Back to Journals » Journal of Inflammation Research » Volume 16
The Value of the Monocyte to High-Density Lipoprotein Cholesterol Ratio in Assessing the Severity of Knee Osteoarthritis: A Retrospective Single Center Cohort Study
Authors Cao J , Hua L, Dong L, Wu Z , Xue G
Received 4 November 2022
Accepted for publication 3 February 2023
Published 11 February 2023 Volume 2023:16 Pages 595—604
DOI https://doi.org/10.2147/JIR.S395229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Jun Cao,1,* Lin Hua,2,* Liang Dong,3 Zhouhuan Wu,4 Guohui Xue2
1Department of Biochemistry and Molecular Biology, School of Medicine, Jiujiang University, Jiujiang, People’s Republic of China; 2Department of Clinical Laboratory, Jiujiang NO.1 People’s Hospital, Jiujiang, People’s Republic of China; 3Department of Rheumatology, Jiujiang NO.1 People’s Hospital, Jiujiang, People’s Republic of China; 4Department of Pharmacology, School of Medicine, Jiujiang University, Jiujiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guohui Xue, Email [email protected]
Background: Inflammatory responses and metabolic abnormalities play essential roles in the pathophysiology of osteoarthritis (OA). Our study aimed to evaluate the association between monocyte-to-high density lipoprotein-cholesterol ratio (MHR) and OA and compared it with other systemic inflammatory markers.
Methods: This study recruited 323 OA cases and age- and sex-matched 283 control participants during the same period. Demographic, clinical, and imaging data and laboratory indicators were obtained from participants’ records. Systemic inflammatory markers were calculated for both cohorts. The diagnostic effectiveness of each index for distinguishing patients with OA was analyzed using receiver operating characteristic (ROC) curves. Spearman’s method and ordered logistic regression were used to analyze the association between each indicator and Kellgren and Lawrence (KL) grade.
Results: MHR was significantly higher (0.38± 0.18 vs 0.25± 0.07, p < 0.0001) in OA patients than healthy controls. MHR had the largest area under the ROC curve for predicting OA. Analysis of ordered logistic regression indicated that MHR was a risk factor for OA radiological severity. Spearman correlation analysis indicated that MHR significantly correlates with the KL grade. Moreover, MHR was significantly higher in early stage patients than in healthy controls.
Conclusion: These results suggest that an elevated MHR could reflect knee OA severity and might be a useful marker for diagnosis and monitoring of OA.
Keywords: osteoarthritis, disease severity, monocyte to high-density lipoprotein cholesterol ratio, inflammation, biomarker
Introduction
Osteoarthritis (OA) is the most prevalent joint disorder in older adults worldwide, with approximately 250 million patients, with knee OA being the predominant type.1 In addition, as the global population ages, the incidence of OA increases yearly, with some studies predicting an increase of more than 40% by 2025.2 Persistent degenerative changes in the articular cartilage can lead to cartilage sclerosis and bone fragmentation. In addition, patients with OA can present with increased inflammatory infiltration, vascularization and fibrosis of infrapatellar fat pad, as well as concurrent inflammation of synovial membrane and meniscal degeneration.3–5These pathological phenotypes can further affect joint homeostasis and promote and perpetuate joint damage. Hence, patients with advanced diseases often require joint replacement surgery, posing significant health and economic challenges to individuals and society.6 Currently, the diagnosis and assessment of OA severity are based on clinical and imaging criteria.7 However, these are usually ineffective in detecting OA until the patient is in an advanced stage.8 Studies have revealed that certain cellular or molecular changes appear much earlier than obvious clinical signs.9 Therefore, the search for OA biomarkers has become a focus in this field.10 Unfortunately, clinically applicable biomarkers for diagnosing OA and assessing its severity are unavailable.11 The underlying molecular mechanisms of OA are not fully understood; however, substantial evidence indicates that inflammatory and metabolic changes play essential roles in the pathophysiology of OA.12 Certain cytokines and mediators produced by joint-infiltrating immune cells can accelerate articular cartilage degeneration, inflammation of synovial membrane, meniscal degeneration and inflammation and fibrosis of the infrapatellar fat pad.13 Among these immune cells, Monocytes (MON) can migrate to tissues and secrete inflammatory cytokines, influencing the level of the inflammatory response. Additionally, their numerical and phenotypic changes can be markers reflecting the degree of inflammation.14 In addition, plasma levels of high-density lipoprotein cholesterol (HDL-C) were significantly and negatively associated with OA risk.15 Thus, the monocyte-to-HDL-C ratio (MHR) can indirectly represent the body’s inflammatory condition, and its association with the development of chronic inflammatory diseases has been established.16 MHR has been associated with prognostic outcomes in diseases such as cardiovascular disease and diabetes.17–19 Nevertheless, no studies have assessed the clinical value of MHR in patients with OA. Therefore, we aimed to evaluate the association between MHR and OA and compared it with other systemic inflammatory markers.
Materials and Methods
Participants and Study Design
This study enrolled 412 patients with OA between February 2015 and May 2022. The diagnostic criteria of the American College of Rheumatology’s revised classification of OA in 1986 was used to determine OA.20
Inclusion criteria involved patients: (1) met the diagnostic criteria for osteoarthritis of the knee; (2) with complete medical records, laboratory data and imaging data; (3) no history of knee trauma or knee surgery. Exclusion criteria involved patients: (1) with other connective tissue and autoimmune diseases; (2) with severe hepatic and/or renal impairment; (3) with severe cardiovascular disease; (4) with malignant neoplasm; (5) with diabetes, hyperthyroidism, or primary or secondary adrenal dysfunction; (6) pregnancy, lactation, or acute or chronic infection; and (7) a history of smoking, violent fractures, or psychiatric disorders. We selected 283 age- and sex-matched healthy participants without any evidence of OA as the control group. Subjects in control group were obtained from the same period of healthy physical examination population, and these individuals did not have any signs and symptoms of arthritis, including: no joint pain, no morning stiffness, no friction sound, no limitation of joint movement, no joint pressure, no joint hypertrophy, no joint deformity, etc. Eighty-nine individuals with OA were excluded due to inadequate laboratory data or meeting the exclusion criteria, and 323 patients were enrolled in the follow-up study. The hospital’s ethics committee approved all procedures.
Radiographic Examination of the Knee Joint for OA
Two experienced radiologists independently analyzed the radiographic images. Radiographs were classified utilizing the Kellgren and Lawrence (KL) classification criteria as follows:21 grade I showed mild bone stenosis; grade II showed significant bone stenosis without the involvement of the joint space; grade III showed moderate narrowing of the joint space; and grade IV showed significant narrowing of the joint space with subchondral bone sclerosis. In patients with OA, the mild, moderate, and severe groups were grouped according to the KL grading criteria, with grades I–II being the mild and moderate groups, and grades III–IV being the severe group.
Data Collection and Laboratory Analysis
Baseline information, including age, sex, and medical and medication history, was collected from the medical records of all participants. A Sysmex XN2000 automatic hematology analyzer was used to measure white blood cells (WBC), neutrophils (NEU), monocytes (MON), lymphocytes (LYM), platelets (PLT), mean corpuscular volume (MCV), mean platelet volume (MPV), and red blood cell distribution width- coefficient of variation (RDW-CV). Albumin (ALB), prealbumin (pALB), alkaline phosphatase (ALP), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), HDL-C, apolipoprotein A1 (APO-A1), and apolipoprotein B100 (APO-B100) levels were measured in fasting venous blood using a Hitachi 7600 automatic biochemical analyzer. C-reactive protein (CRP) levels were determined using an immunoturbidimetric assay with the IMMAGE 800 system.
Calculation of the Derived Systemic Inflammatory Markers
This study systematically evaluated the values of previously reported systemic inflammatory markers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), derived NLR (dNLR), ALB to CRP ratio (ACR), neutrophil to HDL-C ratio (NHR), platelet to HDL-C ratio (PHR), MHR, and ALB to ALP ratio (AAPR), in the diagnosis and severity assessment of OA. The above-derived indicators were obtained using the following formulae: (1) NLR=NEU/LYM (2) PLR=PLT/LYM (3) dNLR=NEU/(WBC-NEU) (4) ACR=ALB/CRP (5) NHR=NEU/HDL-C (6) MHR=MON/HDL-C (7) PHR=PLT/HDL-C and (8) AAPR=ALB/ALP.
Statistical Analysis
A priori power analyses were conducted in G*Power 3.1.9.7 in order to ensure a sufficiently large sample size. All data obtained in this study were processed and analyzed using SPSS 23.0 software. Data of this research were tested for normal distribution using Kolmogorov–Smirnov test. For normally distributed data, independent t-tests were performed to compare differences. Non-normally distributed data were compared using the Mann–Whitney U-test. The chi-squared (χ2) test was used to compare the count data. One-way ANOVA was used for multiple comparisons following a post-hoc Tukey’s HSD test. Spearman’s method was used to analyze the correlations between the indicators. Ordered logistic regression was used to identify the predictors of OA severity. The diagnostic performance of each indicator was evaluated using receiver operating characteristic (ROC) curves. The differences were considered statistically significant at p= 0.05.
Results
Participants’ Baseline and Laboratory Characteristics
The control group included 283 healthy participants with a median age of 58, including 224 women (79.16%) and 59 men (20.85%). The OA group consisted of 323 patients with confirmed knee OA, including 262 (81.11%) women and 61 (18.89%) men, with a median age of 62. There were 96 (29.72%), 142 (43.96%), 73 (22.60%), and 12 (3.72%) patients with OA grades I, II, III, and IV, respectively. Further analysis of the laboratory data revealed insignificant differences in MCV, PLT counts, pALB, TG, TC, LDL-C, and APO-A1 between the two groups (p > 0.05). In contrast, when compared to healthy controls, patients with OA had higher BMI (body mass index), WBC counts, RDW-CV, MPV, NEU counts, MON counts, CRP, ALP, and APO-B100 (p < 0.05) and decreased levels of LYM counts, ALB, and HDL-C (p < 0.05). The demographic and laboratory characteristics of the participants are described in more detail in Table 1.
 |
Table 1 The Demographic Data and Laboratory Indicators of the Two Populations |
The Changes in the Derived Systemic Inflammatory Markers in OA Cases
Compared to the healthy population, patients with OA had higher NLR, dNLR, NHR, and MHR (p < 0.05). Furthermore, ACR and AAPR were significantly lower in patients with OA than in healthy controls (p < 0.05). Meanwhile, PLR and PHR did not differ significantly between the two groups. Figure 1 shows the differences between the derived systemic inflammatory markers in the two populations.
Diagnostic Performance of the Derived Systemic Inflammatory Markers for Differentiating OA Cases from Healthy Individuals
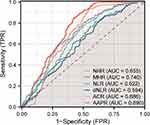
We evaluated the ability of these markers to identify patients with OA by drawing ROC curves for NLR, PLR, dNLR, ACR, NHR, MHR, PHR, and AAPR. The ROC curve results suggested that the MHR had the largest area under the curve at 0.740, with an optimal diagnostic threshold of 0.32. The MHR had a diagnostic sensitivity of 0.848 and a specificity of 0.579. The ROC curves of each indicator are presented in Figure 2, and Table 2 provides a complete list of the diagnostic performance metrics.
 |
Table 2 Diagnostic Performance Characteristics of Hematology-Derived Markers of Osteoarthritis |
The Correlation Between the Derived Systemic Inflammatory Markers and KL Scores of OA Cases
Spearman correlation analysis revealed that NHR, MHR, and PHR were positively correlated with KL scores, with the largest correlation coefficient of 0.4061 for MHR (p < 0.0001). The correlations between the other indicators and the KL scores were insignificant (p > 0.05). Details are depicted in Figure S1.
Differences in MHR Among OA Patients with Different KL Classifications
Patients with KL grades I to IV had significantly higher MHR than healthy controls. Patients graded as IV had a significantly higher MHR than those graded as III, II, or I. Moreover, MHR was higher in cases graded III than in those graded II, with no significant difference in MHR between cases graded II and I (p > 0.05). Additionally, MHR was significantly higher in early-stage patients (grade I and II) than in healthy controls (p < 0.05). Details are depicted in Figure 3.
The Factors with OA Severity
After adjusting for age and sex, we discovered that LYM, MON, HDL-C, NHR, MHR, and PHR were closely associated with KL grade in patients with OA using ordered logistic regression (Table 3).
 |
Table 3 The Factors of Osteoarthritis Severity with or Without Adjustment for Age and Sex (β, Regression Coefficient) |
Discussion
This study confirmed that MHR might provide important information for assessing radiological severity in patients with OA. According to our findings, patients with severe OA had a higher MHR than those with mild-to-moderate OA. Furthermore, MHR values were significantly higher in OA patients with KL grade I or II compared to healthy controls, suggesting that MHR may be a marker for early OA diagnosis. We also demonstrated that MHR was an independent predictor of OA severity. This study indicates that MHR may be useful for the adjunctive diagnosis of OA and the assessment of disease severity.
The risk of suffering from OA is significantly increased in individuals over 40 years, and the prevalence of OA is as high as 50% in those older than 65 years.22 Age is an independent risk factor for OA incidence,23 but it remains unclear whether age correlates with OA severity. Our findings suggest that age is a risk indicator for radiological severity in patients with OA. Age-related changes in chondrocytes and cartilage matrices, such as shortened chondrocyte telomeres, increased β-galactosidase activity, and cartilage matrix loss, as well as changes in the subchondral bone, synovium, meniscus and infrapatellar fat pad, all contribute to the progression of OA.24,25
OA was once thought to be a mechanical response of the joint to increasing loads over time; however, it is now considered an inflammatory damage response process mediated by an integrated involvement of mechanical stress and immune response rather than a simple mechanical wear process.26 The most prevalent hypothesis for the mechanism of OA is a sterile inflammatory reaction induced by degenerating cartilage fragments or remnants of cellular breakdown,27 which can induce the upregulation of pro-inflammatory cytokines.28 It can also induce the upregulation of cartilage oligomeric matrix proteins, proteoglycans, cross-linked C-telopeptides of type II collagen, and matrix metalloproteinases.29 These cytokines and cartilage degradation products have been associated with OA. In addition, some nutrients and vitamins have been shown to associate with OA, Vitamin A and its derivatives retinoic acid regulate the cartilage and skeletal formation and drive OA development.30 However, OA is the consequence of a multifaceted misbalance between synthesis and cleavage of extracellular matrix, and measuring one alone is often inadequate. These markers are primarily used in scientific research rather than clinical settings because they are small, difficult to measure, and expensive. Hence, it is clinically important to find inexpensive, readily available, and suitable serological markers for the early screening and diagnosis of OA in primary care hospitals. Our study aimed to identify markers that would enable primary care hospitals to screen and diagnose in the preclinical or subclinical stage, and to enable people with high-risk factors for OA and early patients with OA to receive further definitive screening and reasonable treatment. Studies on systemic inflammatory indicators in the blood have demonstrated that they can be used as independent factors in the diagnosis, severity grading, and prognostic assessment of the disease.31–33 Therefore, hematology-derived markers are a feasible and promising OA diagnosis and screening method. In this study, we examined the possibility of MHR as a basic hematological marker for the severity of OA.
We confirmed that MHR could be an effective OA biomarker. Circulating MON can enter tissues and differentiate into macrophages, which then participate in the immune response, and its quantitative changes can reflect the progression of certain inflammatory diseases.34 Articular cartilage, infrapatellar fat pad and synovial membrane damage in OA contribute to the production of inflammatory cytokines, which activate macrophages to produce more pro-inflammatory cytokines.35,36 Loukov et al discovered that modulating MON recruitment to the synovium could be a new therapeutic target for OA.37 Consistent with Gao et al,38 we discovered that peripheral MON counts were significantly higher in patients with OA. The increased MON counts may be caused by immune-inflammatory responses. Additionally, it has been discovered that MON can be activated through an inflammasome-mediated pathway that promotes OA progression.39 Furthermore, alterations in lipid structure have recently been revealed to play an essential role in joint degeneration, and conditional knockout mice with low HDL-C expression and high LDL-C expression were more likely to develop ectopic bone formation and OA.40,41 To the best of our knowledge, HDL-C can regulate lipid metabolism while also reducing macrophage-mediated inflammatory responses.42 HDL-C produces anti-inflammatory biological actions on macrophages that exceed its impact on cellular cholesterol. Indeed, HDL-C can activate transcription factor 3, a vital negative regulator of TLR signaling.43 Epidemiological data suggest that the serum concentration of HDL-C is significantly decreased in patients with OA,44 which is consistent with our findings. Moreover, a higher HDL level was directly correlated with a lower risk of OA.45 MHR, derived from the pro-inflammatory factor MON and anti-inflammatory factor HDL-C, is considered a low-cost and simple laboratory indicator of systemic inflammation in several diseases. A study on Behcet’s disease revealed that MHR reflects the overall inflammation level in patients and serves as a marker to predict early vascular involvement.46 Similarly, in psoriasis, MHR indicates a patient’s inflammation level.47 Therefore, MHR can be applied in monitoring disease severity in patients with OA.
This study had some limitations. First, the cohort of patients with OA enrolled was relatively small, with all patients originating from a single center. Future studies should include a larger multicenter population for evaluation. In addition, the potential value of MHR has been identified; however, its relationship with the pathobiology of OA remains elusive, and it is unclear whether its elevation is implicated in an increased risk of cardiovascular involvement in OA. Therefore, an in-depth analysis of the detailed mechanisms of its elevation and progression with OA is needed.
In conclusion, MHR was significantly higher in patients with OA than in controls, even in early-stage patients. It is out-performing, but is complimentary to other inflammatory markers, especially those that are intra-articular in nature compared to systemic markers. To the best of our knowledge, this is the first report to identify MHR as a potential and convenient biomarker of OA.
Data Sharing Statement
All data supporting this study will be provided by the corresponding author upon reasonable request.
Ethics Statement
This study was approved by the Ethics Committee of the Jiujiang No.1 People’s Hospital in accordance with the Declaration of Helsinki (JJSDYRMYY-YXLL-2021-313). Informed consent was waived due to this study’s retrospective nature and the anonymized processing of patient data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the the National Natural Science Foundation of China (grant number 81860166) and the Science and Technology Program of the Jiangxi Provincial Health and Health Commission (grant number SKJP220200272).
Disclosure
The authors report no conflicts of interest in this work.
References
1. James SL, Abate D, Abate KH., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi:10.1016/S0140-6736(18)32279-7
2. Hayes DA, Miller LE, Block JE. Knee Osteoarthritis Treatment with the KineSpring Knee Implant System: a Report of Two Cases. Case Rep Orthop. 2012;2012:1–6. doi:10.1155/2012/297326
3. Heard BJ. The infrapatellar fat pad is affected by injury induced inflammation in the rabbit knee: use of dexamethasone to mitigate damage. Inflammation Res. 2016;65:459
4. López-Franco M. Meniscal degeneration in human knee osteoarthritis: in situ hybridization and immunohistochemistry study. Arch Orthop Trauma Surg. 2016;136:175
5. Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–275. doi:10.1038/s41584-022-00749-9
6. Safiri S, Kolahi -A-A, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819–828. doi:10.1136/annrheumdis-2019-216515
7. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72:149–162. doi:10.1002/acr.24131
8. Xin X, Tan Q, Li F, et al. Potential Value of Matrix Metalloproteinase-13 as a Biomarker for Osteoarthritis. Front Surg. 2021;8:750047. doi:10.3389/fsurg.2021.750047
9. Felson DT, Hodgson R. Identifying and Treating Preclinical and Early Osteoarthritis. Rheum Dis Clin N Am. 2014;40:699–710. doi:10.1016/j.rdc.2014.07.012
10. Valdes AM. Metabolic syndrome and osteoarthritis pain: common molecular mechanisms and potential therapeutic implications. Osteoarthritis Cartilage. 2020;28:7–9. doi:10.1016/j.joca.2019.06.015
11. van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29:143–150. doi:10.1016/j.joca.2020.10.006
12. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi:10.1016/j.cytogfr.2018.10.002
13. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P FH. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi:10.1038/nrrheum.2010.196
14. Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. doi:10.1016/j.immuni.2018.10.005
15. Li H, George DM, Jaarsma RL, Mao X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med. 2016;4:133. doi:10.21037/atm.2016.03.48
16. Rajcan-Separovic E. Whole Exome Sequencing in Recurrent Early Pregnancy Loss (RPL). MHR. 2016;22(5):364.
17. Villanueva DLE, Tiongson MD, Ramos JD, Llanes EJ. Monocyte to High-Density Lipoprotein Ratio (MHR) as a predictor of mortality and Major Adverse Cardiovascular Events (MACE) among ST Elevation Myocardial Infarction (STEMI) patients undergoing primary percutaneous coronary intervention: a meta-analysis. Lipids Health Dis. 2020;19:55. doi:10.1186/s12944-020-01242-6
18. Karatas A, Turkmen E, Erdem E, Dugeroglu H, Kaya Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomark Med. 2018;12:953–959. doi:10.2217/bmm-2018-0048
19. Gökçay Canpolat A, Emral R, Keskin Ç, Canlar Ş, Şahin M, Çorapçioğlu D. Association of monocyte-to-high density lipoprotein-cholesterol ratio with peripheral neuropathy in patients with Type II diabetes mellitus. Biomark Med. 2019;13:907–915. doi:10.2217/bmm-2018-0451
20. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi:10.1002/art.1780290816
21. Emrani PS, Katz JN, Kessler CL, et al. Joint space narrowing and Kellgren–Lawrence progression in knee osteoarthritis: an analytic literature synthesis. Osteoarthritis Cartilage. 2008;16:873–882. doi:10.1016/j.joca.2007.12.004
22. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet. 2011;377:2115–2126. doi:10.1016/S0140-6736(11)
23. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, Hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi:10.1136/annrheumdis-2013-203355
24. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26. doi:10.1016/j.berh.2009.08.006
25. Belluzzi E, Macchi V, Fontanella C, et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int J Mol Sci. 2020;21:6016. doi:10.3390/ijms21176016
26. Geyer M, Schönfeld C. Novel Insights into the Pathogenesis of Osteoarthritis. Curr Rheumatol Rev. 2018;14:98–107. doi:10.2174/1573397113666170807122312
27. Motta F, Barone E, Sica A, Selmi C. Inflammaging and Osteoarthritis. Clin Rev Allergy Immunol. 2022. doi:10.1007/s12016-022-08941-1
28. Molnar V, Matišić V, Kodvanj I, et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021;22:9208. doi:10.3390/ijms22179208
29. Živanović S, Petrović Rackov L, Živanović A, Jevtić M, Nikolić S, Kocić S. Cartilage Oligomeric Matrix Protein - inflammation biomarker in knee osteoarthritis. Bosn J Basic Med Sci. 2011;11:27. doi:10.17305/bjbms.2011.2619
30. Zheng X, Liang J, Li Y, Tu M. Role of Fat-Soluble Vitamins in Osteoarthritis Management. JCR J Clin Rheumatol. 2018;24:132–137. doi:10.1097/RHU.0000000000000587
31. Cai C, Zhang R, Xu X, Li G, Gou H. Diagnostic values of NLR and miR‑141 in patients with osteoarthritis and their association with severity of knee osteoarthritis. Exp Ther Med. 2020;21:74. doi:10.3892/etm.2020.9506
32. Taşoğlu Ö, Şahin A, Karataş G, et al. Blood mean platelet volume and platelet lymphocyte ratio as new predictors of Hip osteoarthritis severity. Medicine. 2017;96:e6073. doi:10.1097/MD.0000000000006073
33. Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol. 2017;36:2689–2695. doi:10.1007/s10067-017-3815-2
34. Sioud P, Ailte F. Targeted Killing of Monocytes/Macrophages and Myeloid Leukemia Cells with Pro-Apoptotic Peptides. Cancers. 2019;11:1088. doi:10.3390/cancers11081088
35. Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555–561. doi:10.1016/j.joca.2020.01.007
36. Dawes JM, Kiesewetter H, Perkins JR, Bennett DL, McMahon SB. Chemokine expression in peripheral tissues from the Monosodium Iodoacetate model of chronic joint pain. Mol Pain. 2013;8:1744.
37. Loukov D, Karampatos S, Maly MR, Bowdish DME. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthritis Cartilage. 2018;26:255–263. doi:10.1016/j.joca.2017.10.018
38. Gao K, Zhu W, Liu W, et al. Diagnostic value of the blood monocyte–lymphocyte ratio in knee osteoarthritis. J Int Med Res. 2019;47:4413–4421. doi:10.1177/0300060519860686
39. Orlowsky EW, Kraus VB. The Role of Innate Immunity in Osteoarthritis: when Our First Line of Defense Goes On the Offensive. J Rheumatol. 2015;42:363–371. doi:10.3899/jrheum.140382
40. Loef M, van de Stadt L, Böhringer S, et al. The association of the lipid profile with knee and hand osteoarthritis severity: the IMI-APPROACH cohort. Osteoarthritis Cartilage. 2022. doi:10.1016/j.joca.2022.05.008
41. de Munter W, van der Kraan PM, van den Berg WB, van Lent PLEM. High systemic levels of low-density lipoprotein cholesterol: fuel to the flames in inflammatory osteoarthritis? Rheumatology. 2016;55:16–24. doi:10.1093/rheumatology/kev270
42. Rye K-A, Barter PJ. Regulation of High-Density Lipoprotein Metabolism. Circ Res. 2014;114:143–156. doi:10.1161/CIRCRESAHA.114.300632
43. De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15:152–160. doi:10.1038/ni.2784
44. Karvonen-Gutierrez CA, Sowers M, Heeringa SG. Sex dimorphism in the association of cardiometabolic characteristics and osteophytes-defined radiographic knee osteoarthritis among obese and non-obese adults: NHANES III. Osteoarthritis Cartilage. 2012;20:614–621. doi:10.1016/j.joca.2012.02.644
45. Garcia-Gil M, Reyes C, Ramos R, et al. Serum Lipid Levels and Risk Of Hand Osteoarthritis: the Chingford Prospective Cohort Study. Sci Rep. 2017;7:3147. doi:10.1038/s41598-017-03317-4
46. Acikgoz N, Kurtoğlu E, Yagmur J, Kapicioglu Y, Cansel M, Ermis N. Elevated Monocyte to High-Density Lipoprotein Cholesterol Ratio and Endothelial Dysfunction in Behçet Disease. Angiology. 2018;69:65–70. doi:10.1177/0003319717704748
47. Sirin MC, Korkmaz S, Erturan I, et al. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An Bras Dermatol. 2020;95:575–582. doi:10.1016/j.abd.2020.02.008
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.