Back to Journals » Patient Preference and Adherence » Volume 17
Experiences of Heart Failure and the Treatment Journey: A Mixed-Methods Study Among Patients with Heart Failure in Sweden
Authors Liljeroos M, Agvall B , Eek D , Fu M
Received 26 April 2023
Accepted for publication 28 July 2023
Published 9 August 2023 Volume 2023:17 Pages 1935—1947
DOI https://doi.org/10.2147/PPA.S418760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Maria Liljeroos,1,2,* Björn Agvall,3,* Daniel Eek,4,* Michael Fu5,*
1Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden; 2Centre for Clinical Research Sörmland, Uppsala University, Eskilstuna, Sweden; 3Department of Research and Development, Region Halland, Halmstad, Sweden; 4Cardiovascular, Renal and Metabolism, Medical Department, BioPharmaceuticals, AstraZeneca, Stockholm, Sweden; 5Section of Cardiology, Department of Medicine, Geriatrics and Emergency Medicine Sahlgrenska University Hospital-Östra Hospital, Gothenburg, Sweden
*These authors contributed equally to this work
Correspondence: Daniel Eek, AstraZeneca AB, Karlebyhus B674, Södertälje, SE-15185, Sweden, Tel +46 31 776 3659, Email [email protected]
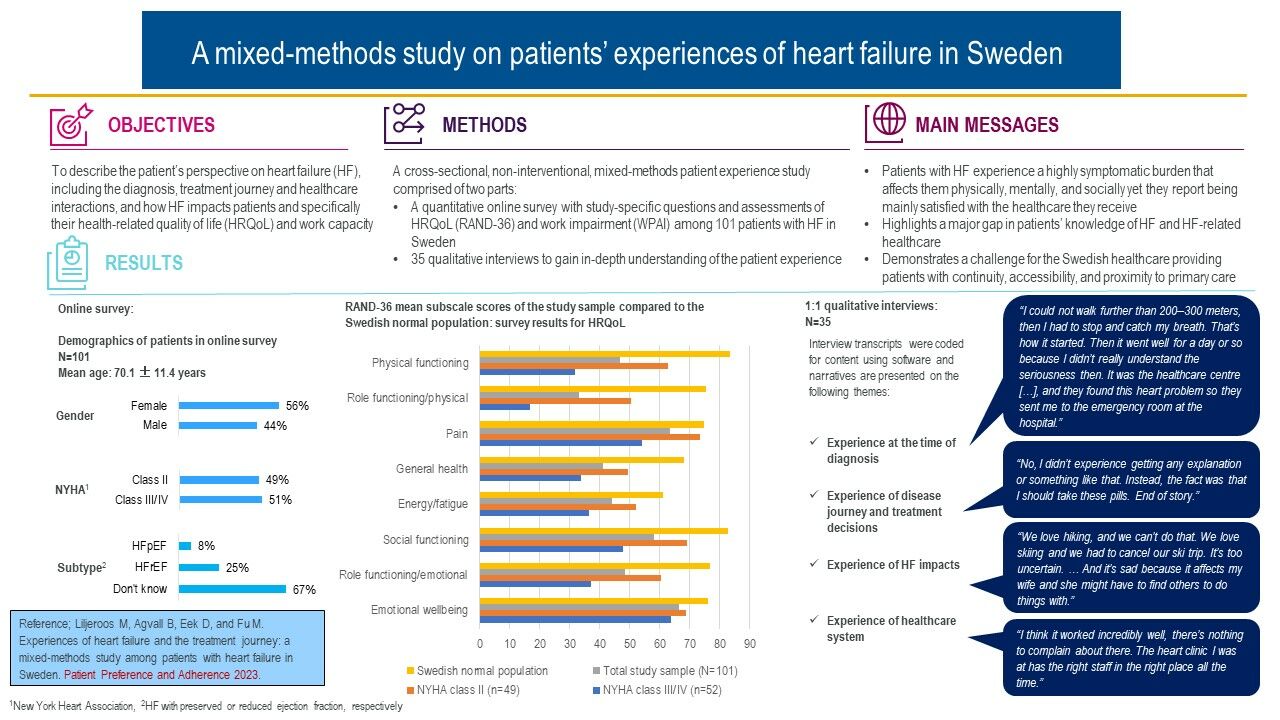
Purpose: Qualitative studies that highlight the patient perspective of heart failure (HF) and its impact on the lives of patients are limited. Our study objective was to describe the patient’s perspective on HF, including the diagnosis, treatment journey and healthcare interactions, and how HF impacts patients’ lives and specifically their health-related quality of life (HRQoL) and work capacity.
Patients and Methods: This cross-sectional, non-interventional, mixed-methods patient experience study comprised: (i) a quantitative online survey with study-specific questions and assessments of HRQoL and work impairment among 101 patients with HF in Sweden and (ii) 35 qualitative interviews to gain in-depth understanding of the patients’ experiences.
Results: Patients were found to experience a highly symptomatic and detrimental impact of HF on their HRQoL and work capacity. Fatigue was the most frequently reported symptom, and it was detrimental to all areas of patients’ lives limiting them mentally, socially, and physically. Two-thirds of patients were not aware of the type of HF they had, one-third did not check their body weight regularly, and around half did not increase their physical exercise as recommended by both guidelines and healthcare practitioners. Patients preferred specialist to primary care, desired greater access to healthcare, and continuity in whom they interact with in primary care.
Conclusion: Patients with HF experience a highly symptomatic burden that affects them physically, mentally, and socially. Our study highlights a major gap in patients’ knowledge about HF and HF-related healthcare. These results demonstrate a challenge for the Swedish healthcare system particularly as regards providing patients with continuity, accessibility, and proximity to primary care.
Keywords: chronic heart failure, health-related quality of life, patient-reported outcomes, qualitative interviews, patient experience
Graphical Abstract:
Plain Language Summary
Studies that investigate heart failure patients’ own perspective on their disease and the treatment journey are limited, especially in Scandinavia. This study tries to fill this gap in previous research. We wanted to understand how patients with heart failure are impacted by their disease, both in terms of how their quality of life is impacted, and how they perceive the healthcare they receive during their treatment journey.
We developed an online survey and recruited 101 Swedish patients to respond. In the survey, we asked questions about disease experiences, heart failure treatments, healthcare quality, quality of life and work capacity. We also invited 35 of the patients to individual in-depth interviews to understand their disease and treatment experiences even better.
Results showed that patients’ quality of life were severely impacted by their heart failure. Fatigue was the most frequently mentioned symptom, and patients described in detail how their experiences of feeling fatigued prevent them from engaging in activities that they before getting ill loved to do. Patients felt mentally, socially, and physically impacted by their heart failure symptoms, and we present several patient quotes that demonstrate how patients feel.
When asked about health care, patients preferred specialist to primary care and wanted greater access to healthcare and wished that they could see the same nurses and physicians in primary care instead of meeting new staff so frequently. Taken together, these results demonstrate challenges for the Swedish healthcare system.
Introduction
Heart failure (HF) is a life-threatening, chronic condition increasing in worldwide prevalence and causing high mortality, hospitalization, disability, and poor health-related quality of life (HRQoL).1 Despite improvements in HF medical treatment and standards of care as recommended by HF guidelines,2,3 poor HRQoL and increased healthcare utilization are frequent concerns among HF patients.4,5
Health-related quality of life reflects the multidimensional impact of a clinical condition and its treatment on the daily lives of patients.6 Patients with HF experience various physical and emotional symptoms, including dyspnea, fatigue, oedema, sleeping difficulties, depression, and chest pain.1 This symptomatology limits the daily physical and social activities of patients7,8 and markedly impairs their HRQoL compared to patients with other chronic diseases and the healthy population.9,10 Maintaining a good HRQoL is as important as survival to most patients living with chronic and progressive illnesses,11 and those with advanced HF have been found to be willing to trade survival time for improved symptom control and better HRQoL.12 Prognosis can be improved by patients implementing lifestyle changes, including weight control and increased physical exercise.13 Nevertheless, HRQoL is subjective and does not merely reflect objective clinical or physiological status, but a patient’s subjective perceptions of the impact of a clinical condition on their lives.14
Qualitative studies highlighting the patient perspective of HF and the treatment journey, and how these impact patients’ HRQoL and physical capacity are limited, especially in Scandinavia. For instance, in a recent systematic review of peer-reviewed qualitative research studies published in English between 2007 and 2020 on patients’ experiences of HF,15 of the 35 identified studies, only four came from Scandinavia, and none focused on the treatment journey and HRQoL. Our objective, therefore, was to increase the understanding of how HF and associated treatments are perceived from the patient’s perspective by investigating the patients’ experiences of their treatment journey and interactions with healthcare providers (HCPs), from diagnosis through subsequent disease stages. We used a mixed-methods study design to facilitate both a quantitative and a qualitative understanding of how HF impacts patients’ HRQoL and physical capacity.
Materials and Methods
Study Design and Participants
This cross-sectional, non-interventional, patient experience study adopted a quantitative online survey and subsequent qualitative in-depth interviews with a subset of survey respondents.
Patients in Sweden were recruited through advertisements in clinicians’ receptions, on Facebook, and in various magazines, including the member magazine of one of Sweden’s largest patient advocacy groups (PAGs). Adverts called for patients with heart failure to a study aiming at better understanding the experience of patients with heart failure in Sweden and provided potential respondents with a web link to general information about the study, informed consent, and eligibility criteria.
Eligible respondents had to declare an age ≥18 years, a HF diagnosis in New York Heart Association (NYHA) classes II‒IV, and that they were neither participating concurrently in a clinical trial nor had undergone major surgery during the last three months.
The survey unlocked for eligible and consenting patients, at the end of which patients were asked to indicate their willingness to participate in a follow-up interview for which they would be reimbursed with a €50 electronic gift card. No reimbursement was provided for completing the survey.
The study was approved by the Swedish Ethical Review Authority, Stockholm Medicine Department 4 (reference number 2021-01969).
Survey Structure
The survey, which launched in July 2021 and remained open for 20 weeks, used closed response options only and consisted of: 1) a study-specific questionnaire with 19 questions on demography, comorbidities, medications, symptoms, HF impact, patients’ diagnosis experiences, and HCP interactions; 2) the RAND 36-Item Health Survey 1.0 (RAND-36v1),16 a generic patient-reported outcome instrument (PRO) that measures HRQoL; and, 3) the Work Productivity and Activity Impairment questionnaire (WPAI),17 a generic PRO that measures disease impact on work and activity impairment.
Qualitative, In-Depth, One-to-One Interviews
To get a good representation of the survey respondents by the interviewed patients, patients that indicated their willingness to participate in the interviews were grouped according to their NYHA class (II/III/IV) and RAND-36 General Health score (Low/Medium/High within the study sample). Thirty-five patients were selected randomly based on the survey completion timestamp (every second, third or fourth patient was selected depending on the number of patients per profile).
Before being interviewed, patients had to provide formal proof of diagnosis and disease stage (NYHA class) by either HCP certification or a screenshot from their electronic health journal. Two interviewers (one male and one female) from IQVIA (third-party vendor), with good understanding of HF, trained in concept elicitation and in-depth interview techniques, conducted telephone interviews lasting approximately 60 minutes using a standardized, semi-structured discussion guide comprising open-ended and prompted questions to explore patients’ experiences with HF and its treatment. The following domains were covered in the discussion guide: diagnosis and disease progression (eg “Can you describe to me what the process of being diagnosed was like for you?” and “How do you feel your healthcare provider(s) managed the process of your diagnosis?”); disease journey and treatments (eg “Can you describe to me what happened to you in terms of your healthcare following the diagnosis?” and “What do you know about the available treatments for this disease?”); impacts (eg “Can you describe for me what life has been like with HF since your diagnosis?” and “What has been the most difficult thing for you to adapt to/change in relation to this disease?”); and, quality of care (eg “What did your healthcare provider discuss with you about the disease and how to manage it?” and “What changes would you make to improve the care you are receiving?”). Mock interviews were carried out prior to the study interviews for interviewers to familiarize themselves with the discussion guide and to ensure that all domains could be covered within a one-hour timeframe. Study interviews were audio-recorded and subsequently transcribed for coding and analysis.
Data Analysis
The RAND-36 and WPAI questionnaires (see Supplementary Methods) were scored according to their guidelines.16,17 Results for the eight RAND-36 subscales were compared to reference values from the general Swedish population.18 The minimal clinically important difference for the subscales ranges between 3 and 5 points.19
Verbatim interview transcripts were translated into English, and coded and analyzed using MaxQDA qualitative research software.20 Given the broad study objective of describing patients’ experiences of their disease and the treatment journey, an inductive thematic analysis approach was used where the goal of coding was to enable identification of the concepts (signs, symptoms, and HRQoL impacts) most important and relevant to patients for their experiences of heart failure and their treatment journey. Coders reviewed each transcript to identify text that included concept expressions and tagged selected text with a code. The codes were organized within a coding framework established at the start and refined/expanded during the coding process. Two coders were involved in coding the aggregate of transcripts, and inter-rater agreement was evaluated between coders to ensure coding consistency. Once the coding process was completed, outputs listing the number of occurrences of the codes were generated and served as the basis for both the results and thematic and contextual data analysis.21
Results
Demographics, Comorbidities and Current Treatments
In total, 435 potential patients accessed the link of whom 288 (66%) aborted without completing the screener, 46 (11%) were not eligible, and 101 (23%) completed the survey. Of the latter, 77 (76%) declared an interest in interview participation.
The mean age of the survey respondents was 70.1±11.4 years and 56% were female. Forty-nine percent and 51% were in NYHA classes II and III/IV, respectively, with only five patients in NYHA IV. The NYHA III/IV patients were slightly older and more were female. The majority (67%) did not know their HF type, 25% reported having HFrEF and 8% had HFpEF (HF with reduced or preserved ejection fraction, respectively) (Table 1).
 |
Table 1 Self-Reported Demography, Heart Failure Subgroup, Medical Treatments, Comorbidities, and NYHA Class Reported by Survey Respondents |
Hypertension (43%) and previous myocardial infarction (21%) were the most commonly self-reported comorbidities followed by diabetes (15%). Patients were well-treated according to previous guideline recommendations3 with beta-blockers (82%), angiotensin-converting-enzyme inhibitors (ACEI)/angiotensin-receptor-blockers (ARB)/angiotensin-receptor-neprilysin inhibitors (ARNI) (88%), mineralocorticoid-receptor antagonists (MRA) (48%), and diuretics (52%).
Experience of Heart Failure Symptoms
Fatigue was the most commonly reported listed symptom followed by dyspnea, swollen ankles and legs, palpitations, and chest pain. As expected, more symptoms were reported by NYHA III/IV than NYHA II patients. Patients who reported “no” or “very little” problems regarding dyspnea were predominantly in class NYHA II, whereas those with “moderate”, “severe” or “very severe” problems were mainly NYHA III/IV patients (Table 2).
 |
Table 2 Frequencies of Current, Main Heart Failure Symptoms and Severity of Shortness of Breath (Dyspnea) Experienced by Survey Respondents by Self-Reported Disease Stage |
Experience of the Healthcare System
Patients’ experiences of the healthcare system were studied in relation to both the time of diagnosis and under current treatment (see Supplementary Table 1). The majority of patients (80%) reported being diagnosed by a cardiologist and in hospital (79%) vs primary healthcare (21%). Similarly, almost all patients (97%) underwent electrocardiography and echocardiography (88%) diagnostic tests, although 45% were unaware if their natriuretic peptide levels had been evaluated. Before diagnosis, 50% of patients had experienced fatigue, 40% dyspnea, and 33% palpitations (Table 2).
Almost half of the patients (48%) underwent routine HF management at hospital, 37% in primary care, and 15% in a combination of these settings. The majority of patients (94%) clearly understood how to take their HF treatment as prescribed, and 92% claimed compliance. When asked if they thought their treatment was efficacious at relieving symptoms, only 7% reported no symptom relief at all, 21% reported being helped only a little bit, whereas the majority (73%) experienced moderate to significant symptom relief.
Experience of Lifestyle Changes to Cope with Heart Failure
As reported in Table 3, 13% of surveyed patients claimed no need to implement any lifestyle changes to cope with HF, while the remainder (87%) had adopted ≥1 of the listed lifestyle changes. The mean number of changes was 2.2 with two being adopted by over half of the patients (weight control [63%] and increased physical exercise [53%]). There were age differences in lifestyle change implementation: younger patients implemented more changes than older patients. The frequency at which patients checked their body weight varied greatly; across all NYHA classes and both age groups, there were patients who claimed to never check their weight and many who did so multiple times per week (Table 3).
 |
Table 3 Survey Respondents’ Adopted Lifestyle Changes to Adapt to Heart Failure by Self-Reported Disease Stage and Age |
Health-Related Quality of Life (RAND-36) and Work Productivity and Activity Impairment (WPAI)
Survey results for HRQoL are displayed by NYHA class and compared to the Swedish general population in Figure 1. The scores for all RAND-36 subscales for the total patient sample were much lower than reference values. Differences, ranging from 9.8 (emotional well-being) to 42.2 (role-functioning/physical), underscore the detrimental impact of HF on patients’ HRQoL. There were also large differences in the RAND-36 subscale scores between NYHA II and NYHA III/IV patients (Figure 1).
Among the survey population, 16% of patients were employed. They reported a 29% absenteeism due to HF over the last seven days. When at work, a 39% presenteeism due to HF was reported, rendering a total work productivity loss of 50%. Irrespective of employment status, all patients (n=101) responded to the degree to which their normal everyday activities were impaired by HF over the last seven days. On average, 54% of patients’ everyday activities were reported being missed due to HF, and the activity impairment increased with disease stage (38% in NYHA II and 69% in NYHA III/IV, respectively).
Qualitative Interviews
Nineteen (54%) of the 35 patients randomly selected for being interviewed were male and aged 30‒89 years. Fourteen (40%) patients were either by HCP certification or their electronic health journal confirmed being diagnosed with HF in NYHA class II, 20 (57%) in III, and one (3%) in IV. Eleven (31%) and 4 (11%) patients had HFrEF and HFpEF, respectively, while 20 (57%) were unaware of their HF type. Hypertension (n=10, 29%) and diabetes (n=5, 14%) were the most common comorbidities. Twenty-eight patients (80%) used beta-blockers, 22 (63%) diuretics, 29 (83%) ACEI/ARB/ARNI, 3 (9%) sodium-glucose-cotransporter-2 inhibitors (SGLT2i), and 20 (57%) MRA. Twenty-nine patients (83%) were retired or unemployed, 4 (11%) were on sick leave, and one (3%) was working. Fifteen patients (43%) lived with their spouse, 15 (43%) lived alone, and 5 (14%) lived with their family.
In the following, which includes only a few representative quotes describing patients’ experiences (see also Supplementary Table 2), we outline results from the interviews in relation to the domains covered within the discussion guide. Twenty-one patients contributed to the reported 37 quotes (1, 2, 3 and 4 quotes each from 12, 5, 1, and 3 patient(s)).
Experience at the Time of Diagnosis
Most patients had been diagnosed by cardiologists when seeking healthcare triggered by experiencing symptoms; very few were diagnosed during routine healthcare visits. Symptomatology prior to diagnosis was multifaceted, although fatigue, dyspnea, swollen legs, pain, and heart-related symptoms were common. A typical patient description was:
I could not walk further than 200‒300 meters, then I had to stop and catch my breath. That’s how it started. Then it went well for a day or so because I didn’t really understand the seriousness then. It was the healthcare center…[…]…, and they found this heart problem so they sent me to the emergency room at the hospital. (Male, NYHA II)
Electrocardiography and echocardiography were the most commonly used diagnostic tests followed by X-rays. Whereas one-third of patients were concerned about the diagnosis process, the majority were satisfied or very satisfied. Upon diagnosis, the most frequently evoked feelings were anxiety/worry and acceptance, as well as shock, sadness, and relief. Some patients felt numb by their lack of understanding of HF and its consequences.
Well, actually, I may not have understood what it meant. Not really. (Female, NYHA III)
Patients discussed their diagnosis more with family, friends, and co-workers than with HCPs, although those who did found discussions to be very supportive. Forty percent of patients were referred to a PAG; of these, one-third became members.
Experience of Disease Journey and Treatment Decisions
Sixteen patients (46%) discussed treatment options with their HCPs; most (n=19 [54%]) were given a prescription and informed about side effects. In the beginning of their treatment journey, very few mentioned having discussed the importance of weight control (n=5 [14%]) and fluid intake restrictions (n=4 [11%]) with their HCPs.
Most patients (n=20 [57%]) felt supported by their HCPs, 12 (34%) did not; these two groups differed in their level of satisfaction with information received and ease of contacting the HCP. The majority (n=27 [77%]) started treatment almost immediately post diagnosis and developed routines to follow complex treatment regimens. Almost all who were asked reported adhering strictly to the prescribed treatment, and the reason for being compliant was because they trusted their HCPs. Eighteen patients (51%) had been on treatment for 1‒5 years, 5 (14%) for 5‒10 years, and 5 (14%) for >10 years. The majority (n=19 [54%]) considered the information they received to be adequate, although 11 (31%) wished they had received more information about HF and its treatment.
No, there were no alternatives. I don’t want to whine about the care staff in any way, they’ve been great in every way. But when it comes to heart failure, I haven’t received any proper information… (Female, NYHA III)
Patients had a good understanding of their current treatments regarding rationale, purpose, and how to take them yet a very low understanding of alternative treatments. Twelve patients (34%) reported side effects (eg hypotension, frequent urination, swelling, reduced stamina, and tiredness). The majority (n=24 [69%]) felt better with treatment in terms of symptom relief.
Experience of Heart Failure Impacts
Patients spontaneously mentioned 21 unique HF impacts on their lives during the interviews: fatigue, changes in diet, walking, and physical exertion were separate impacts spontaneously mentioned by >10 patients. Fatigue was the key impact for almost all (n=28 [80%]) patients: it prevented them from doing activities, exercise, working or functioning socially; was constantly present every day; affected them both mentally and socially with a constant need to rest; and, worsened with disease progression.
Yes, I’m very tired. My ability to take initiative is bad and I don’t have any energy. …However, one does get depressed as well. …It impacts me to the extent that I lack initiative. That is, if I sit down on a chair, I can remain there for hours without doing anything. (Female, NYHA III)
During their treatment journey, HCPs advised patients to make lifestyle changes to live with HF, primarily weight control and dietary changes. Twenty-two patients (63%) reported trying to control their weight (eg by frequent weight monitoring) and half of the patients (n=17 [49%]) reported making dietary changes, eg eating more fresh fruits and vegetables, eating less red meat and/or more fish, and reducing carbohydrate, sugar, and salt intake.
Table 4 summarizes all impacts mentioned during the interviews.
 |
Table 4 Frequencies of Heart Failure Impacts on Patients’ Lives as Reported in Interviews |
Experience of the Healthcare System
The majority of patients (n=20 [57%]) received healthcare from cardiologists, but most saw multiple HCPs (eg both cardiologists and nurses). Nineteen patients (54%) saw primary care physicians and 6 (17%) regularly visited both specialists and primary care physicians. Patients tended to be more pleased with specialist than primary care, although the majority (n=26 [74%]) were pleased with their healthcare and HCP interactions in both settings. Staff continuity and proximity were crucial for patients’ healthcare satisfaction, driving the preference for specialist vs primary care; specialist care was perceived as superior with regards to continuity, accessibility, and knowledgeable staff.
…I think there’s a big shortcoming in this, that when you’re referred to your healthcare center again, nothing happens after that. (Male, NYHA III)
Many patients (n=26 [74%]) reported a moderate to strong understanding of HF, its management, and associated comorbidities primarily due to their HCPs; only 17% reported poor understanding due to receiving unsatisfactory information from HCPs. Patients used multiple sources to research HF, the Internet being their primary source, followed by PAGs and HCPs.
Notably, the majority of patients (74%) were pleased with the healthcare and disease information they received, and the HCP relationship, accessibility and continuity were vital for treatment satisfaction. The few patients who were dissatisfied (n=9 [26%]) complained of inaccessibility, healthcare discontinuity, and too brief visits to their physician with a handful of patients requiring more patient-friendly terminology from their physicians.
Discussion
Using this combined survey-deep interview approach, we found that patients experience a high symptom burden and detrimental impact of HF on HRQoL. Interestingly, two-thirds of patients were unaware of their HF type, one-third did not check body weight regularly, and about half still did not increase physical exercise levels despite national/global HF guidelines and HCP advice. Despite general satisfaction with their healthcare, patients desired greater HCP access and continuity in the HCP interactions.
Our sample was relatively representative despite comprising slightly younger patients (70.1±11.4) and a greater number of females (56%) than in a recently published study of a real-world hospital cohort (mean age 77.5±12.6, 45% females).22 Our sample was, however, representative of contemporary treatment (beta-blockers [82%], ACEI/ARB/ARNI [88%] and MRA [48%] vs 88% beta-blockers, 76% ACEI/ARB/ARNI and 48% MRA in the real-world hospital cohort, respectively), although we observed less treatment with diuretics (52% vs 80%). Very few patients were treated with SGLT2i, probably because these were added to guidelines23 whilst the survey was open and received reimbursement in Sweden after the survey had closed. Both the younger age of our sample and the shift to a less symptomatic population may have been influenced by the first, Internet-based part of our study that required patients to have Internet access and the ability to work online.
Patients experienced a high symptomatic burden, particularly fatigue, dyspnea, swollen ankles and legs, and palpitations causing a severe, detrimental impact on their HRQoL, work productivity, and daily activities. Fatigue was the most troublesome as it was constant and affected almost all patients mentally, physically, and socially, a striking finding since most patients were treated according to previous guideline recommendations.3
Possible explanations are limited lifestyle changes and self-care: 13% of patients claimed no need for any lifestyle changes to cope with HF, and only about half checked their body weight and increased their physical exercise. Since non-pharmacological interventions are important for symptomatic relief, especially regarding HRQoL and physical capacity, poor or inadequate self-care and other, non-pharmacological measures may contribute to inadequate symptomatic relief and poor HRQoL despite optimal treatment according to previous guidelines.3
Our data highlight a major gap in patients’ knowledge about HF and HF-associated comorbidities. Almost two-thirds of patients were unaware their HF type and only 25% and 8% reported having HFrEF or HFpEF, respectively, a huge knowledge gap compared to the real-world HF cohort, where 41% and 40% of patients reported having HFrEF and HFpEF, respectively.22 Moreover, patients reported almost all comorbidities less frequently than in the real-world setting,4 including hypertension (43% vs 59%), previous myocardial infarction (21% vs 39%), diabetes (15% vs 34%), and chronic kidney disease (4% vs 46%).
Our finding of a limited patient knowledge reflects that of a previous study where 44% of patients were unsure as to whether they had HF,24 highlighting that HF patients do indeed lack knowledge about their condition and consequently, have a limited understanding of HF. This warrants greater and better-quality information and patient education to increase awareness of HF, particularly regarding a patient’s self-care and other non-pharmacological interventions since only about half of our patients paid regular attention to these healthcare factors.
The service level of the healthcare system may also contribute to the demonstrated gap in patient knowledge about HF and HF-related comorbidities. It should not be assumed that HF patients necessarily understand complex disease information correctly since many are elderly, fragile, or may even have difficulties reading any information they do receive. Therefore, HCPs have an important responsibility to use patient-friendly terminology to ensure that patients are well-informed, and to consider repeated information or teach-back strategies.25
Few patients mentioned having discussed the importance of weight control and water intake restrictions with their HCPs early in their treatment journey. Although not all patients were probed about this, it is surprising that these common measures of self-care were not spontaneously mentioned by more patients. Fewer than half of the patients enrolled discussed treatment options with their HCPs (most received a prescription and information about side effects) and one-third wished they had received more information about HF and treatment strategies. Although the majority were pleased with their healthcare, patients desired better HCP continuity, accessibility, and proximity, all of which were vital for treatment satisfaction and thus driving patient preference for specialist rather than primary care because the former was perceived to be superior in continuity, accessibility, and knowledgeable staff. These patient experiences, concerns, and wishes reflect shortcomings in the current Swedish healthcare system and constitute a crucial challenge, namely how to enhance accessibility, continuity and the competence in primary healthcare for HF patients. Referring stable HF patients to primary care for ongoing, guideline-driven treatment and follow-up is both advantageous and cost-effective.26,27 Thus, good accessibility and continuity of primary care with competent staff are fundamental success factors in aiding patient adherence to guidelines and recommended strategies, both pharmacological and non-pharmacological.26
In terms of study strengths, our study was conducted by a third-party vendor, which minimizes patient inclusion bias. The mixed-methods approach allowed collection of both quantitative and qualitative data on HRQoL, which have been lacking in previous studies. Compared to the Swedish general population, our sample’s HRQoL scores were dramatically lower on all RAND-36 subscales, and the interview data allowed a qualitative understanding of how HRQoL is impacted by HF. The study also contributes to the existing knowledge by illuminating the patient perspective on their treatment journey and not only on HF. In terms of limitations, the relatively moderate sample size and the requirement for Internet access/usage skills may limit generalization of our findings. Despite targeting the real-world setting, subjects who were older, more extremely symptomatic and/or with limited Internet access/skills may not have been enrolled. Another limitation is that the diagnosis was not confirmed but self-reported for survey respondents. Still, there were no external incentives to participate in the survey, why we do not believe that any respondents participated for other reasons than reporting on their HF. Monetary reimbursement was only provided for the subsequent interview, for which patients’ diagnoses were confirmed by patients’ HCP or electronic medical records. With no exceptions, all patients that were invited to the interview managed to prove their diagnosis.
Conclusion
Patients with HF in Sweden experience a high symptomatic burden that affects them physically, mentally, and socially. There is also a major patient knowledge gap about HF and HF-related healthcare that challenges the healthcare system, particularly in its provision of the necessary continuity, accessibility, and proximity of primary care for the HF patient.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Informed Consent
The study was approved by the Swedish Ethical Review Authority, Stockholm Medicine Department 4 (reference number 2021-01969) and complies with the Declaration of Helsinki. All eligible subjects provided their written consent to participate in the survey and/or the follow-up interview. Participants’ informed consent included publication of anonymized responses.
Acknowledgments
The authors are grateful to Helena Goike and Susanna Jerström at AstraZeneca for logistical support and valuable comments on the manuscript; to Åsa Lindelöf and Joacim Folkesson at IQVIA for performing the interviews; to Anjaly Maria Jose and Nidhi Saiwal at IQVIA for coding the interview data; to Kimberly Kelly at IQVIA for leading the coding of the qualitative data team and conducting the analysis and synthesis of these data; and to Obinna Onwude at IQVIA for analyzing the survey data. Dr Grażyna Söderbom at Klipspringer AB is acknowledged for editorial support funded by AstraZeneca.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by AstraZeneca, which sponsored the study and editorial support.
Disclosure
ML has received personal fees from AstraZeneca, Novartis and Boehringer Ingelheim. BA has received personal fees from AstraZeneca and Boehringer Ingelheim. DE is a full-time employee of the study sponsor AstraZeneca and owns stocks in the company. MF has received grants and personal fees from AstraZeneca, Novartis Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Vifor-Fresenius. The authors report no other conflicts of interest in this work.
References
1. Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev. 2017;22:
2. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:
3. Ponikowski P, Voors AA, Anker SD, et al; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:
4. Yasin ZM, Anderson PD, Lingman M, et al. Receiving care according to national heart failure guidelines is associated with lower total costs: an observational study in Region Halland, Sweden. Eur Heart J Qual Care Clin Outcomes. 2021;7:
5. Ødegaard KM, Hallén J, Lirhus SS, Melberg HO, Halvorsen S. Incidence, prevalence, and mortality of heart failure: a nationwide registry study from 2013 to 2016. ESC Heart Fail. 2020;7:1917–1926. doi:10.1002/ehf2.12773
6. Westlake C, Dracup K, Creaser J, et al. Correlates of health-related quality of life in patients with heart failure. Heart Lung. 2002;31:
7. Boyd KJ, Murray SA, Kendall M, Worth A, Frederick Benton T, Clausen H. Living with advanced heart failure: a prospective, community based study of patients and their carers. Eur J Heart Fail. 2004;6:
8. Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:
9. Juenger J. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):
10. van Jaarsveld CH, Sanderman R, Miedema I, Ranchor AV, Kempen GI. Changes in health-related quality of life in older patients with acute myocardial infarction or congestive heart failure: a prospective study. J Am Geriatr Soc. 2001;49:
11. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:
12. Stevenson LW, Hellkamp AS, Leier CV, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:
13. Jonkman NH, Westland H, Groenwold RH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail. 2016;22:
14. Freedland KE, Rich MW, Carney RM. Improving quality of life in heart failure. Curr Cardiol Rep. 2021;23:159. doi:10.1007/s11886-021-01588-y
15. Austin RC, Schoonhoven L, Clancy M, Richardson A, Kalra PR, May CR. Do chronic heart failure symptoms interact with burden of treatment? Qualitative literature systematic review. BMJ Open. 2021;11:e047060. doi:10.1136/bmjopen-2020-047060
16. RAND. 36-item short form survey instrument (SF-36); 2022. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html.
17. WPAI coding 2019; Margaret Reilly Associates Inc. Available from: http://www.reillyassociates.net/WPAI_Coding.html.
18. Ohlsson-Nevo E, Hiyoshi A, Norén P, Möller M, Karlsson J. The Swedish RAND-36: psychometric characteristics and reference data from the Mid-Swed Health Survey. J Patient Rep Outcomes. 2021;5:66. doi:10.1186/s41687-021-00331-z
19. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:
20. Rädiker S, Kuckartz U. Focused analysis of qualitative interviews with MAXQDA. Step by step. Berlin: MAXQDA Press; 2020. Available from: https://www.maxqda-press.com/catalog/books/focused-analysis-of-qualitative-interviews-with-maxqda.
21. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:
22. Wideqvist M, Cui X, Magnusson C, Schaufelberger M, Fu M. Hospital readmissions of patients with heart failure from real world: timing and associated risk factors. ESC Heart Fail. 2021;8:
23. McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599. doi:10.1093/eurheartj/ehab368
24. Kobayashi M, Wilcke C, Girerd N. Assessment of patient knowledge, awareness, and adherence in heart failure in a real-life setting: insights from data acquired in pharmacies. J Clin Med. 2022;11:863. doi:10.3390/jcm11030863
25. Centrella-Nigro AM, Alexander C. Using the teach-back method in patient education to improve patient satisfaction. J Contin Educ Nurs. 2017;48:
26. Luttik ML, Jaarsma T, van Geel PP, et al. Long-term follow-up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH-2 study. Eur J Heart Fail. 2014;16:
27. Schou M, Gustafsson F, Videbaek L, et al; NorthStar Investigators, all members of The Danish Heart Failure Clinics Network. Extended heart failure clinic follow-up in low-risk patients: a randomized clinical trial (NorthStar). Eur Heart J. 2013;34:
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Assessing the Validity of the Long-Term Conditions Questionnaire (LTCQ) in Women During Pregnancy and the First Year Following Birth
Kelly L, Fitzpatrick R, Kurinczuk JJ, Rivero-Arias O, Alderdice F
Patient Related Outcome Measures 2022, 13:221-228
Published Date: 19 October 2022
Impact of Belimumab on Patient-Reported Outcomes in Systemic Lupus Erythematosus: Insights from Clinical Trials and Real-World Evidence
Gomez A, Enman Y, Parodis I
Patient Related Outcome Measures 2023, 14:1-13
Published Date: 19 January 2023

