Back to Journals » Journal of Pain Research » Volume 15
A Bibliometric and Knowledge Map Analysis of Osteoarthritis Signaling Pathways from 2012 to 2022
Received 15 August 2022
Accepted for publication 17 November 2022
Published 6 December 2022 Volume 2022:15 Pages 3833—3846
DOI https://doi.org/10.2147/JPR.S385482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Baijun Li,1 Jie Zheng2
1Institution of Acupuncture-moxibustion and Massage, Shaanxi University of Chinese Medicine, Shaanxi, People’s Republic of China; 2Shaanxi Key Laboratory of Acupuncture and Herbal Medicine, Shaanxi, People’s Republic of China
Correspondence: Jie Zheng, Institution of Acupuncture-moxibustion and Massage, Shaanxi University of Chinese Medicine, Shaanxi, 712046, People’s Republic of China, Tel +86 138 9298 0566, Email [email protected]
Background: Osteoarthritis(OA) is one of the most common joint diseases, and signaling pathways play an essential role in the occurrence and development of OA, so it is significant to study OA with signaling pathways as an entry point.
Purpose: This study aims to visualize and map the knowledge of OA-related signaling pathway research between 2012 and 2022, summarise and analyze the current research status and potential development trends in the domain, and provide a reference for future OA-related research.
Methods: Retrieve relevant literature from the Web of Science database and use VOSviwer and CiteSpace software to visualize authors, institutions, country distribution, references, and keywords. The results are interpreted and analyzed in conjunction with the results obtained.
Results: According to the search strategy, a total of 4894 articles were published between January 2012 and January 2022; during these ten years, the number of reports increased annually, and the research became further intensive; through this analysis, it was found that China is the most prolific country in this field; The institution with the most articles was Xi’an Jiaotong University from China, and the most prolific author was Tang Chih Hsin; Among the cited references, the reports of Glyn-Jones S and Hunter DJ were ranked first and second respectively. In the keyword analysis, cartilage and expression were the popular keywords; Animal model, akt, and platelet-rich plasma had the highest centrality; Burst analysis revealed pi3k, senescence, Ampk, and exosomes had received more attention in recent years of research.
Conclusion: This study analyzes and summarizes the current research status and development trend of relevant signaling pathways in OA from the perspective of bibliometric and visual analysis, which can help researchers to keep track of hot topics and conduct more in-depth exploration of research hotspots and frontier knowledge areas.
Keywords: osteoarthritis, signaling pathway, inflammation, articular cartilage, mechanism, CiteSpace, VOSviwer
Introduction
Osteoarthritis (OA) is the most common chronic bone and joint disease worldwide, with approximately 10% of the global population suffering from OA.1 The pathogenesis of OA is closely related to age, and of the 240 million people with OA, about 18% of patients over 60 years of age are women, and about 10% are men.2 The pathological mechanism of OA is still highly uncertain, and joint swelling, stiffness, and recurrent pain seriously affect patients’ daily activities.3,4 In the advanced stages of OA, patients will face irreversible damage to the bone and joints; this ultimately leads to serious consequences of disability and a consequent decline in work and quality of life, causing economic pressure on society and financial and psychological stress on patients and their families.5,6 Scholars have conducted numerous studies on the pathogenesis and risk factors of OA and proposed it is associated with gender, age, metabolic disorders, strain, and genetics; With gender-related differences in prevalence linked to sex hormones, estrogen by interfering with the transformation between cartilage and bone, and smaller cartilage volumes in women compared to men;7,8 With aging problems such as loss of joint cartilage, more laxity in joints, and abnormal stresses on the joints leading to abnormal loading increase the prevalence of the disease in post-menopausal women.9 Obesity influences joint bones, cartilage, and synovial membranes by increasing the mechanical load on the joints and by the secretion of adipokines and inflammatory factors from adipose tissue.10 Damage and overuse can cause cartilage deterioration and biomechanical changes.11 While Genetic factors leading to OA may be associated with altered gene expression in cartilage tissue.12 These factors usually affect tissues such as bone, articular cartilage, ligaments, synovium, and muscle and activate related signaling pathways such as nuclear factor-κB (NF-κB) signaling pathway, nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway,13 and Wnt signaling pathway.14 These signaling pathways regulate cell proliferation and apoptosis and promote the release of inflammatory mediators, causing the breakdown of articular cartilage, degeneration, subchondral osteosclerosis, synovitis, bone redundancy,15,16 and other pathological changes, leading to the occurrence and development of OA. As a complex disease with multiple tissues and signaling pathways acting together in the whole joint,17 it is necessary to explore and study the molecular mechanisms and signaling pathways in depth, which is conducive to a comprehensive understanding of the pathogenesis of OA and provides new reference ideas for the treatment and prevention of OA.
CiteSpace and VOSviewer are commonly used bibliometric software in the medical field. Using bibliometric methods for qualitative and quantitative analysis of research literature in a specific field can reveal more clearly the knowledge structure and trends of research in the area.18 VOSviewers clustered topics in different colors to construct a more intuitive collaborative network graph,19 The country, institution, and author collaboration network maps in the CiteSpace and VOSviewers software provide a visual representation of their collaborative relationships and geographical distribution. Their publication quantity and centrality results help to identify influential countries, institutions, and authors over time, as well as the state of disciplinary development in the field, helping researchers to find collaborators and institutions with similar research interests and speeding up the flow of knowledge. The analysis of cited references helps to filter out the research directions of influential articles in the relevant field, which is useful in identifying popular research areas and exploring potential trends. CiteSpace’s co-occurrence, clustering, and burst detection of keywords can analyze current hot spots and predict cutting-edge trends.20,21
Many researchers have investigated the OA signaling pathway, but there is a lack of bibliometric analysis of OA signaling pathway studies; Therefore, this study is the first bibliometric analysis in this area, with the aim of providing relevant researchers with a completely new perspective in order to obtain essential information.
Methods
Data Source and Retrieval Method
The data for this study were obtained from the Web Of Science (WoS) core collection. The search terms were “osteoarthritis” and “signaling pathways”; the type of literature was “article” and “review”; the publication time frame was set from 2012 to 2022; the language was restricted to English. The retrieved documents are exported from WoS in plain text format with a selection of full-text records and cited references.
Analysis Tool and Parameter Settings
We use Microsoft Office Excel software to graph trends in the number of articles issued and to summarize related data. VOSviewer 1.6.18 maps the author, institution, and country collaboration network with the minimum number of documents of an author set to 10, the country analysis set to 5, and institution analysis set to 10, in addition to the ranking of country publications via Scimago Graphica software. CiteSpace 5.8 R3 software to analyze the keywords and references, CiteSpace-related parameter settings: Year Per Slice set to 1, g-index: k=25, Top N=50, Pruning method was Pathfinder, selecting log-likelihood ratio(LLR) algorithm labels for keyword clustering.
Results
Search Results
The retrieval method is described in Table 1. The search results in 109,447 articles on the topic of OA and 2,452,024 articles related to signaling pathways. by combining these two groups of terms, the final result was 4894 articles. After the selected documents were de-duplicated using the “Remove Duplicates” function in CiteSpace software, 4893 articles were finally included in this analysis.
 |
Table 1 Retrieval Method |
Analysis of Annual Publication and Citations
The annual trend of publications and their citations on OA signaling pathway research is shown in Figure 1, with a steady increase in the number of publications and citations; from 217 in 2012 to a peak of 874 in 2021, citations also increased explosively from 156 in 2012 to 26,720 in 2021, indicating that signaling pathway research is accelerating in the OA field and receiving sustained attention from researchers.
 |
Figure 1 Annual publication and citation trends for OA signaling pathway research. |
Analysis of Country Distribution
Eighty-two countries have researched this field, of which 47 have published more than five papers. Figure 2 shows the visual network mapping of country publications and citations constructed based on VOSviewer data using Scimago Graphica software. Figure 3 presents a map of the country’s cooperation network using VOSviewer software. Table 2 provides the top 10 countries in terms of publications and citations, with China (n=2376) being the country with the most publications, followed by the United States (n=1019), the United Kingdom (n=341), South Korea (n=215), and Japan (n=214), with the United States ranking second in terms of publications but with the highest number of citations. These three countries have formed close cooperation with other countries with high publication volumes, which has played an essential role in promoting the development of research in this field and has shown that cooperation is conducive to academic output.
 |
Table 2 Top 10 Countries with the Most Published Articles and Citations |
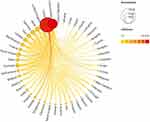 |
Figure 2 National publication quantity and citation visualization network mapping for OA signaling pathway research. |
 |
Figure 3 Map of national collaborative network for OA signaling pathway research. |
Analysis of Research Institutions
Among the institutions involved in OA signaling pathway research, 227 have more than ten publications. The VOSviewer software was used to construct an optical network for these institutions that reached the threshold (Figure 4). Table 3, and Table 4 show the top 10 institutions regarding the number of publications and citations. The institutions with the highest number of publications were mainly from China, with Xi’an Jiaotong University having the highest number of publications (n=125), followed by Shanghai Jiaotong University (n=120) and Zhejiang University (n=119); Rush University had the highest frequency of citations (n=3488), followed by Shanghai Jiaotong University (n=2806), and Oxford University (n=2347). The high ranking of these institutions in the number of publications and frequency of citations is sufficient to illustrate that their research results greatly influence the field.
 |
Table 3 Top 10 Institutions for OA Signaling Pathway Research in Terms of the Number of Publications |
 |
Table 4 Top 10 Institutions for OA Signaling Pathway Research in Terms of the Number of Citations |
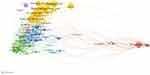 |
Figure 4 A collaborative network of publishing institutions for OA signaling pathway research. |
Analysis of Authors
The author collaboration network visualization in VOSviewer can identify the core authors in the field and visually show the collaborative relationships among authors. Setting the minimum posting threshold to 10, a total of 122 authors meet the criteria. Table 5 lists the top 10 authors in terms of the number of publications and citations, and Figure 5 shows the collaboration network of the 122 authors. Tang Chih Hsin (n=47) is the author with the most publications, while Guo Xiong (n=37) and Wang Wei (n=35) ranked second and third, respectively. Among Citations, Chen Di (n=1333), Martel Pelletier, Johanne (n=1085), and Pelletier Jean Pierre (n=1085) have the highest frequency of citations. However, the number of their publications is not in the leading position, their articles are heavily cited, and these data show that their articles have a significant influence and outstanding contribution to the field.
 |
Table 5 Top 10 Authors in Terms of the Number of Articles and Citations for OA Signaling Pathways |
 |
Figure 5 A collaborative network of publishing authors for OA signaling pathway research. |
Analysis of Cited Reference
Figure 6 shows the visual network diagram of cited references drawn by CiteSpace software. Table 6 shows the top 10 cited references, Glyn-Jones S’s article being the most cited, which systematically summarizes the mechanisms of cartilage, synovial and inflammatory factors in the pathogenesis of OA;22 Hunter DJ’s article also summarizes OA pain mechanisms and surgical therapies;23 Robinson WH ranked third, and his article mentioned that Low-grade inflammation is a non-negligible factor in OA and can activate signaling pathways such as JAK-STAT as well as NF-KB leading to the development and progression of OA.24 The references in Table 5 are mainly reviews, and the first and second-ranked articles were published in the prestigious Lancet journal. Analysis of the other highly cited references in the table reveals that the study of signaling pathways in OA inflammation mechanisms is an important focus.
 |
Table 6 Top 10 Cited References for OA Signaling Pathway Research |
 |
Figure 6 Cited reference visualization network diagram for OA signaling pathway research. |
Analysis of Keywords
Keywords Co-Occurrence
Keywords are a high summary of the research content of an article, and counting the frequently used keywords helps to discover the research hotspots in the field. In contrast, the high-centered keywords have a pivotal connecting role and can usually be regarded as the critical turning point of the research direction in the field.25 We used CiteSpace software for co-occurrence analysis of keywords and obtained a visualization map (Figure 7); Table 7 shows the frequency and centrality ranking of keywords, where cartilage, expression, and OA were the keywords with the highest number of occurrences and were popular studies in the field. The animal model (0.26), akt (0.16), and platelet-rich plasma (0.15) have high centrality and are key connection points for the shift of OA signaling pathway research hotspots.
 |
Table 7 Top 10 Keywords Used in the Study for OA Signaling Pathways in Terms of Frequency and Centrality |
 |
Figure 7 Keyword co-occurrence network mapping for OA signaling pathway research. |
Keywords Cluster
The clustering was performed using the log-likelihood ratio (LLR) algorithm based on the keyword’s co-occurrence network. In the cluster analysis, when Modularity Q > 0.3, Weighted Mean Silhouette S > 0.5 indicates that the clustering is credible and compelling. In this study, we obtained a total of 20 clusters (Figure 8), Q=0.7722, S=0.9146, in which #0akt, #1wnt, #2nuclear factor kappa b, #15mapk, #13long non-coding RNA, #16microrna, #17dna, #9nerve growth factor, #10matrix metalloproteinase 13 are relevant factors and signaling pathways involved in pathological changes in OA. #3bone, #8rheumatoid arthritis, #14hip, #19bone marrow are studies of the locus of OA and similar diseases; #5progression, #6apoptosis, #7oxidative stress, #12inhibition, #18pain are studies of the mechanism of OA; #4older adult, #11prevalence, are studies of the population and incidence of OA.
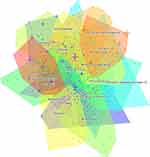 |
Figure 8 Visual mapping of keyword clustering for OA signaling pathway research. |
Burst Keywords
CiteSpace’s keyword burst detection identifies keywords that are suddenly being used a lot at a stage, and to some extent, it can indicate a shift in research frontiers as well as research hotspots. Figure 9 shows the top 20 ranked keywords in terms of burst strength. In the graph, Begin and End indicate the start and end time of the emergence, and the higher the Strength value suggests, the more significant the influence of the emergent term; blue indicates the research cycle of the emergent keyword, while red is the burst cycle. Human articular chondrocyte (15.7), rheumatoid arthritis (14.65), and osteoarthritic cartilage (9.12) are the top three keywords for Strength intensity, and human articular chondrocyte, osteoarthritic cartilage bursts lasted for a more extended period from 2012 to 2016. It indicates that articular cartilage is an essential carrier of signaling pathways in OA, and researchers have conducted a series of studies on signaling pathways around cartilage tissue.PI3K, senescence, AMPK, and exosomes are popular keywords that continue to this day and have excellent research potential in this field.
 |
Figure 9 Visual mapping of the burst keywords for OA signaling pathway research. |
Discussion
Basic Information About the Research
The current study is the first to summarize and analyze the research on the OA signaling pathway through a new perspective of bibliometrics. In the trend graph of 4894 annual publications and citations, The year-by-year increasing trend indicates that it has become a trend to use signaling pathways as the entry point to explore the pathogenesis and treatment of OA.
A total of 82 countries or regions have published papers on this occasion, with closer links between the cooperation of countries. China conducted much research on OA signaling pathways and was the country with the most significant number of publications, which may be associated with the relatively large population and high prevalence in China. The number of citations of the US articles is higher than that of the Chinese, which means that their research results are highly recognized and convincing in the field.
The main force of institutional publications are universities and their affiliated hospitals, there are more inter-institutional collaborations, among which Xi`an Jiao Tong University and Shanghai Jiao Tong University in China publish the most articles, and Rush University ranks first in terms of citation frequency. However, the number of literature is not as high as the top two, indicating that Rush University’s research results are widely recognized and have a significant reference value.
In the analysis of author postings, the author with the most postings was Tang Chih-Hsin, and there is close teamwork between authors, but there is a lack of collaborative communication between author teams, which should be strengthened in the future.
Research Hotspots and Trends
The current cited references analysis found that highly cited references are mainly reviews, lacking high-impact clinical validation and evidence of efficacy. From the first ten highly cited references, it can be seen that inflammatory mechanisms are an essential concern in OA, OA involves complex regulatory mechanisms of inflammatory signaling pathways, and Single signaling pathways often do not clearly define their pathogenesis; therefore, the study of the mechanisms of inflammatory signaling pathways in OA tissues is a popular trend and still needs to be explored in further depth.
The keyword co-occurrence analysis showed that cartilage is the critical research organization of the OA signaling pathway, which is a crucial component of the joint, and cartilage damage can often be considered a symbol of OA, chondrocyte apoptosis, autophagy, and abnormal expression of inflammatory factors in cartilage can activate relevant signaling pathways to accelerate OA development and progression.26 In addition, there is a growing interest in studying signaling pathways in bone, bone marrow, synovium, and other tissues. Signaling pathways in the hip and knee are more generally studied. Animal models and akt platelet-rich plasma were the top three keywords for centrality; Akt, wnt, and nuclear factor kappa b (NF-κB) are the first three larger clusters in the cluster analysis; PI3K, senescence, ampk, and exosomes as emergent terms in the recent two years have excellent research potential in this field. The research on OA signaling pathways is biased toward animal studies indicating that the relevant mechanism research is still in the exploration stage. Akt is an essential messenger in the PI3K/AKT/mTOR signaling pathway, which regulates the development of OA by mediating the inflammatory response, cell proliferation, and autophagy;27,28 PI3K, as a signaling protein with catalytic activity, regulates chondrocyte injury and autophagy by activating the downstream effector molecule Akt;26 Platelet-rich plasma contains autologous growth factors that promote cell proliferation and differentiation29 and is effective in the recovery of skeletal and muscular diseases, especially cartilage tissue;30 Wnt is an extracellularly secreted glycoprotein, and wnt has a role in promoting osteogenic tissue formation and regulates OA by acting alone or in combination with other signaling pathways.31,32 The NF-κB pathway is a vital regulator of the catabolic effects of inflammatory factors and affects OA chondrocyte synthesis and metabolism by regulating the expression of matrix-degrading enzymes.33,34 AMP-activated protein kinase (AMPK) is involved in the regulation of cellular energy metabolism, aging, inflammation, and mechanical damage inhibits AMPK activity, causing a disruption in the homeostasis of cartilage, subchondral bone, and synovium, triggering synovial degeneration, subchondral bone remodeling, and synovial inflammation,35,36 AMPK signaling pathway also interacts with NF-KB, mTOR, PI3K-Akt, and other signaling pathways to promote the development of OA.37 Senescence is an important influencing factor for the occurrence of OA,38 studies have demonstrated that oxidative stress and mitochondrial dysfunction lead to cell signaling malfunction and accelerate cellular senescence,39 while those senescent cells that lose their dividing function and cannot be cleared in time can disrupt joint homeostasis and prevent normal joint function.40,41 Exosomes are molecular transduction carriers from chondrocytes, synovial fluid, or other tissues that have important regulatory roles in the pathophysiology of OA.42 It has been demonstrated that exosomes secreted by MSCs may have a repairing effect on OA through signaling pathways such as AKT, ERK, and AMPK, and there are now many biological techniques such as 3D biomaterials and Nanoparticles that use exosomes for repairing OA cartilage.25,43
Limitations
Although a detailed search strategy was formulated, it was impossible to ensure that each article was relevant to the specified topic because of the large volume of data and the inability to check and filter each document; In addition, we only searched one database, WOSCC, which may lead to the inclusion of literature data is not very comprehensive, but this database is an internationally recognized knowledge database with significant influence and authority, it contains relatively complete literature in the field of medicine, in total this analysis can describe the current status of OA signaling pathway research and research trends.
Conclusion
In summary, this study provides new insights and valuable information on the OA signaling pathway, the number of publications has been increasing over the past decade as OA signaling pathway mechanisms are not fully defined, and OA signaling pathway exploration continues in-depth. Collaboration among authors’ teams and between research institutions still needs to be strengthened; In terms of research trends, the study of signaling pathways in the joint tissues, especially cartilage tissue, under inflammatory stimulation is still the main focus of research in this field, in addition, PI3K, Wnt, Akt, platelet-rich plasma, ampk, nuclear factor kappa B, exosomes in OA signaling pathway research is the popular and emerging research, in the future, the mechanism of aging, inflammation, autophagy under the action of related signaling pathways can be explored in depth, to find more effective prevention and treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China Project (No.81804162), The General Project of the Key R&D Program of Shaanxi Provincial Department of Science and Technology (2022SF-184), Research Project of the Young Innovation Team of Shaanxi Provincial Department of Education (21JP034).
Disclosure
The authors declare that they have no financial interest or other potential conflicts of interest in this research work.
References
1. Grassel S, Zaucke F, Madry H. Osteoarthritis: novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J Clin Med. 2021;10(9):48. doi:10.3390/jcm10091938
2. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–325. doi:10.1016/j.joca.2017.11.014
3. Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: a Review. JAMA. 2021;325(6):568–578. doi:10.1001/jama.2020.22171
4. Zhang P, Li K, Kamali A, et al. Small molecules of herbal origin for osteoarthritis treatment: in vitro and in vivo evidence. Arthritis Res Ther. 2022;24(1):105. doi:10.1186/s13075-022-02785-y
5. Mu P, Feng J, Hu Y, Xiong F, Ma X, Tian L. Botanical Drug Extracts Combined With Biomaterial Carriers for Osteoarthritis Cartilage Degeneration Treatment: a Review of 10 Years of Research. Front Pharmacol. 2021;12:789311. doi:10.3389/fphar.2021.789311
6. Bernetti A, Mangone M, Paolucci T, et al. Evaluation of the efficacy of intra-articular injective treatment with reticular hyaluronic acid (Mo.Re. Technology) in amateur athletes with over-use gonarthrosis. Med Sport. 2020;73:127–139. doi:10.23736/S0025-7826.20.03648-0
7. Xu J, Yan Z, Wu G, Zheng Y, Liao X, Zou F. Identification of key genes and pathways associated with sex difference in osteoarthritis based on bioinformatics analysis. J Musculoskelet Neuronal Interact. 2022;22(3):393–400.
8. Maleki-Fischbach M, Jordan JM. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12(4):212. doi:10.1186/ar3091
9. Chaganti RK, Lane NE. Risk factors for incident osteoarthritis of the Hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99–104. doi:10.1007/s12178-011-9088-5
10. Chang J, Liao Z, Lu M, Meng T, Han W, Ding C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthritis Cartilage. 2018;26(7):864–871. doi:10.1016/j.joca.2018.03.004
11. Bernetti A, Agostini F, Alviti F, et al. New Viscoelastic Hydrogel Hymovis MO.RE. Single Intra-articular Injection for the Treatment of Knee Osteoarthritis in Sportsmen: safety and Efficacy Study Results. Front Pharmacol. 2021;12:673988. doi:10.3389/fphar.2021.673988
12. Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14(10):19805–19830. doi:10.3390/ijms141019805
13. Wang L, He C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front Immunol. 2022;13:967193. doi:10.3389/fimmu.2022.967193
14. Maeda K, Kobayashi Y, Koide M, et al. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int J Mol Sci. 2019;20:22. doi:10.3390/ijms20225525
15. Rai V, Dilisio MF, Samadi F, Agrawal DK. Counteractive Effects of IL-33 and IL-37 on Inflammation in Osteoarthritis. Int J Environ Res Public Health. 2022;19:9. doi:10.3390/ijerph19095690
16. Jin X, Dong X, Sun Y, Liu Z, Liu L, Gu H. Dietary Fatty Acid Regulation of the NLRP3 Inflammasome via the TLR4/NF-kappaB Signaling Pathway Affects Chondrocyte Pyroptosis. Oxid Med Cell Longev. 2022;2022:3711371. doi:10.1155/2022/3711371
17. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi:10.1002/art.34453
18. Hao T, Chen X, Li G, Yan J. A bibliometric analysis of text mining in medical research. Soft Computing. 2018;22(23):7875–7892. doi:10.1007/s00500-018-3511-4
19. Van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
20. Zheng Z, Xu W, Xue Q. Research Hotspots and Trends Analysis of Patellar Instability: a Bibliometric Analysis from 2001 to 2021. Front Surg. 2022;9:870781. doi:10.3389/fsurg.2022.870781
21. Shao B, Qin YF, Ren SH, et al. Structural and Temporal Dynamics of Mesenchymal Stem Cells in Liver Diseases From 2001 to 2021: a Bibliometric Analysis. Front Immunol. 2022;13:859972. doi:10.3389/fimmu.2022.859972
22. Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. The Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
23. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. The Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
24. Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi:10.1038/nrrheum.2016.136
25. Xu L, Gu H, Zhang Y. Research Hotspots of the Rehabilitation Medicine Use of sEMG in Recent 12 Years: a Bibliometric Analysis. J Pain Res. 2022;15:1365–1377. doi:10.2147/JPR.S364977
26. Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi:10.1097/BOR.0b013e328349c2b1
27. Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28(4):400–409. doi:10.1016/j.joca.2020.02.027
28. Manning BD, Toker A. AKT/PKB Signaling: navigating the Network. Cell. 2017;169(3):381–405. doi:10.1016/j.cell.2017.04.001
29. Wang J, Sun Y, Liu Y, et al. Effects of platelet-rich fibrin on osteogenic differentiation of Schneiderian membrane derived mesenchymal stem cells and bone formation in maxillary sinus. Cell Commun Signal. 2022;20(1):88. doi:10.1186/s12964-022-00844-0
30. Medina-Porqueres I, Martin-Garcia P, Sanz-De-Diego S, et al. Clinical and Functional Outcome of Meniscal Injuries Treated with Platelet-Rich Plasma: a Single-Center Case Series. Int J Environ Res Public Health. 2022;19:12. doi:10.3390/ijerph19127118
31. Maeda K, Kobayashi Y, Koide M, et al. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int J Mol Sci. 2019;20:22.
32. Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):97. doi:10.1186/s12964-019-0411-x
33. Choi MC, Jo J, Park J, Kang HK, Park Y. NF-kappaB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells. 2019;8:7. doi:10.3390/cells8070734
34. Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. doi:10.2174/138945010791011938
35. Rabinovitch RC, Samborska B, Faubert B, et al. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017;21(1):1–9. doi:10.1016/j.celrep.2017.09.026
36. Wang J, Li J, Song D, et al. AMPK: implications in osteoarthritis and therapeutic targets. Am J Transl Res. 2020;12(12):7670–7681.
37. Yi D, Yu H, Lu K, et al. AMPK Signaling in Energy Control, Cartilage Biology, and Osteoarthritis. Front Cell Dev Biol. 2021;9:696602. doi:10.3389/fcell.2021.696602
38. Zhang C, Jiang S, Lu Y, Yuan F. Butorphanol tartrate mitigates cellular senescence against tumor necrosis factor-alpha (TNF-alpha) in human HC-A chondrocytes. Bioengineered. 2022;13(3):5434–5442. doi:10.1080/21655979.2021.2024651
39. Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021;17(1):47–57. doi:10.1038/s41584-020-00533-7
40. Huang H, Lei H, Yang F, Fan X, Dang Q, Li Y. Activation of the bile acid receptor GPBAR1 (TGR5) ameliorates interleukin-1beta (IL-1beta)- induced chondrocytes senescence. Biomed Pharmacother. 2018;106:1713–1719. doi:10.1016/j.biopha.2018.06.154
41. Bi J, Cai W, Ma T, et al. Protective effect of vildagliptin on TNF-alpha-induced chondrocyte senescence. IUBMB Life. 2019;71(7):978–985. doi:10.1002/iub.2049
42. Zhou Q, Cai Y, Jiang Y, Lin X. Exosomes in osteoarthritis and cartilage injury: advanced development and potential therapeutic strategies. Int J Biol Sci. 2020;16(11):1811–1820. doi:10.7150/ijbs.41637
43. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi:10.1016/j.biomaterials.2019.02.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
