Back to Journals » Patient Related Outcome Measures » Volume 13
Assessing the Validity of the Long-Term Conditions Questionnaire (LTCQ) in Women During Pregnancy and the First Year Following Birth
Authors Kelly L, Fitzpatrick R, Kurinczuk JJ, Rivero-Arias O, Alderdice F
Received 9 June 2022
Accepted for publication 31 August 2022
Published 19 October 2022 Volume 2022:13 Pages 221—228
DOI https://doi.org/10.2147/PROM.S376070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Liana Bruce
Laura Kelly,1,2 Ray Fitzpatrick,1 Jennifer J Kurinczuk,3 Oliver Rivero-Arias,3 Fiona Alderdice2,3
1Health Services Research Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK; 2Harris Manchester College, University of Oxford, Oxford, UK; 3NIHR Policy Research Unit in Maternal and Neonatal Health and Care, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK
Correspondence: Fiona Alderdice, NIHR Policy Research Unit in Maternal and Neonatal Health and Care, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Richard Doll Building, Old Road Campus, Headington, Oxford, OX3 7LF, United Kingdom, Tel +44 0 1865617901, Email [email protected]
Background: The aim of this study was to validate a generic patient-reported outcome measure, the Long-Term Conditions Questionnaire (LTCQ), among pregnant and postpartum women living with a pre-existing long-term condition (LTC).
Methods: Cognitive interviews were conducted with women who were currently pregnant or had given birth within the past year and living with a pre-existing LTC (n=11) and with healthcare professionals working in maternal care (n=11) to explore the acceptability of LTCQ items. An online survey was subsequently administered among women who were pregnant or had given birth within the past year and living with a pre-existing LTC (n=718). Tests of validity were performed including assessing correlations between the LTCQ and reference measures, the Well-being in Pregnancy (WiP) Questionnaire and the EuroQol EQ-5D-5L. Internal consistency was assessed using the Cronbach’s alpha statistic.
Results: All LTCQ items were considered relevant and appropriate for use with women who were pregnant or had given birth within the past year. The most commonly reported LTC among the online survey sample (n=718) was a mental health condition (n=350, 48.7%) followed by joint, bone and connective tissues (n= 212, 29.5%) and gastrointestinal (n=143, 19.9%) condition. Data indicated LTCQ scores behaved in a predictable pattern, demonstrating poorer scores for women reporting a greater number of LTCs; mean (SD) scores, one LTC= 61.86 (17.8), two LTCs= 55.29 (16.0), three LTCs= 49.84 (15.52) and four LTCs= 44.94 (12.2). Poorer scores were also reported for women living with at least one mental health condition compared to those reporting no mental health condition, mean score = 66.18 (SD 16.7) v 48.64 (SD 13.3), p< 0.001 respectively. As anticipated, LTCQ scores demonstrated significant correlations in the expected direction with both the EQ-5D-5L and WiP scores. For all LTCQ items, the Cronbach’s alpha statistic was 0.93.
Conclusion: Data presented here indicate that the LTCQ, which assesses living well with one or more LTC, is suitable for use among pregnant and postpartum women, from both the woman’s perspective and from the perspectives of maternity healthcare professionals. Use of the LTCQ would facilitate the identification of unmet needs within this high-risk cohort and support the exploration of how LTCs may affect women throughout the pregnancy and post-natal period. Understanding unmet needs within this cohort of women provides an opportunity to link up specialist care within maternity services and enhance personalised care.
Keywords: maternity care, qualitative interviews, patient-reported outcomes, questionnaire, chronic conditions, pregnancy, postpartum
Introduction
Providing adequate and effective care for pregnant and postpartum women living with pre-existing long-term health conditions (LTCs) is an important consideration in healthcare. Whilst assessing the prevalence of LTCs among this cohort of women is often difficult, 10% of women in the UK have reported having a pre-existing LTC which complicated their pregnancy.1 Through the use of routine UK health-care datasets, estimates of the prevalence of pre-existing multi-morbidity (defined as two or more LTCs) among pregnant women have ranged from 19.8% to 46.2% with mental health conditions being highly prevalent.2
Improving poor maternal outcomes due to pre-existing LTCs needs to be addressed within maternity care. When considering maternal deaths within the UK, 65% of women who died during the years 2017–2019 were recorded as having a pre-existing medical problem, with 33% of women known to have a pre-existing mental health problem.3 Yet many of these women experience fragmented care and inadequate communication between healthcare professionals/ specialities.1,3 To improve outcomes, women at risk need to be identified and improvements made in the provision of care. Better Births, the maternity strategy for England, highlights the need for safe, personalised birth plans for all women building on a woman’s needs and choices.4 Pregnancy and the transition to parenthood is a time of great physical and psychological change for a woman with pre-existing LTCs. Therefore clear evidence of unmet needs and maternal concerns related to her LTC is needed to provide safe, personalised maternity care. To realise this, clinicians and researchers need good quality and well-validated standardised measures that assess the impact of living with LTCs at their disposal.5–7
The Long-Term Conditions Questionnaire (LTCQ) is a patient-reported outcome measure that was developed to capture the concept of living well with one or more LTC.8 The questionnaire consists of 20 items which measure the impacts of living with one or more mental or physical LTC and is suitable for use in both health and social care settings. Responses to items are scored from 0 (most negative response) to 4 (most positive response). All 20 items are scored as a single composite measure, with higher scores indicating more positively reported lives. To date, the LTCQ has undergone an extensive process of development and validation. The original selection of candidate items was initially informed by literature reviews, interviews with professional stakeholders, interviews with people living with LTCs and refined through subsequent expert and patient feedback.9–11 Validation surveys have been conducted with primary care and social care recipients in addition to patients using memory clinic services.8,12 It is however unknown how the LTCQ performs among pregnant and postpartum women who are living with pre-existing LTCs. The purpose of this analysis was to assess whether the LTCQ is suitable and acceptable for use within this cohort of women without compromising psychometric integrity.
Methods
Ethical approval for the research was granted by the University of Oxford’s Medical Sciences Interdivisional Research Ethics Committee (Reference Number: R61498/RE001). Participants provided informed consent and confirmation of eligibility through signing an online consent form to take part in the qualitative interviews.
Data reported here were collected during a wider study to explore the acceptability and feasibility of using health and well-being measures among pregnant and postpartum women living with pre-existing LTCs.13 The first stage of the wider research involved qualitative interviews to confirm the suitability (and make modifications where necessary) of selected instruments for use in this population and the second stage of the research consisted of an online survey to obtain data to carry out appropriate psychometric validation.
Stage 1: Cognitive Interviews
Qualitative interviews were carried out with 1) women, aged 18 or over and living within the UK, who were currently pregnant or had given birth within the past year and living with a (self-reported) LTC and, 2) healthcare professionals with experience of caring for women during and/or after pregnancy who were living with a pre-existing LTC. Healthcare professionals took part in the interviews in parallel with the pregnant and postpartum women to provide insight into their interpretation of items and perceived suitability within clinical practice. Participants were recruited through a number of support groups available to new mothers (for example, National Childbirth Trust and Mumsnet) and a range of condition specific organisations (for example, Diabetes UK). Healthcare professionals were recruited via existing networks and relevant professional bodies (for example, the Royal College of Midwives and the Royal College of Obstetricians and Gynaecologists). Full participant recruitment details have been previously reported.13
Interviews consisted of two parts. The first part explored experiences of care for women with pre-existing LTCs whilst the later part took the form of a cognitive interview. During the cognitive interview phase, participants were shown three measures (the Euroqol EQ-5D-5L,14 the LTCQ and, the Well-being in Pregnancy (WiP) questionnaire15) and asked to comment on their understanding and usefulness of each item. In relation to the LTCQ, probing questions were asked to identify any gaps in content when measuring living well with an LTC.16
Interview transcripts were transcribed verbatim, and data were managed using qualitative software (QSR NVIVO 11). Pre-specified codes were generated for data which gave insight into the meaning and interpretation of specific items and further codes were generated in cases where additional topics or concepts arose which may not already be incorporated within the existing LTCQ. Data were summarized according to each questionnaire item within an Excel document (Microsoft) facilitating item interpretation and comparisons across the sample.
Stage 2: Online Validation Survey
Data from the 718 survey respondents who reported living with a LTC formed the basis of the analysis. The online survey was administered using Qualtrics software to women who were either currently pregnant or had given birth within the past year. Participants were required to be 18 years old or over and living within the UK. Women who self-reported living with a LTC before their current or last pregnancy were asked to complete the Euroqol EQ-5D-5L,14 the LTCQ and, if currently pregnant, a modified version of the WiP questionnaire (which included a newly developed five item standalone LTC module).15,17 Women were also asked demographic questions, questions relating to experiences of maternity care and experiences of postpartum care, where applicable.
In addition to recruitment procedures outlined for Stage 1, participants were recruited through paid social media advertisements (Facebook and Instagram). All advertisements included a link to further information about the research and the survey link. Further information regarding Stage 2 study participants and procedure can be found here.17
Additional Assessments
Additional reference measures of well-being and quality of life were administered in the online survey with a view to assessing construct validity. The EQ-5D-5L is a generic health-related quality of life measure containing five questions. Each question covers the following domains: mobility, self-care, usual activities, pain and depression/anxiety.14 Each question has five response options, and the scores of the five domains can be transformed into a single index value with the score typically ranging from 0 (death) to 1 (best possible health). Mean (SD) EQ-5D-5L utility scores for pregnant women have previously been reported as 0.84 (0.17) in China and 0.87 (0.16) in the UK.18,19 For the 0–30 day postpartum period, mean (95% CI) EQ-5D-5L utility scores for women experiencing vaginal births have previously been reported as ranging between 0.57 (0.52, 0.61) to 0.93 (0.92, 0.94).20 For women experiencing caesarean section, utility scores ranging between 0.28 (0.18, 0.38) and 0.85 (0.82, 0.89) have been reported.20
The WiP questionnaire was initially developed to measure positive and negative emotions and thoughts about pregnancy.15 Responses are scored on a Likert scale (“All the time” to “At no time”). Total scores are obtained through summing the scores of each item with some item responses inverted to ensure higher scores correspond to higher well-being. The mean WiP score has previously been observed as 51.47 (SD 8.62) with no floor or ceiling effects for pregnant women.15 Since its initial development, and as part of the wider study, the WiP has been modified with questionnaire items amended and the development of additional items to assess well-being in pregnancy for women living with a LTC.17 The revised questionnaire contains 12 items measuring three core WiP scales; 1) Concerns over support after birth, 2) Positive pregnancy and, 3) Confidence about motherhood, and a five item standalone LTC specific scale.
Analysis
Differences between groups were examined using independent t-tests (for example, pregnant/ postpartum or partner/ no partner) and analysis of variance (ANOVA) in cases where more than two subgroups existed (for example, age group). Correlations between the LTCQ and the other included measures were assessed using Pearson correlation coefficients (r). Internal consistency was assessed using the Cronbach’s alpha statistic.21
Results
Qualitative Interviews
Participants included five pregnant and six postpartum women with pre-existing LTCs (mean age, 32.9, SD 3.8). Eleven healthcare professionals took part and roles included specialist midwives (hypertension and renal disease, diabetes and infant feeding coordinator), a GP (special interest in perinatal health and high-risk pregnancies), three obstetricians, an obstetric physician, a consultant perinatal psychiatrist and two health visitors. See13 for further details.
Pregnant/postpartum women and healthcare professionals considered items to be easily understood, relevant and likely to capture useful data when completed by pregnant or postpartum women who are living with one or more LTC. Whilst some concerns were expressed by healthcare professionals regarding the length of the questionnaire potentially adding to patient burden, the depth of granularity of the measure was welcomed when trying to understand any limitations to living well with their LTC. Pregnant/postpartum women and healthcare professionals welcomed the inclusion of questions that were pertinent to both mental and physical health.
Whilst all items were considered relevant by both women and health professionals, it was noted by some healthcare professionals that negative responses to items relating to social factors, such as “Felt safe at home” or “Felt that your home is suitable”, may be difficult to address. Obstetricians, for example, felt that these questions were more appropriate for people who are considered frail. Conversely, a health visitor advised that these questions were important questions which needed to be addressed. Women felt it was important to address responses to items asking about social aspects of living with a LTC as they touched on a sense of coping which may otherwise not be explored. No amendments were made to items validated in the original version of the LTCQ.
Online Validation Survey
A total of 718 women with a self-reported LTC completed the online survey. The average age was 32.04 (SD 4.8) years old with 421 (58.6%) women having remained in education until ≥ 19 years old. The large majority reported having a partner (n= 657, 91.5%) and were White British (n= 647, 90.1%). There was a high degree of multi-morbidity with 331 (46.1%) women reporting living with more than one LTC. The most commonly reported LTC was a mental health condition (n=350, 48.7%) followed by joint, bone and connective tissues (n= 212, 29.5%) and gastrointestinal (n=143, 19.9%) conditions (Table 1).
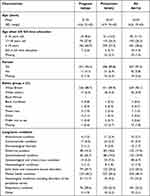 |
Table 1 Participant Characteristics |
No floor or ceiling effects were present, defined as more than 20% of responses achieving the minimum or maximum possible LTCQ score. For all LTCQ items, the Cronbach’s alpha statistic was 0.93. No significant differences in LTCQ scores were reported between women who were currently pregnant and women who had given birth within the past year (see Table 2). There were however significant differences in LTCQ scores according to age, relationship status and type or number of conditions reported. Women who reported having a partner demonstrated significantly higher LTCQ scores when compared to women who reported having no partner (mean score = 58.36 v 48.87 respectively, t= 4.76, p<0.001) and those who reported living with at least one mental health condition reported lower LTCQ scores compared to those reporting no mental health condition, mean score = 66.18 (SD 16.7) v 48.64 (SD 13.3) respectively, t=−15.58, p<0.001.
 |
Table 2 Long Term Conditions Questionnaire Score Comparisons Between Subsamples |
Significant differences in LTCQ scores were reported when comparing women according to the number of reported LTCs (p<0.001). Post-hoc tests demonstrated significant differences in scores between women reporting one LTC and those reporting two, three or more than four LTCs (all p<0.01) with scores becoming progressively more negative (worse) with the greater number of LTCs reported.
As anticipated, LTCQ scores demonstrated significant moderate to high correlations with the EQ-5D-5L scores (r= 0.68, p<0.01). WIP domains ranged from low to medium correlations with LTCQ scores (r= 0.26–0.47), however, the WiP-LTC bolt on module for women with LTC had high correlations of 0.70 (p<0.01) with the LTCQ total score (Table 3).
 |
Table 3 Correlation Coefficients Between All Well-Being Measures |
Discussion
This short report assessed the suitability of the LTCQ for use among pregnant and postpartum women living with one or more pre-existing LTC. Women regarded items acceptable and relevant to living with a LTC during or post pregnancy and supported the inclusion of items relating to both mental and physical health. Whilst healthcare professionals viewed the LTCQ items as relevant for women within their care, it was noteworthy that those working within the community setting, for example health visitors, rated items measuring social well-being more relevant than those working in the more traditional medical setting.
In keeping with recent estimates regarding pre-existing multi-morbidity,2 women reported a high degree of multi-morbidity with the most common LTC being a mental health condition. Analysis also indicated that LTCQ scores progressively worsened with the number of conditions reported and were poorer for women reporting living with a mental health condition compared to those reporting a physical health condition alone. These findings indicate the LTCQ can detect expected differences between sub-groups within this cohort and are supported by similar findings within the initial LTCQ validation survey.8
Results from our wider study have indicated that women and healthcare professionals support the use of health and well-being measures to improve care and their use in woman-centred outcome assessment.13 Determining the acceptability of the LTCQ among women who are pregnant or in the first year following birth is an important step in strengthening women centred care. Use of the measure can help to identify unmet needs and support the delivery of holistic care to high risk women in maternity services across multidisciplinary teams.
While this study supports the acceptability of the LTCQ within this population group, the survey was cross-sectional by design. Future research which follows women throughout the pregnancy and postpartum period will provide insight into how responses change across this timeframe, in addition to identifying pertinent timepoints at which to administer the instrument.
Conclusion
This study reports findings from research conducted with women living with LTCs who were either pregnant or had recently given birth and with healthcare professionals in maternity care. Data presented here indicate that the LTCQ, which assesses living well with one or more LTC, is suitable for use among pregnant and postpartum women. Use of the LTCQ would facilitate the identification of unmet needs within this high-risk cohort and support the exploration of how LTCs may affect women throughout the pregnancy and post-natal period. Women living with LTCs can experience fragmented care during pregnancy and childbirth. Introducing such measures to maternity care links up specialist care needs with maternity care needs from a woman’s perspective thus enhancing personalised care and supporting communication across care pathways.
Data Sharing Statement
Due to ethical concerns, the interview transcripts cannot be made openly available.
Ethics Approval and Consent to Participate
The manuscript complies with the declaration of Helsinki. Ethical approval was granted by the University of Oxford’s Medical Sciences Interdivisional Research Ethics Committee (Reference Number: R61498/RE001). All participants provided written consent via a secure online consent form prior to taking part.
Acknowledgments
The authors would like to thank Diabetes UK, Diabetes Support Forum UK, National Childbirth Trust, Mumsnet, Netmums, Mums Like Us, The Royal College of Midwives and The Royal College of Obstetricians and Gynaecologists who helped with recruitment by circulating the study recruitment link. The authors would also like to thank all participants who volunteered their time to take part in the study and to our patient and public representative, Antoinette Powell, for her helpful and insightful contribution to this study.
Funding
This study was funded internally within the Nuffield Department of Population Health, University of Oxford. LK receives funding from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford Health NHS Foundation Trust. This research was part-funded by the National Institute for Health Research (NIHR) Policy Research Programme, conducted through the Policy Research Unit in Maternal and Neonatal Health and Care, PR-PRU-1217-21202. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Disclosure
ORA is a member of the EuroQol Group that holds copyright of EQ-5D family instruments. The other authors declare that they have no competing interests in this work.
References
1. Knight M, Bunch K, Tuffnell D, et al. MBRRACE-UK saving lives, improving mothers’ care - lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity; 2015.
2. Lee SI, Azcoaga-Lorenzo A, Agrawal U, et al. Epidemiology of pre-existing multimorbidity in pregnant women in the UK in 2018: a cross-sectional study. Lancet. 2021;398:S7. doi:10.1016/S0140-6736(21)02550-2
3. Knight M, Bunch K, Tuffnell D, et al. Saving lives, improving mothers’ care - lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2017–19. Oxford, England; 2021.
4. National Maternity Review. Better births: improving outcomes in England. A five year forward view for maternity care. NHS England; 2016. Available from: https://www.england.nhs.uk/wp-content/uploads/2016/02/national-maternity-review-report.pdf.
5. Lee SI, Eastwood KA, Moss N, et al. Protocol for the development of a core outcome set for studies of pregnant women with pre-existing multimorbidity. BMJ Open. 2021;11:e044919.
6. Nijagal MA, Wissig S, Stowell C, et al. Standardized outcome measures for pregnancy and childbirth, an ICHOM proposal. BMC Health Serv Res. 2018;18:953. doi:10.1186/s12913-018-3732-3
7. CROWN. Core outcome sets in women’s and newborn health | the CROWN initiative; 2022. http://www.crown-initiative.org/core-outcome-sets/.
8. Potter CM, Batchelder L, A’Court C, et al. Long-Term Conditions Questionnaire (LTCQ): initial validation survey among primary care patients and social care recipients in England. BMJ Open. 2017;7:e019235.
9. Kelly L, Potter C, Hunter C, et al. Refinement of the Long-Term Conditions Questionnaire (LTCQ): patient and expert stakeholder opinion. Patient Relat Outcome Meas. 2016;7:183–193. doi:10.2147/PROM.S116987
10. Potter C, Kelly L, Gibbons E, et al. Item generation for the long-term conditions questionnaire (LTCQ): qualitative interviews with patients.
11. Hunter C, Fitzpatrick R, Jenkinson C, et al. Perspectives from health, social care and policy stakeholders on the value of a single self-report outcome measure across long-term conditions: a qualitative study. BMJ Open. 2015;5:e006986.
12. Potter CM, Peters M, Cundell M, McShane R, Fitzpatrick R. Use of the Long-Term Conditions Questionnaire (LTCQ) for monitoring health-related quality of life in people affected by cognitive impairment including dementia: pilot study in UK memory clinic services. Qual Life Res. 2021;30:1641–1652. doi:10.1007/s11136-021-02762-z
13. Kelly L, Kurinczuk JJ, Rivero-Arias O, Fitzpatrick R, Gibbons EAF. Exploring the use of health and wellbeing measures during pregnancy and the first year following birth in women living with pre-existing long-term conditions: qualitative interviews with women and healthcare professionals. BMC Health Serv Res. 2021;21:21. doi:10.1186/s12913-020-06006-7
14. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736. doi:10.1007/s11136-011-9903-x
15. Alderdice F, McNeill J, Gargan P, Perra O. Preliminary evaluation of the Well-being in Pregnancy (WiP) questionnaire. J Psychosom Obs Gynaecol. 2017;38:133–142. doi:10.1080/0167482X.2017.1285898
16. SAGE Publications I. Cognitive Interviewing in Practice: Think-Aloud, Verbal Probing, and Other Techniques. Cognitive Interviewing. Thousand Oaks: SAGE Publications, Inc. USA: SAGE Publications, Inc; 2005.
17. Kelly L, Kurinczuk JJ, Fitzpatrick R, et al. Refinement of the Well-being in Pregnancy (WiP) questionnaire: cognitive interviews with women and healthcare professionals and a validation survey. BMC Pregnancy Childbirth. 2022;22:325. doi:10.1186/s12884-022-04626-x
18. Wu H, Sun W, Chen H, et al. Health-related quality of life in different trimesters during pregnancy. Health Qual Life Outcomes. 2021;19(1):182. doi:10.1186/s12955-021-01811-y
19. Heslin M, Chua KC, Trevillion K, Nath S, Howard LM, Byford S. Psychometric properties of the five-level EuroQoL-5 dimension and Short Form-6 dimension measures of health-related quality of life in a population of pregnant women with depression. BJPsych Open. 2019;5(6):e88. doi:10.1192/bjo.2019.71
20. Kohler S, Sidney Annerstedt K, Diwan V, et al. Postpartum quality of life in Indian women after vaginal birth and cesarean section: a pilot study using the EQ-5D-5L descriptive system. BMC Pregnancy Childbirth. 2018;18:427. doi:10.1186/s12884-018-2038-0
21. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi:10.1007/BF02310555
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
ACTIVE PREGNANCY: Workshop on Promotion of Physical Activity in Pregnancy for Exercise Professionals

Santos-Rocha R, Pajaujiene S, Szumilewicz A
Journal of Multidisciplinary Healthcare 2022, 15:2077-2089
Published Date: 14 September 2022
Association of Opioid Use Disorder and Provision of Highly Effective Inpatient Postpartum Contraception
Malhotra T, Sheyn D, Arora K
Open Access Journal of Contraception 2023, 14:95-102
Published Date: 20 June 2023
Experiences of Heart Failure and the Treatment Journey: A Mixed-Methods Study Among Patients with Heart Failure in Sweden
Liljeroos M, Agvall B, Eek D, Fu M
Patient Preference and Adherence 2023, 17:1935-1947
Published Date: 9 August 2023
Exploring Motivations Regarding Dietary Intake Intentions in Gestational Diabetes Mellitus: Development and Validation of a Questionnaire
Di J, Zhu Q, Wu L, Tan J, Gao Y, Liu J
Patient Preference and Adherence 2023, 17:2939-2948
Published Date: 14 November 2023
Assessment of Levels of Anxiety and Fear of Covid-19 in a Population of Pregnant Women in Spain
Muñoz-Vela FJ, Fernández-Carrasco FJ, Gómez-Salgado J, Allande-Cussó R, Marques Monteiro Dias Mendes IM, Martins Teixeira-da-Costa EI, Vázquez-Lara JM, Fagundo-Rivera J, Rodríguez-Díaz L
Psychology Research and Behavior Management 2023, 16:4665-4676
Published Date: 16 November 2023
