Back to Journals » Open Access Journal of Contraception » Volume 14
Association of Opioid Use Disorder and Provision of Highly Effective Inpatient Postpartum Contraception
Authors Malhotra T , Sheyn D, Arora K
Received 16 March 2023
Accepted for publication 10 June 2023
Published 20 June 2023 Volume 2023:14 Pages 95—102
DOI https://doi.org/10.2147/OAJC.S411092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Igal Wolman
Tani Malhotra,1,2 David Sheyn,3 Kavita Arora1,4
1Department of Obstetrics and Gynecology, MetroHealth Medical Center, Cleveland, OH, USA; 2Department of Obstetrics and Gynecology, University Hospitals Cleveland Medical Center, Cleveland, OH, USA; 3Department of Urology, University Hospitals Cleveland Medical Center, Cleveland, OH, USA; 4Department of Obstetrics and Gynecology, University of North Carolina – Chapel Hill, Chapel Hill, NC, USA
Correspondence: Tani Malhotra, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University Hospitals Cleveland Medical Center, 11100 Euclid Ave, Cleveland, OH, 44106, USA, Tel +1 216-778-4444, Email [email protected]
Abstract: We sought to examine the rates of the inpatient provision of postpartum long-acting and permanent methods (IPP LAPM) of contraception in patients with opioid use disorder (OUD). This is a retrospective cross-sectional regression analysis of the National Inpatient Sample between 2012 and 2016. Patients with a diagnosis of OUD that delivered and received postpartum permanent contraception or long acting reversible contraception placement during the same hospitalization were identified. Regression analyses were performed to identify the demographic and clinical factors associated with long acting and permanent contraception method utilization. Of the 22,294 patients with OUD who delivered during the study period, 2291 (10.3%) received IPP LAPM. The majority of patients (1989) (86.6%) with OUD who chose inpatient provision of long acting or permanent methods after delivery received permanent contraception. After adjusting for covariates, patients with OUD had an overall decreased probability of receiving IPP LAPM (aOR=0.89, 95% CI: 0.85– 0.95), decreased probability of receiving permanent contraception (aOR: 0.82, 95% CI: 0.78– 0.88), but an increased probability of receiving long-acting reversible contraception (aOR: 1.29, 95% CI: 1.04– 1.60) compared to patients without OUD. This study highlights the continued need to ensure appropriate measures (such as antepartum contraceptive counseling, availability of access to inpatient LAPM, and removal of Medicaid policy barriers to permanent contraception) are in place so that the contraceptive needs of patients with OUD are fulfilled.
Keywords: opioid use disorder, substance use disorder, pregnancy, disparities, contraception, postpartum, intrauterine device, implant, permanent contraception, surgical contraception
Introduction
The provision of postpartum contraception is associated with the prevention of short-interval pregnancies and resultant maternal and neonatal morbidity and mortality including preterm birth, preeclampsia, and low birth weight.1,2 Long-acting and permanent methods of contraception (LAPM) are the most effective methods of contraception and thus result in the greatest reduction in number of short-interval pregnancies.3 Overall, rates of LAPM use postpartum have increased in the last decade, mostly due to increased utilization of long-acting reversible contraception (LARC).4
Opioid use disorder (OUD) in pregnancy is associated with increased hospital length of stay, stillbirth, preterm delivery, and neonatal abstinence syndrome and impacts approximately 0.76% of pregnancies in the United States.5 While 45% of pregnancies in the general population were unplanned in 2011, patients with opioid use disorders (OUD) have rates of unintended pregnancies approaching 75–90%.6–11 Thus, access to reliable contraception in the postpartum period is especially important in patients with OUD given the higher rates of unintended pregnancy in this population and a majority of unintended pregnancies occurring within the first year after delivery.3 Yet, postpartum provision of contraception in this population ranges from 21% to 25% versus 72% for the general population.3,12,13 The national rates of LAPM contraception receipt for patients with OUD are not well described with some studies conducted in individual states suggesting rates range from 8% to 28%; however, national data is lacking.12,14,15 Further, as only about 43% of patients with OUD attend their postpartum visit, inpatient provision (IPP) is crucial for ensuring access to those patients with OUD who desire LAPM.12,16
Given that rates of IPP LAPM provision in patients with OUD are largely unknown, we aimed to determine the national rate and trends over time of IPP LAPM provision in those with OUD. We hypothesized that rates of IPP LAPM provision in patients with OUD would be lower than that of the general population.
Materials and Methods
We performed a retrospective cross-sectional study utilizing the National Inpatient Sample (NIS) between the years 2012–2016. The NIS is the largest all-payer database; represents a 20% random sample of discharges from hospitals in the United States, and represents over 95% of the US population due to self-weighting.17 Diagnosis-Related Group (DRG) codes were used to identify patients who underwent spontaneous vaginal, operative vaginal, or cesarean delivery; patients with OUD; and patients receiving IPP LAPM. Patients who had a hysterectomy during the delivery admission were excluded. Covariates included in the regression analysis were age, race, United States census region, hospital location, hospital size, urbanization level, median household income, pre-existing comorbidities using Elixhauser measures, pregnancy- and delivery-related comorbidities, length of hospitalization, and non-opioid substance use.18 These covariates were chosen, given their association with contraceptive decision-making and access. Details regarding specific DRG codes utilized for this analysis have been previously published.19
We evaluated differences between patients with and without OUD who received for LAPM contraception as well as LAPM breakdown among patients with OUD using Wilcoxon rank-sum, Student’s t-test, the Kruskal–Wallis test, and Fisher’s exact test where appropriate. Multivariable logistic regression was performed to determine the association of demographic, geographic, comorbidity, and pregnancy-complication variables on use of LAPM contraception among patients with OUD. Regression results are reported in terms of unadjusted and adjusted odds ratios (OR and aOR, respectively) and 95% confidence intervals (95% CI). In line with the NIS data use agreement, exact numbers are not listed for cells with less than 10 patients.20 Statistical significance was set at p <0.05. All statistical analysis was performed using STATA version 14.1 (Stata Corp, College Station, TX). We complied with relevant data protection and privacy regulations. Studies using the NIS database are considered exempt by the Institutional Review Board of University Hospitals Cleveland Medical Center.
Results
During the study period, there were 3,573,018 unweighted deliveries, of which 22,294 (0.6%) patients had a diagnosis of OUD at the time of delivery. Two thousand two hundred ninety-one (10.3%) patients with a diagnosis of OUD received IPP LAPM compared to 246,826 (6.9%) of those without OUD (p<0.001) (Table 1).
 |
Table 1 Characteristics of Patients with and without Opioid Use Disorder Who Received Inpatient Postpartum Provision of Long Acting and Permanent Contraception Methods |
However, after adjusting for confounders, OUD was found to be associated with an overall decreased probability of utilization of IPP LAPM (aOR=0.89, 95% CI: 0.85–0.95). This was primarily driven by the fact that the majority of LAPM in this cohort were permanent contraception and OUD was associated with a decreased probability of receiving permanent contraception (aOR: 0.82, 95% CI: 0.78–0.88) but showed an increased probability of receiving long-acting reversible contraception (LARC) (aOR: 1.29, 95% CI: 1.04–1.60. The results after multivariable logistic regression for utilization of LAPM in patients with OUD are shown in Table 2.
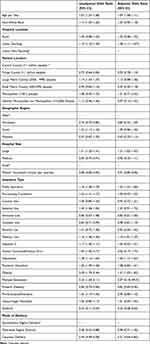 |
Table 2 Unadjusted and Adjusted Variables Associated with Utilization of Inpatient Postpartum Provision of Long Acting and Permanent Contraception Methods in Patients with Opioid Use Disorder |
Discussion
In this study of the National Inpatient Sample from 2012 to 2016, patients with OUD had an 11% lower probability of utilizing IPP LAPM for postpartum contraception compared to those without OUD. Importantly, participants were less likely to undergo permanent contraception but more likely to receive LARC.
The reason for the difference in rates of IPP LAPM utilization between patients with and without OUD is likely to be multifactorial. A prior study of attitudes towards contraception in women with OUD noted that if contraception was freely provided, 17% would choose a LARC method and more women with OUD would choose contraceptive injections (41%) than any other method. Another study found that 33–42% of patients with OUD would be unlikely to choose a LARC.7,9 This is comparable to the findings in one study of the general population in which 32–53% of patients receiving care at a federally qualified health center had low acceptability of LARCs.21 Differences in clinician counseling due to implicit biases may impact access and uptake of LAPM.22 Additionally, policy-level barriers to IPP LAPM may also play a role in our study findings. Rates of IPP LARC were noted to increase after policy changes requiring Medicaid coverage of IPP LARCs in South Carolina.1 Patients with OUD may also be less likely to receive permanent contraception inpatient than the general population due to the lack of a valid Medicaid sterilization consent form given barriers to prenatal care.23
The main strength of this study is that it relied on data from the NIS, which provides a representative sample of the hospitals across the US, thus increasing the reliability and generalizability of our results. We acknowledge the limitations inherent with our retrospective methodology utilizing solely inpatient administrative data. While changes in Medicaid coverage for inpatient LARCs that occurred during our study period resulted in overall increases in IPP LARC, additional studies are needed to better understand the impact of Medicaid expansion to cover IPP LARC on rates of utilization specifically in patients with OUD.
Conclusion
Patients with opioid use disorder (OUD) are less likely than patients without OUD to receive inpatient provision of long acting or permanent methods of contraception. Potential areas for improvement include antepartum contraceptive counseling, availability of access to inpatient LAPM, and removal of Medicaid policy barriers to permanent contraception. This study highlights the continued need to ensure that the contraceptive goals for patients with OUD are being achieved.
Acknowledgment
This study was presented at the virtual 41st Annual Pregnancy Meeting of the Society for Maternal Fetal Medicine January 25–30th, 2021.
Disclosure
Dr. Arora is funded by 1R01HD098127 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) branch of the National Institutes of Health (NIH). This manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Sheyn receives research support from Renalis (Cleveland, OH). Renalis had no involvement in the design or drafting of this study. Dr Sheyn also reports personal fees from Caldera Medical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Liberty A, Yee K, Darney BG, et al. Coverage of immediate postpartum long-acting reversible contraception has improved birth intervals for at-risk populations. Am J Obstet Gynecol. 2020;222:
2. Thiel de Bocanegra H, Chang R, Menz M, Howell M, Darney P. Postpartum contraception in publicly-funded programs and interpregnancy intervals. Obstet Gynecol. 2013;122(2 Pt 1):296–303.
3. White K, Teal S, Potter JE. Contraception after delivery and short interpregnancy intervals among women in the United States. Obstet Gynecol. 2015;126(6):1471–1477.
4. Moniz MH, Chang T, Heisler M, et al. Inpatient postpartum long-acting reversible contraception and sterilization in the United States, 2008-2013. Obstet Gynecol. 2017;129(6):1078–1085.
5. Malhotra T, Sheyn T, Arora KS. Opioid use disorder at delivery hospitalization in the United States: 2012-2016. Am J Addict. 2023:65.
6. Parlier AB, Fagan B, Ramage M, Galvin S. Prenatal care, pregnancy outcomes, and postpartum birth control plans among pregnant women with opiate addictions. South Med J. 2014;107(11):676–683.
7. Fischbein RL, Lanese BG, Falletta L, Hamilton K, King JA, Kenne DR. Pregnant or recently pregnant opioid users: contraception decisions, perceptions and preferences. Contracept Reprod Med. 2018;3:4.
8. Collier KW, MacAfee LK, Kenny BM, Meyer MC. Does co-location of medication assisted treatment and prenatal care for women with opioid use disorder increase pregnancy planning, length of interpregnancy interval, and postpartum contraceptive uptake? J Subst Abuse Treat. 2019;98:73–77.
9. Matusiewicz AK, Melbostad HS, Heil SH. Knowledge of and concerns about long-acting reversible contraception among women in medication-assisted treatment for opioid use disorder. Contraception. 2017;96(5):365–369.
10. Smith C, Morse E, Busby S. Barriers to reproductive healthcare for women with opioid use disorder. J Perinat Neonatal Nurs. 2019;33(2):E3–E11.
11. Heil SH, Jones HE, Arria A, et al. Unintended pregnancy in opioid-abusing women. J Subst Abuse Treat. 2011;40(2):199–202.
12. Krans EE, Kim JY, James AE, Kelley DK, Jarlenski M. Postpartum contraceptive use and interpregnancy interval among women with opioid use disorder. Drug Alcohol Depend. 2018;185:207–213.
13. Patton BP, Krans EE, Kim JY, Jarlenski M. The impact of Medicaid expansion on postpartum health care utilization among pregnant women with opioid use disorder. Subst Abus. 2019;40(3):371–377.
14. MacAfee LK, Harfmann RF, Cannon LM, et al. Sexual and reproductive health characteristics of women in substance use treatment in Michigan. Obstet Gynecol. 2020;135(2):361–369.
15. Charron E, Rennert L, Mayo RM, Eichelberger KY, Dickes L, Truong KD. Contraceptive initiation after delivery among women with and without opioid use disorders: a retrospective cohort study in a statewide Medicaid population, 2005-2016. Drug Alcohol Depend. 2021;220:108533.
16. Kotha A, Chen BA, Lewis L, Dunn S, Himes KP, Krans EE. Prenatal intent and postpartum receipt of long-acting reversible contraception among women receiving medication-assisted treatment for opioid use disorder. Contraception. 2019;99(1):36–41.
17. Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318(20):2011–2018.
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
19. Sheyn D, Arora KS. Changes in rates of inpatient postpartum long-acting reversible contraception and sterilization in the USA, 2012-2016. Matern Child Health J. 2021;25(10):1562.
20. Publishing with HCUP Data. Healthcare cost and utilization project (HCUP). Available from: www.hcup-us.ahrq.gov/db/publishing.jsp.
21. Paul R, Huysman BC, Maddipati R, Madden T. Familiarity and acceptability of long-acting reversible contraception and contraceptive choice. Am J Obstet Gynecol. 2020;222(4S):
22. Kathawa CA, Arora KS. Implicit bias in counseling for permanent contraception: historical context and recommendations for counseling. Health Equity. 2020;4(1):326–329.
23. Block-Abraham D, Arora KS, Tate D, Gee RE. Medicaid consent to sterilization forms: historical, practical, ethical, and advocacy considerations. Clin Obstet Gynecol. 2015;58(2):409–417.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
ACTIVE PREGNANCY: Workshop on Promotion of Physical Activity in Pregnancy for Exercise Professionals

Santos-Rocha R, Pajaujiene S, Szumilewicz A
Journal of Multidisciplinary Healthcare 2022, 15:2077-2089
Published Date: 14 September 2022
Assessing the Validity of the Long-Term Conditions Questionnaire (LTCQ) in Women During Pregnancy and the First Year Following Birth
Kelly L, Fitzpatrick R, Kurinczuk JJ, Rivero-Arias O, Alderdice F
Patient Related Outcome Measures 2022, 13:221-228
Published Date: 19 October 2022
The Pendulum: The Need to Develop a Safe, Effective, and Equitable Management Strategy for Opioids in Cancer Patients
Noreika D, Konecny M
Risk Management and Healthcare Policy 2024, 17:1079-1082
Published Date: 25 April 2024
Patient Perceived Quality of Virtual Group Contraception Counseling
Ereme K, Akullo K, Class QA, Hinz E
Open Access Journal of Contraception 2024, 15:99-105
Published Date: 4 July 2024
