14) and Scientific Program of Shanghai Pudong Hospital (YJRCJJ201808).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine. 2014;42(12):698–702. doi:10.1016/j.mpmed.2014.09.007
2. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi:10.1038/s41581-020-0278-5
3. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33(7):1497–1499. doi:10.2337/dc09-2310
4. Izzo A, Massimino E, Riccardi G, et al. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients. 2021;13(1):183. doi:10.3390/nu13010183
5. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc. 2016;17(9):846–851. doi:10.1016/j.jamda.2016.07.007
6. Kawada T. Mortality risk of sarcopenia in older subjects. J Am Med Dir Assoc. 2021;22(9):1883. doi:10.1016/j.jamda.2021.04.011
7. Xu J, Wan CS, Ktoris K, et al. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. 2022;68(4):361–376. doi:10.1159/000517099
8. Takahashi F, Hashimoto Y, Kaji A, et al. Sarcopenia is associated with a risk of mortality in people with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:783363. doi:10.3389/fendo.2021.783363
9. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi:10.1016/j.jamda.2013.11.025
10. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/S0140-6736(19)31138-9
11. Tsekoura M, Kastrinis A, Katsoulaki M, et al. Sarcopenia and its impact on quality of life. Adv Exp Med Biol. 2017;987:213–218.
12. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi:10.1093/ageing/afz046
13. Wu X, Li X, Xu M, et al. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China Health and Retirement Longitudinal Study. PLoS One. 2021;16(3):e0247617. doi:10.1371/journal.pone.0247617
14. Ethgen O, Beaudart C, Buckinx F, et al. The Future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. 2017;100(3):229–234. doi:10.1007/s00223-016-0220-9
15. Wen CY, Lien AS, Jiang YD. Sarcopenia in elderly diabetes. J Diabetes Investig. 2022;13(6):944–946. doi:10.1111/jdi.13752
16. Huang S, Xiang C, Song Y. Identification of the shared gene signatures and pathways between sarcopenia and type 2 diabetes mellitus. PLoS One. 2022;17(3):e0265221. doi:10.1371/journal.pone.0265221
17. Mesinovic J, Zengin A, De Courten B, et al. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–1072. doi:10.2147/DMSO.S186600
18. Lin JA, Hou JD, Wu SY. Effect of sarcopenia on mortality in type 2 diabetes: a long-term follow-up propensity score-matched diabetes cohort study. J Clin Med. 2022;11(15):4424. doi:10.3390/jcm11154424
19. Ghafouri-Fard S, Khoshbakht T, Hussen BM, et al. Emerging role of non-coding RNAs in senescence. Front Cell Dev Biol. 2022;10:869011. doi:10.3389/fcell.2022.869011
20. Nair KS. Aging muscle. Am J Clin Nutr. 2005;81(5):953–963. doi:10.1093/ajcn/81.5.953
21. Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405–410. doi:10.1097/01.mco.0000134362.76653.b2
22. Mccormick R, Vasilaki A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology. 2018;19(6):519–536. doi:10.1007/s10522-018-9775-3
23. Son JW, Lee SS, Kim SR, et al. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES. Diabetologia. 2017;60(5):865–872. doi:10.1007/s00125-016-4196-9
24. Wu H, Liu M, Chi VTQ, et al. Handgrip strength is inversely associated with metabolic syndrome and its separate components in middle aged and older adults: a large-scale population-based study. Metabolism. 2019;93:61–67. doi:10.1016/j.metabol.2019.01.011
25. Mitchell WK, Williams J, Atherton P, et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi:10.3389/fphys.2012.00260
26. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi:10.1371/journal.pone.0007038
27. Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. 2019;10(1):5–6. doi:10.1007/s41999-018-0134-3
28. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813–1818. doi:10.2337/db05-1183
29. Par A, Hegyi JP, Vancsa S, et al. Sarcopenia - 2021: pathophysiology, diagnosis, therapy. Orv Hetil. 2021;162(1):3–12. doi:10.1556/650.2021.32015
30. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73(1):13–18. doi:10.1253/circj.CJ-08-0961
31. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15(1):12–22. doi:10.1097/MCO.0b013e32834dd297
32. Proctor MJ, Mcmillan DC, Horgan PG, et al. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One. 2015;10(3):e0116206. doi:10.1371/journal.pone.0116206
33. Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. 2020;12(5):1293. doi:10.3390/nu12051293
34. Sinha-Hikim I, Cornford M, Gaytan H, et al. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91(8):3024–3033. doi:10.1210/jc.2006-0357
35. Jang HC. Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab J. 2016;40(3):182–189. doi:10.4093/dmj.2016.40.3.182
36. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol a Biol Sci Med Sci. 2006;61(10):1059–1064. doi:10.1093/gerona/61.10.1059
37. Meier NF, Lee DC. Physical activity and sarcopenia in older adults. Aging Clin Exp Res. 2020;32(9):1675–1687. doi:10.1007/s40520-019-01371-8
38. Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034–1045. doi:10.1258/ebm.2010.010014
39. Dang M, Shore-Lorenti C, Mcmillan LB, et al. Associations of serum 25-hydroxyvitamin D with physical performance and bone health in overweight and obese older adults. Int J Environ Res Public Health. 2019;16(3):509. doi:10.3390/ijerph16030509
40. Mirhosseini N, Vatanparast H, Mazidi M, et al. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. doi:10.1210/js.2017-00472
41. Lucato P, Solmi M, Maggi S, et al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. Maturitas. 2017;100:8–15. doi:10.1016/j.maturitas.2017.02.016
42. Hirani V, Cumming RG, Naganathan V, et al. Longitudinal associations between vitamin d metabolites and sarcopenia in older Australian men: the concord health and aging in men project. J Gerontol a Biol Sci Med Sci. 2017;73(1):131–138. doi:10.1093/gerona/glx086
43. Uchitomi R, Oyabu M, Kamei Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. 2020;12(10):3189. doi:10.3390/nu12103189
44. Remelli F, Vitali A, Zurlo A, et al. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12):2861. doi:10.3390/nu11122861
45. Kalyani RR, Metter EJ, Egan J, et al. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi:10.2337/dc14-1166
46. US Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services; 2014.
47. Maddatu J, Anderson-baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101–107. doi:10.1016/j.trsl.2017.02.004
48. Larsson L, Orlander J. Skeletal muscle morphology, metabolism and function in smokers and non-smokers. A study on smoking-discordant monozygous twins. Acta Physiol Scand. 1984;120(3):343–352. doi:10.1111/j.1748-1716.1984.tb07394.x
49. Wust RC, Morse CI, De Haan A, et al. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol. 2008;104(1):103–110. doi:10.1007/s00421-008-0792-9
50. Degens H, Veerkamp JH. Changes in oxidative capacity and fatigue resistance in skeletal muscle. Int J Biochem. 1994;26(7):871–878. doi:10.1016/0020-711X(94)90079-5
51. Orlander J, Kiessling KH, Larsson L. Skeletal muscle metabolism, morphology and function in sedentary smokers and nonsmokers. Acta Physiol Scand. 1979;107(1):39–46. doi:10.1111/j.1748-1716.1979.tb06440.x
52. Degens H, Gayan-Ramirez G, Van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191(6):620–625. doi:10.1164/rccm.201410-1830PP
53. Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. 2019;234(6):8152–8161. doi:10.1002/jcp.27603
54. Argyropoulou D, Geladas ND, Nomikos T, et al. Exercise and nutrition strategies for combating sarcopenia and type 2 diabetes mellitus in older adults. J Funct Morphol Kinesiol. 2022;7(2). doi:10.3390/jfmk7020048
55. Giha HA, Alamin OAO, Sater MS. Diabetic sarcopenia: metabolic and molecular appraisal. Acta Diabetol. 2022;59(8):989–1000. doi:10.1007/s00592-022-01883-2
56. Russell ST, Rajani S, Dhadda RS, et al. Mechanism of induction of muscle protein loss by hyperglycaemia. Exp Cell Res. 2009;315(1):16–25. doi:10.1016/j.yexcr.2008.10.002
57. Purnamasari D, Tetrasiwi EN, Kartiko GJ, et al. Sarcopenia and chronic complications of type 2 diabetes mellitus. Rev Diabet Stud. 2022;18(3):157–165. doi:10.1900/RDS.2022.18.157
58. Goossens GH, Blaak EE, Theunissen R, et al. Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol. 2012;50(3):142–149. doi:10.1016/j.molimm.2012.01.005
59. Mcbride MJ, Foley KP, D’souza DM, et al. The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice. Am J Physiol Endocrinol Metab. 2017;313(2):E222–E232. doi:10.1152/ajpendo.00060.2017
60. Hirata Y, Nomura K, Senga Y, et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight. 2019;4(4). doi:10.1172/jci.insight.124952
61. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–1244. doi:10.1016/j.jhep.2016.07.040
62. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–1984. doi:10.1016/j.biocel.2005.04.018
63. Yoshida T, Delafontaine P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. 2020;9(9):1970. doi:10.3390/cells9091970
64. Lawrence JC. mTOR-dependent control of skeletal muscle protein synthesis. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S177–S185. doi:10.1123/ijsnem.11.s1.s177
65. Bassil MS, Gougeon R. Muscle protein anabolism in type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2013;16(1):83–88. doi:10.1097/MCO.0b013e32835a88ee
66. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–829. doi:10.1016/S2213-8587(14)70034-8
67. Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110(45):18162–18167. doi:10.1073/pnas.1317049110
68. Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10(9):504–516. doi:10.1038/nrneph.2014.112
69. Bataille S, Chauveau P, Fouque D, et al. Myostatin and muscle atrophy during chronic kidney disease. Nephrol Dial Transplant. 2021;36(11):1986–1993. doi:10.1093/ndt/gfaa129
70. Du J, Wang X, Miereles C, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113(1):115–123. doi:10.1172/JCI18330
71. Baczek J, Silkiewicz M, Wojszel ZB. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients. 2020;12(8):2401. doi:10.3390/nu12082401
72. Buch A, Carmeli E, Boker LK, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age--an overview. Exp Gerontol. 2016;76:25–32. doi:10.1016/j.exger.2016.01.008
73. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi:10.1038/nri2925
74. Perry BD, Caldow MK, Brennan-Speranza TC, et al. Muscle atrophy in patients with type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc Immunol Rev. 2016;22:94–109.
75. Zhang X, Li H, He M, et al. Immune system and sarcopenia: presented relationship and future perspective. Exp Gerontol. 2022;164:111823. doi:10.1016/j.exger.2022.111823
76. Goyal R, Faizy AF, Siddiqui SS, et al. Evaluation of TNF-alpha and IL-6 levels in obese and non-obese diabetics: pre- and postinsulin effects. N Am J Med Sci. 2012;4(4):180–184. doi:10.4103/1947-2714.94944
77. King DE, Mainous AG, Buchanan TA, et al. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26(5):1535–1539. doi:10.2337/diacare.26.5.1535
78. Vozarova B, Weyer C, Hanson K, et al. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9(7):414–417. doi:10.1038/oby.2001.54
79. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol a Biol Sci Med Sci. 2002;57(5):M326–332. doi:10.1093/gerona/57.5.M326
80. Rong YD, Bian AL, Hu HY, et al. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18(1):308. doi:10.1186/s12877-018-1007-9
81. Haddad F, Zaldivar F, Cooper DM, et al. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi:10.1152/japplphysiol.01026.2004
82. Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507–1512. doi:10.2337/dc06-2537
83. Ma JF, Sanchez BJ, Hall DT, et al. STAT 3 promotes IFN γ/ TNF α-induced muscle wasting in an NF -κB-dependent and IL −6-independent manner. EMBO Mol Med. 2017;9(5):622–637. doi:10.15252/emmm.201607052
84. Ip WKE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356(6337):513–519. doi:10.1126/science.aal3535
85. Omura T, Araki A. Skeletal muscle as a treatment target for older adults with diabetes mellitus: the importance of a multimodal intervention based on functional category. Geriatr Gerontol Int. 2022;22(2):110–120. doi:10.1111/ggi.14339
86. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi:10.1038/nature04634
87. Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6(3):197–207. doi:10.1002/jcsm.12043
88. Aragno M, Mastrocola R, Catalano MG, et al. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53(4):1082–1088. doi:10.2337/diabetes.53.4.1082
89. Scicchitano BM, Pelosi L, Sica G, et al. The physiopathologic role of oxidative stress in skeletal muscle. Mech Ageing Dev. 2018;170:37–44. doi:10.1016/j.mad.2017.08.009
90. Zhang L, Kimball SR, Jefferson LS, et al. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46(11):1500–1509. doi:10.1016/j.freeradbiomed.2009.03.001
91. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618–5623. doi:10.1073/pnas.0501559102
92. Abbatecola AM, Paolisso G, Fattoretti P, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15(10):890–895. doi:10.1007/s12603-011-0366-0
93. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi:10.1111/j.1469-7793.2000.t01-1-00203.x
94. Bua E, Johnson J, Herbst A, et al. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79(3):469–480. doi:10.1086/507132
95. Chen H, Ma J, Liu A, et al. The association between sarcopenia and fracture in middle-aged and elderly people: a systematic review and meta-analysis of cohort studies. Injury. 2020;51(4):804–811. doi:10.1016/j.injury.2020.02.072
96. Hyatt HW, Powers SK. The role of calpains in skeletal muscle remodeling with exercise and inactivity-induced atrophy. Int J Sports Med. 2020;41(14):994–1008. doi:10.1055/a-1199-7662
97. Huang J, Zhu X. The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol Res. 2016;65(4):547–560. doi:10.33549/physiolres.933087
98. Suzuki A, Yabu A, Nakamura H. Advanced glycation end products in musculoskeletal system and disorders. Methods. 2022;203:179–186. doi:10.1016/j.ymeth.2020.09.012
99. Haus JM, Carrithers JA, Trappe SW, et al. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. doi:10.1152/japplphysiol.00670.2007
100. Forbes JM, Sourris KC, De Courten MP, et al. Advanced glycation end products (AGEs) are cross-sectionally associated with insulin secretion in healthy subjects. Amino Acids. 2014;46(2):321–326. doi:10.1007/s00726-013-1542-9
101. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J. 2014;61(1):61–70. doi:10.1507/endocrj.EJ13-0244
102. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67–81. doi:10.1530/JOE-15-0533
103. Waqas K, Chen J, Trajanoska K, et al. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with sarcopenia. J Clin Endocrinol Metab. 2022;107(2):e793–e803. doi:10.1210/clinem/dgab632
104. Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–1345. doi:10.1002/jmri.21754
105. Scott D, Shore-Lorenti C, Mcmillan LB, et al. Calf muscle density is independently associated with physical function in overweight and obese older adults. J Musculoskelet Neuronal Interact. 2018;18(1):9–17.
106. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi:10.1038/nature05482
107. Pellegrinelli V, Rouault C, Rodriguez-Cuenca S, et al. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes. 2015;64(9):3121–3134. doi:10.2337/db14-0796
108. Bittel DC, Bittel AJ, Tuttle LJ, et al. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J Diabetes Complications. 2015;29(2):250–257. doi:10.1016/j.jdiacomp.2014.11.003
109. Meex RCR, Blaak EE, Van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev. 2019;20(9):1205–1217. doi:10.1111/obr.12862
110. Jimenez-Gutierrez GE, Martínez-Gómez LE, Martínez-Armenta C, et al. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. 2022;11(15):2359. doi:10.3390/cells11152359
111. Csapo R, Malis V, Sinha U, et al. Age-associated differences in triceps surae muscle composition and strength - an MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet Disord. 2014;15:209. doi:10.1186/1471-2474-15-209
112. Ticinesi A, Nouvenne A, Cerundolo N, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633. doi:10.3390/nu11071633
113. Bouter KE, Van Raalte DH, Groen AK, et al. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152(7):1671–1678. doi:10.1053/j.gastro.2016.12.048
114. Utzschneider KM, Kratz M, Damman CJ, et al. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2016;101(4):1445–1454. doi:10.1210/jc.2015-4251
115. Allin KH, Tremaroli V, Caesar R, et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61(4):810–820. doi:10.1007/s00125-018-4550-1
116. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi:10.1038/nature11450
117. Kootte RS, Levin E, Salojarvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611–619 e616. doi:10.1016/j.cmet.2017.09.008
118. Strasser B, Wolters M, Weyh C, et al. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients. 2021;13(6):2045. doi:10.3390/nu13062045
119. Park S, Yuan H, Zhang T, et al. Long-term silk peptide intake promotes skeletal muscle mass, reduces inflammation, and modulates gut microbiota in middle-aged female rats. Biomed Pharmacother. 2021;137:111415. doi:10.1016/j.biopha.2021.111415
120. Houghton MJ, Kerimi A, Mouly V, et al. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2019;33(2):1887–1898. doi:10.1096/fj.201801209R
121. Van Krimpen SJ, Jansen FAC, Ottenheim VL, et al. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target. Nutrients. 2021;13(4):1115. doi:10.3390/nu13041115
122. Liu Y, Wang Y, Ni Y, et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31(1):77–91 e75. doi:10.1016/j.cmet.2019.11.001
123. Burini RC, Anderson E, Durstine JL, et al. Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med Health Sci. 2020;2(1):1–6. doi:10.1016/j.smhs.2020.03.004
124. Liu HW, Chang SJ. Moderate exercise suppresses NF-kappaB Signaling and activates the SIRT1-AMPK-PGC1alpha axis to attenuate muscle loss in diabetic db/db mice. Front Physiol. 2018;9:636. doi:10.3389/fphys.2018.00636
125. Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. doi:10.1007/s12603-018-1139-9
126. Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33(1):17–26. doi:10.1016/j.cger.2016.08.002
127. Reidy PT, Mahmassani ZS, Mckenzie AI, et al. Influence of exercise training on skeletal muscle insulin resistance in aging: spotlight on muscle ceramides. Int J Mol Sci. 2020;21(4):1514. doi:10.3390/ijms21041514
128. Kimura T, Okamura T, Iwai K, et al. Japanese radio calisthenics prevents the reduction of skeletal muscle mass volume in people with type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001027. doi:10.1136/bmjdrc-2019-001027
129. Zhao D, Shi W, Bi L, et al. Effect of short-term acute moderate-intensity resistance exercise on blood glucose in older patients with type 2 diabetes mellitus and sarcopenia. Geriatr Gerontol Int. 2022;22(8):653–659. doi:10.1111/ggi.14437
130. Zhang XZ, Xie WQ, Chen L, et al. Blood flow restriction training for the intervention of sarcopenia: current stage and future perspective. Front Med. 2022;9:894996. doi:10.3389/fmed.2022.894996
131. Shang X, Scott D, Hodge AM, et al. Dietary protein intake and risk of type 2 diabetes: results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am J Clin Nutr. 2016;104(5):1352–1365. doi:10.3945/ajcn.116.140954
132. Manders RJ, Little JP, Forbes SC, et al. Insulinotropic and muscle protein synthetic effects of branched-chain amino acids: potential therapy for type 2 diabetes and sarcopenia. Nutrients. 2012;4(11):1664–1678. doi:10.3390/nu4111664
133. Low S, Wang J, Moh A, et al. Amino acid profile of skeletal muscle loss in type 2 diabetes: results from a 7-year longitudinal study in asians. Diabetes Res Clin Pract. 2022;186:109803. doi:10.1016/j.diabres.2022.109803
134. Hamarsland H, Nordengen AL, Nyvik Aas S, et al. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: a randomized controlled trial. J Int Soc Sports Nutr. 2017;14:43. doi:10.1186/s12970-017-0202-y
135. Jayanama K, Theou O, Godin J, et al. Association of fatty acid consumption with frailty and mortality among middle-aged and older adults. Nutrition. 2020;70:110610. doi:10.1016/j.nut.2019.110610
136. Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–412. doi:10.3945/ajcn.110.005611
137. Kiecolt-glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26(6):988–995. doi:10.1016/j.bbi.2012.05.011
138. Ganapathy A, Nieves JW. Nutrition and sarcopenia-what do we know? Nutrients. 2020;12(6):1755. doi:10.3390/nu12061755
139. Tamura Y, Omura T, Toyoshima K, et al. Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients. 2020;12(11):3367. doi:10.3390/nu12113367
140. Hashimoto Y, Takahashi F, Kaji A, et al. Eating speed is associated with the presence of sarcopenia in older patients with type 2 diabetes: a cross-sectional study of the KAMOGAWA-DM cohort. Nutrients. 2022;14(4):759. doi:10.3390/nu14040759
141. Hamstra SI, Roy BD, Tiidus P, et al. Beyond its psychiatric use: the benefits of low dose lithium supplementation. Curr Neuropharmacol. 2022. doi:10.2174/1570159X20666220302151224
142. Atkinson RA, Srinivas-Shankar U, Roberts SA, et al. Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J Gerontol A Biol Sci Med Sci. 2010;65(11):1215–1219. doi:10.1093/gerona/glq118
143. Papanicolaou DA, Ather SN, Zhu H, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17(6):533–543. doi:10.1007/s12603-013-0335-x
144. Neto WK, Gama EF, Rocha LY, et al. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age. 2015;37(1):9742. doi:10.1007/s11357-014-9742-0
145. Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 2018;465:134–142. doi:10.1016/j.mce.2017.06.013
146. Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled Phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153–161. doi:10.1007/s13539-011-0034-6
147. Solomon ZJ, Mirabal JR, Mazur DJ, et al. Selective androgen receptor modulators: current knowledge and clinical applications. Sex Med Rev. 2019;7(1):84–94. doi:10.1016/j.sxmr.2018.09.006
148. Wu CN, Tien KJ. The impact of antidiabetic agents on sarcopenia in type 2 diabetes: a literature review. J Diabetes Res. 2020;2020:9368583. doi:10.1155/2020/9368583
149. Massimino E, Izzo A, Riccardi G, et al. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: current evidence and underlying mechanisms. Cells. 2021;10(8):1958. doi:10.3390/cells10081958
150. Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016;101(4):1754–1761. doi:10.1210/jc.2015-3754
151. Cetrone M, Mele A, Tricarico D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr Diabetes Rev. 2014;10(4):231–237. doi:10.2174/1573399810666140918121022
152. Mele A, Calzolaro S, Cannone G, et al. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol Res Perspect. 2014;2(1):e00028. doi:10.1002/prp2.28
153. Sanz-Canovas J, Lopez-Sampalo A, Cobos-Palacios L, et al. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health. 2022;19(14):8677. doi:10.3390/ijerph19148677
154. Yokota T, Kinugawa S, Hirabayashi K, et al. Pioglitazone improves whole-body aerobic capacity and skeletal muscle energy metabolism in patients with metabolic syndrome. J Diabetes Investig. 2017;8(4):535–541. doi:10.1111/jdi.12606
155. Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care. 2007;30(11):2773–2778. doi:10.2337/dc07-0717
156. Sencan C, Dost FS, Ates Bulut E, et al. DPP4 inhibitors as a potential therapeutic option for sarcopenia: a 6-month follow-up study in diabetic older patients. Exp Gerontol. 2022;164:111832. doi:10.1016/j.exger.2022.111832
157. Doucet J, Chacra A, Maheux P, et al. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011;27(4):863–869. doi:10.1185/03007995.2011.554532
158. Bamba R, Okamura T, Hashimoto Y, et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J Cachexia Sarcopenia Muscle. 2022;13(1):574–588. doi:10.1002/jcsm.12814
159. Yabe D, Shiki K, Suzaki K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021;11(4):e045844. doi:10.1136/bmjopen-2020-045844
160. Kumar A, Davuluri G, Welch N, et al. Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy. Free Radic Biol Med. 2019;145:284–299. doi:10.1016/j.freeradbiomed.2019.09.031
161. Lee SJ, Mcpherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98(16):9306–9311. doi:10.1073/pnas.151270098
162. Dasarathy S, Mccullough AJ, Muc S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54(5):915–921. doi:10.1016/j.jhep.2010.08.032
163. Tsai CH, Huang PJ, Lee IT, et al. Endothelin-1-mediated miR-let-7g-5p triggers interleukin-6 and TNF-α to cause myopathy and chronic adipose inflammation in elderly patients with diabetes mellitus. Aging. 2022;14(8):3633–3651. doi:10.18632/aging.204034
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.