Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Vitamin E Supplement Protects Against Gestational Diabetes Mellitus in Mice Through nuclear factor-erythroid factor 2-related factor 2/heme oxygenase-1 Signaling Pathway
Received 11 November 2022
Accepted for publication 14 February 2023
Published 1 March 2023 Volume 2023:16 Pages 565—574
DOI https://doi.org/10.2147/DMSO.S397255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Bozhu Lin,1 Xiaorong Zhang2
1Gynaecology and Obstetrics Department, Longyan People’s Hospital, Longyan, People’s Republic of China; 2Neonatal Department, Longyan People’s Hospital, Longyan, People’s Republic of China
Correspondence: Xiaorong Zhang, Neonatal Department, Longyan People’s Hospital, No. 31, Denggaoxi Road, Xinluo District, Longyan, 364000, People’s Republic of China, Tel +86-13605936060, Email [email protected]
Background: Gestational diabetes mellitus (GDM) is the most common pregnant disorder worldwide. In this study, we aimed to explore whether vitamin E (VE) treatment alone could protect against GDM in a mouse model.
Methods: 6-week-old C57BL/6J female mice were fed on high-fat diet for two weeks and continued with high-fat diet after pregnancy to induce GDM. The pregnant mice were orally administrated with 2.5, 25 or 250 mg/kg VE twice per day during pregnancy together with high-fat diet. Oral glucose tolerance test, insulin amounts, oxidative stress and inflammation were then measured.
Results: Only 250 mg/kg VE could improve glucose tolerance and insulin level in pregnant mice. VE (250 mg/kg) effectively inhibited GDM-induced hyperlipidemia, and secretion of inflammatory cytokines such as tumor necrosis factor-α and interleukin-6. VE also significantly ameliorated maternal oxidative stress at the late stage of pregnancy, and also improved reproductive outcomes, including increasing the litter size and birth weight in GDM mice. Moreover, VE also activated GDM-reduced nuclear factor-erythroid factor 2-related factor 2 (Nrf2) / heme oxygenase-1 signaling pathway in the maternal liver tissues of GDM mice.
Conclusion: Our data clearly demonstrated that 250 mg/kg VE twice a day during pregnancy could significantly ameliorate the symptoms of GDM by alleviating oxidative stress, inflammation, hyperglycemia, and hyperlipidemia through Nrf2/HO-1 signaling pathway in GDM mice. Thus, additional VE supplement might be beneficial to GDM.
Keywords: gestational diabetes mellitus, vitamin E, fetus, oxidative stress, Nrf2
Introduction
Gestational diabetes mellitus (GDM) is a medical condition occurring in the late stage of pregnancy, where placenta-derived hormones prevent pregnant women from using insulin effectively and eventually lead to increased glucose in the body and insulin resistance, making GDM the most common medical pregnant disorder throughout the world.1–3 Incidence rate of GDM generally increases as a result of gradually increasing marriage age as the obesity epidemic spreads. Therefore, GDM has become a major global health problem. Because it impairs both pregnant women and their offspring, it has attracted increasing research attention. However, many questions remain unsolved for GDM management.
Nutrition intervention for GDM patients has been considered the most common therapy.4,5 Nutritional quantity and quality have a great effect on the growth of the fetus, thus medical nutrition therapy is the primary therapy for 30–89% of GDM patients.6–8 Vitamin E is a well-known nutrition that usually serves as an antioxidant to prevent the formation of reactive oxygen species (ROS). Due to its anti-oxidative activity, VE is recognized as a beneficial nutrient to human health, including cancer, aging, and arthritis.9–11 VE also prevents platelet hyper-aggregation, which leads to atherosclerosis.12 In addition, it also helps reduce the production of prostaglandins such as thromboxane, which causes platelet clumping. Moreover, VE was reported to be associated with GDM.13 GDM patients had a significantly lower level of VE than normal pregnant women,14 which gave us a hint that low VE levels might be the key reason of developing diabetes during pregnancy. Therefore, additional VE supplements might be beneficial to GDM patients. However, current studies only indicated that the co-supplement of VE with another agent was helpful to GDM. For example, co-supplement of VE and magnesium or zinc for 6 weeks alleviated fasting plasma glucose and reduced lipid profile in GDM patients.15,16 Therefore, we aimed to investigate whether VE supplementation alone could exhibit a protective effect against GDM.
Materials and Methods
Animal Procedure
The experimental design was shown in Figure 1. 6-week-old female C57BL/6J mice were on a high-fat diet (D12331, 58% kcal from fat; Research Diets, New Brunswick, NJ) for two weeks, then mated with background-matched male mice. The day when a mucous vaginal plug was presented was regarded as gestation day (GD) 0. Pregnant mice were orally treated with 2.5, 25, 250 mg/kg VE twice a day during pregnancy together with a high-fat diet. The mice fed on a standard diet (D12329, 11% kcal) were defined as wild-type (WT) mice. At GD19, 6 mice per group were sacrificed and their blood samples, visceral fat tissues, and liver tissues were collected for ELISA and Western blot analysis. After delivery (GD22-25), the litter size and birth weight of the fetus in each group (10 pregnant mice for each group) were counted and weighed. This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH). All the animal procedures were approved by the Ethics Committee of Longyan People’s Hospital (approval number #2020.b82).
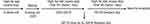 |
Figure 1 Schematic illustration of the experimental procedures. |
Oral Glucose Tolerance Test (OGTT)
After fasting for 16 h, mice were orally administrated with 2 g/kg glucose. Then blood glucose levels were measured using a glucometer (TERUMO, Tokyo, Japan) at indicated time points as previously described.17
Western Blot
Visceral fat tissues and liver tissues were collected and lysed in RIPA buffer. Nuclear protein was extracted from the liver tissues as previously described.18 Western blot was performed as previously described.19 β-actin was used as a loading control (Sigma-Aldrich, St. Louis, MO, USA). The primary antibodies including interleukin-6 (IL-6), the cell-bound precursor of tumor necrosis factor-α (TNF-α), adiponectin, nuclear factor-erythroid factor 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) were purchased from Abcam (Cambridge, MA). Histone-3 antibody was purchased from Cell Signaling Technology (Danvers, MA).
Biochemical Indexes Analysis
Blood was collected from treated mice. Then serum was acquired after centrifugation of the blood at 1000 g for 10 min at 4°C, and all the biochemical indexes, including total cholesterol (TCh), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using Total Cholesterol Assay Kit (STA-384, Cell Biolabs), Serum Triglyceride Quantification Kit (STA-396, Cell Biolabs), HDL and LDL/VLDL Cholesterol Assay Kit (STA-391, Cell Biolabs), respectively.18
Elisa
Insulin secretion in the serum was determined using the high-sensitivity PLUS insulin kit (Morinaga-Seikagaku Co. Ltd., Yokohama, Japan) according to the manufacturer’s instructions. The levels of oxidative stress markers, including malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione (GSH), and catalase (CAT) were measured using Lipid Peroxidation MDA Assay Kit (S0131), Total Superoxide Dismutase Assay Kit (S0109), Total Glutathione Peroxidase Assay Kit (S0058), Glutathione Reductase Assay Kit (S0055), and Catalase Assay Kit (S0051) (Beyotime Biotechnology, China), respectively.20
Statistical Analysis
The quantitative data were shown as means ± standard deviation (SD). Differences among different groups were calculated with ANOVA analysis followed with appropriate post hoc tests by GraphPad 7.0. P < 0.05 was regarded as significant difference.
Results
VE Ameliorates Diabetes-Induced Glucose Intolerance in Pregnant Mice
First, to determine the effective dose of VE in pregnant mice, we treated pregnant mice with three different doses (2.5, 25, and 250 mg/kg). OGTT results demonstrated that, compared to WT mice, GDM mice displayed glucose intolerance (Figure 2A and B). Neither 2.5 nor 25 mg/kg VE exerted any significant effect on glucose tolerance, whereas only 250 mg/kg VE significantly ameliorated glucose intolerance (Figure 2A and B). Compared with the control-treated GDM mice, the total blood glucose in the VE administration group was significantly decreased. In GDM mice, the serum insulin content was lower than that of WT, and serum insulin was also elevated after VE administration (Figure 2C). These results demonstrated that 250 mg/kg VE significantly ameliorated GDM-induced glucose intolerance. Thus, 250 mg/kg was chosen for the following experiments.
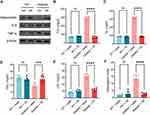
VE Inhibits Dysfunction of Adipocytokine Expression and Hyperlipidemia in Pregnant GDM Mice
GDM usually leads to hyperlipidemia, which was reflected by the low expression of adiponectin in visceral fat (Figure 3A), elevated TCh (Figure 3B, WT+water: 68.83±4.35 mg/dl; WT+VE: 76.67±4.46 mg/dl; Diabetic+water: 292.70±15.79 mg/dl), Triglyceride (TG) (Figure 3C, WT+water: 88.17±4.62 mg/dl; WT+VE: 85.67±5.72 mg/dl; Diabetic+water: 316.30±11.00 mg/dl), LDL (Figure 3E, WT+water: 15.66±2.86 mg/dl; WT+VE: 19.50±4.03 mg/dl; Diabetic+water: 64.83±6.65 mg/dl) and atherogenic index (Figure 3F, WT+water: 1.75±0.66; WT+VE: 2.66±1.97; Diabetic+water: 13.58±2.80), as well as decreased HDL level (Figure 3D, WT+water: 30.67±2.94 mg/dl; WT+VE: 28.33±3.27 mg/dl; Diabetic+water: 18.33±5.28 mg/dl) in the serum of GDM mice. VE administration remarkably reversed the abnormal expressions of these markers, which suggested that VE could effectively ameliorate GDM-caused hyperlipidemia, as evidenced by decreased levels of TCh (Diabetic+VE: 67.17±10.87 mg/dl), TG (Diabetic+VE: 87.17±5.60 mg/dl), LDH (Diabetic+VE: 23.67±4.89 mg/dl), and atherogenic index (Diabetic+VE: 2.50±0.77), in addition with increased HDL level (Diabetic+VE: 28.83±5.57 mg/dl) (Figure 3A–F). Furthermore, compared to WT mice, GDM mice displayed activated inflammation, as evidenced by the elevated expressions of inflammatory cytokines including TNF-α and IL-6 (Figure 3A). The activated inflammation was partially reversed after VE treatment, as evidenced by the decreased expression of TNF-α and IL-6 (Figure 3A). These data indicated that VE could ameliorate GDM-induced inflammation and hyperlipidemia.
VE Reduces Maternal Oxidative Stress in Pregnant GDM Mice at the Late Stage of Pregnancy
GDM could cause oxidative stress in mice, so we evaluated the anti-oxidative effect of VE in GDM mice. Consistently, GDM mice displayed severe oxidative stress, as reflected by the increased levels of serum MDA (Figure 4A, WT+water: 11.65±3.04 mM; WT+VE: 14.01±3.30 mM; Diabetic+water: 30.92±6.33 mM) and liver MDA (Figure 4B, WT+water: 19.33±6.83 nmol/mg protein; WT+VE: 18.82±3.19 nmol/mg protein; Diabetic+water: 34.00±3.74 nmol/mg protein), and reduced activities of SOD (Figure 4C, WT+water: 41.50±5.96 U/mg protein; WT+VE: 40.17±5.12 U/mg protein; Diabetic+water: 18.08±6.39 U/mg protein), GPx (Figure 4D, WT+water: 23.92±2.20 mU/mg protein; WT+VE: 22.08±3.53 mU/mg protein; Diabetic+water: 13.50±2.59 mU/mg protein), GSH (Figure 4E, WT+water: 32.25±2.75 nM/mg protein; WT+VE: 34.50±2.42 nM/mg protein; Diabetic+water: 23.58±1.96 nM/mg protein) and CAT (Figure 4F, WT+water: 21.84±4.35 U/mg protein; WT+VE: 20.50±2.88 U/mg protein; Diabetic+water: 14.25±2.04 U/mg protein) in the liver, in comparison with those in WT pregnant mice (Figure 4A–F), while elevated oxidative stress was significantly decreased after VE administration in GDM mice, as demonstrated by the decreased levels of serum MDA (Diabetic+VE: 16.67±3.88 mM) and liver MDA (Diabetic+VE: 25.05±4.14 nmol/mg protein), and increased activities of SOD (Diabetic+VE: 41.25±5.71 U/mg protein), increased GPx activity (Diabetic+VE: 23.91±3.43 mU/mg protein), GSH (Diabetic+VE: 30.83±3.97 nM/mg protein), and CAT (Diabetic+VE: 29.17±3.52 U/mg protein) in the liver (Figure 4A–F). These data suggested that VE effectively alleviated GDM-induced oxidative stress in GDM mice.
VE Alleviates GDM Reproductive Outcomes
Next, we evaluated the protective effect of VE treatment on reproductive outcomes in the GDM mice. GDM mice delivered smaller litter size (Figure 5A, WT+water: 8.30±1.42; WT+VE: 9.00±1.05; Diabetic+water: 6.20±1.14) and heavier birth weight than those from the WT pregnant mice (Figure 5B, WT+water: 1.185±0.047 g; WT+VE: 1.143±0.088 g; Diabetic+water: 1.363±0.056 g). VE treatment could increase the litter size (Diabetic+VE: 8.10±1.37) and decrease the birth weight (Diabetic+VE: 1.231±0.059 g) (Figure 5A and B). However, VE administration showed no effect on litter size and birth weight in WT pregnant mice.
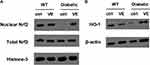
Effects of VE on Nrf2 Activation and HO-1 Expression in the Fetus of Pregnant GDM Mice
We further explored the detailed mechanism of VE treatment on GDM by analyzing the Nrf2/HO-1 signaling pathway, which regulates several crucial anti-oxidative genes and maintains redox homeostasis. Western blot results demonstrated that GDM mice displayed lower expression levels of nuclear Nrf2 and HO-1 in the liver tissues of fetus from GDM mice, with no change in total Nrf2 (Figure 6A and B), suggesting that the redox state was affected. Interestingly, VE treatment increased their expression in the GDM mice, indicating that VE enhanced the resistance to oxidative stress in the fetus of GDM mice.
Discussion
GDM is the most common pregnant disorder that endangers both the pregnant women and their offspring without timely and proper treatment, such as the increased risk of T2DM.21,22 Therefore, proper management of GDM is important to minimize maternal and neonatal complications. Current conventional treatments for GDM include a special diet, daily exercise, and monitoring blood glucose. If special diet and exercise are not sufficient to control blood glucose, some doctors prescribe oral medications for pregnant women to control their blood glucose, such as sulfonylureas,23,24 metformin,25,26 because oral anti-diabetic drugs are safe during pregnancy and less harmful to fetal development than poorly controlled diabetes. While others use insulin injections directly instead of oral medication, because they believe more research is needed to show that oral medications are as safe and effective as insulin injections for controlling GDM.27–29 Even using insulin also carries the risk of over-injection leading to hypoglycemia.30,31 Therefore, if special diet or exercise could well control blood glucose, neither oral anti-diabetic drugs nor insulin-injection is needed.
VE is an essential nutrient that is widely used as an anti-oxidative agent to reduce body-generated excessive free radicals.32 Endogenous or exogenous ROS could break the redox balance in the body, eventually contributing to various diseases, hence VE supplement could effectively suppress lipoprotein oxidation and inhibit redox imbalance-related diseases, such as Alzheimer’s disease,33 cardiovascular disease,34–36 neurologic disease.37 A meta-analysis revealed that GDM patients showed a lower level of VE than healthy pregnant women,38 which implied that VE might be involved in the development of GDM. Because previous publications indicated that VE co-supplemented with other supplements, such as omega-3 fatty acid,38,39 magnesium 40 or zinc,41 ameliorated the symptoms and complications in GDM pregnant women and their offspring, VE was regarded as the necessary supplement for the treatment of GDM.42 Our data clearly demonstrated that VE supplement alone could effectively alleviate the inflammation, oxidative stress, hyperglycemia, hyperlipidemia and reproductive outcomes of GDM mice. It should be noted that low doses of VE (2.5 or 25 mg/kg) showed no effect on glucose tolerance in GDM mice, and only a high dose of VE (250 mg/kg) significantly increased glucose tolerance and insulin activity. Surprisingly, we found that 250 mg/kg VE also improved glucose tolerance and insulin activity in WT pregnant mice, which suggested that VE might also improve glucose tolerance and insulin sensitivity not only in pregnant mice with GDM but also in healthy pregnant mice. We speculated that the insulin amount and insulin sensitivity were altered even in healthy pregnant women due to the hormones produced by the placenta, which explained the effects of a high dose of VE on the glucose tolerance and insulin amount in non-glucose intolerant pregnant women. Although previous publications indicated that VE had no effect on improving lipid profile in Menopausal women,43 based on different mechanisms between Menopause and GDM, we strongly recommend the effect of VE on GDM.
VE is a well-known anti-oxidant,44 that can suppress oxidative stress to alleviate various diseases.45 Our data also verified that VE could significantly decrease oxidative stress, leading to alleviated GDM symptoms. Current results also demonstrated that although VE was supplemented throughout the entire pregnancy, its anti-oxidative stress effect was only reflected in the late stage of pregnancy instead of early or middle stage, which is also the period when GDM occurs.
Moreover, VE was reported to be the property against low-grade inflammation.34,45,46 Which was consistent with our results showing that 250 mg/kg VE inhibited GDM-induced inflammation in GDM mice. Although one reviewer indicated that VE might have an anti-oxidative effect on GDM,14 its conclusion is still that VE concentration was much lower in the serum of GDM patients than that of in healthy women, which also hints that support VE might also have a protective effect on GDM patients.
The mechanism of VE-improved GDM might be complex. In the current study, we only explored the effects of VE on the Nrf2 signaling pathway, because the most prominent protective effect of VE was to inhibit excessive oxidative stress as an anti-oxidant. As expected, VE could effectively increase the nuclear translocation of Nrf2 to initiate the Nrf2/HO-1 signaling pathway, which modulated some anti-oxidative genes at the transcriptional level to maintain redox homeostasis.
The current work also has some limitations. First, other signaling pathways involved in oxidative stress and inflammation could be examined for their participation in the protective effect of VE against GDM. Second, different regimens of VE treatment could be considered in the future to reduce the dose of VE to a clinically acceptable dose by repeated administration or increased treatment period.
Conclusion
Our data demonstrated that 250 mg/kg VE twice a day during pregnancy could significantly ameliorate the symptoms of GDM in mice, by alleviating oxidative stress, inflammation hyperglycemia, and hyperlipidemia through the Nrf2 signaling pathway in GDM mice. In addition, VE showed protective effects on the offspring by increasing the litter size and reducing the birth weight. The current study suggests that VE supplements might have a beneficial effect on GDM.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
There is no funding to report.
Disclosure
The authors declared that they have no conflict of interest.
References
1. Kautzky-Willer A, Harreiter J, Winhofer-Stöckl Y, et al. Gestationsdiabetes (GDM) (Update 2019) [Gestational diabetes mellitus (Update 2019)]. Wien Klin Wochenschr. 2019;131:91–102. German. doi:10.1007/s00508-018-1419-8
2. Senat M-V, Deruelle P. Le diabète gestationnel. Gynecol Obstet Fertil. 2016;44:244–247. doi:10.1016/j.gyobfe.2016.01.009
3. Berger H, Gagnon R, Sermer M, et al. RETIRED: diabetes in pregnancy. J Obstet Gynaecol Can. 2016;38:667–79.e1. doi:10.1016/j.jogc.2016.04.002
4. American Diabetes Association. American Diabetes Association Workshop-Conference on gestational diabetes: summary and recommendations. Diabetes Care. 1980;3:499–501. doi:10.2337/diacare.3.3.499
5. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–B167.
6. Langer O. Maternal glycemic criteria for insulin therapy in gestational diabetes mellitus. Diabetes Care. 1998;21 Suppl 2:B91–8.
7. Gunderson EP. Gestational diabetes and nutritional recommendations. Curr Diab Rep. 2004;4:377–386. doi:10.1007/s11892-004-0041-5
8. Reader DM. Medical nutrition therapy and lifestyle interventions. Diabetes Care. 2007;30:S188–S93.
9. Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14:e157–e65.
10. Jandak J, Steiner M, Richardson PD. Reduction of platelet adhesiveness by vitamin E supplementation in humans. Thromb Res. 1988;49:393–404. doi:10.1016/0049-3848(88)90242-3
11. Kline K, Lawson KA, Yu W, Sanders BG Vitamin E and cancer. Vitam Horm. 2007;76:435–461.
12. Freedman JE, Keaney JF. Vitamin E inhibition of platelet aggregation is independent of antioxidant activity. J Nutr. 2001;131:374S–7S. doi:10.1093/jn/131.2.374S
13. Ma H, Qiao Z, Li N, Zhao Y, Zhang S. The relationship between changes in vitamin A, vitamin E, and oxidative stress levels, and pregnancy outcomes in patients with gestational diabetes mellitus. Ann Palliat Med. 2021;10:6630–6636. doi:10.21037/apm-21-1036
14. Sharifipour F, Abedi P, Ciahkal SF, Jahanfar S, Mohaghegh Z, Zahedian M. Serum vitamin E level and gestational diabetes mellitus: a systematic review and meta-analysis. J Diabetes Metab Disord. 2020;19:1787–1795.
15. Maktabi M, Jamilian M, Amirani E, Chamani M, Asemi Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018;17:163.
16. Ostadmohammadi V, Samimi M, Mobini M, et al. The effect of zinc and vitamin E cosupplementation on metabolic status and its related gene expression in patients with gestational diabetes. J Matern Fetal Neonatal Med. 2019;32:4120–4127.
17. Sugiyama C, Yamamoto M, Kotani T, Kikkawa F, Murata Y, Hayashi Y. Fertility and pregnancy-associated ß-cell proliferation in mice deficient in proglucagon-derived peptides. PLoS One. 2012;7:e43745.
18. Chen Y, Tang J, Zhang Y, et al. Astaxanthin alleviates gestational diabetes mellitus in mice through suppression of oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:2517–2527.
19. Ozaki KI, Awazu M, Tamiya M, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016;310:E643–E651.
20. Liu Z, Iyer MR, Godlewski G, et al. Functional selectivity of a biased cannabinoid-1 receptor (CB(1)R) antagonist. ACS Pharmacol Transl Sci. 2021;4:1175–1187.
21. Guillén-Sacoto MA, Barquiel B, Hillman N, Burgos M, Herranz L. Gestational diabetes mellitus: glycemic control during pregnancy and neonatal outcomes of twin and singleton pregnancies. Endocrinol Diabetes Nutr. 2018;65:319–327.
22. Xiao Z, Wang Y, Thai PN, Li X, Lu X, Pu J. Mechanisms linking hyperglycemia in pregnancy to the offspring cardiovascular system dysfunction. STEMedicine. 2021;2:e91.
23. Niu X, Yang H, Zhang H, et al. Study on association between gestational diabetes mellitus and sulfonylurea receptor-1 gene polymorphism. Zhonghua fu Chan ke za Zhi. 2005;40:159–163.
24. Rodenstein MS, Bianco ME, Ramchal MU, Murias M, Silton RL, Josefson JL. Long‐term follow‐up of children with in utero exposure to sulfonylurea medications. Obes Sci Pract. 2021;7:487–493.
25. Gray SG, McGuire TM, Cohen N, Little PJ. The emerging role of metformin in gestational diabetes mellitus. Diabetes Obes Metab. 2017;19:765–772.
26. Moore LE, Briery CM, Clokey D, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med. 2007;52:1011–1015.
27. Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113:193–205.
28. Magon N, Seshiah V. Gestational diabetes mellitus: non-insulin management. Indian J Endocrinol Metab. 2011;15:284.
29. Goetzl L, Wilkins I Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22:403–406.
30. Nachum Z, Ben-Shlomo I, Weiner E, Shalev E Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy: randomised controlled trial. BMJ. 1999;319:1223–1227.
31. Walkinshaw SA. WITHDRAWN: very tight versus tight control for diabetes in pregnancy. Cochrane Database Syst Rev. 2007;2007:CD000226–CD.
32. Ungurianu A, Zanfirescu A, Nitulescu G, Margina D. Vitamin E beyond Its Antioxidant Label. Antioxidants. 2021;10:634.
33. Lloret A, Esteve D, Monllor P, Cervera-Ferri A, Lloret A. The effectiveness of vitamin E treatment in Alzheimer’s disease. Int J Mol Sci. 2019;20:879.
34. Sozen E, Demirel T, Ozer NK. Vitamin E: regulatory role in the cardiovascular system. IUBMB Life. 2019;71:507–515.
35. Meydani M. Vitamin E modulation of cardiovascular disease. Ann N Y Acad Sci. 2004;1031:271–279.
36. Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17:e56–e65.
37. Sokol RJ. Vitamin E deficiency and neurologic disease. Annu Rev Nutr. 1988;8:351–373.
38. Jamilian M, Dizaji SH, Bahmani F, et al. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can j Diabetes. 2017;41:143–149.
39. Taghizadeh M, Jamilian M, Mazloomi M, Sanami M, Asemi Z. A randomized-controlled clinical trial investigating the effect of omega-3 fatty acids and vitamin E co-supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. J Clin Lipidol. 2016;10:386–393.
40. Maktabi M, Jamilian M, Amirani E, Chamani M, Asemi Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018;17:1–6.
41. Ostadmohammadi V, Samimi M, Mobini M, et al. The effect of zinc and vitamin E cosupplementation on metabolic status and its related gene expression in patients with gestational diabetes. J Mater Fetal Neonat Med. 2019;32:4120–4127.
42. Bieri JG, Corash L, Hubbard VS. Medical uses of vitamin E. N Engl J Med. 1983;308:1063–1071.
43. Rezasoltani P, Elliyoun N, Ziaie T, Sobhani A, Kazemnezhjad Leyli E, Kazemi Aski S. Double-blind controlled trial of vitamin E effects on serum lipid profile in menopausal women. Diabetes Metab Syndr Obes. 2021;14:1053–1060.
44. Lee GY, Han SN The role of vitamin E in immunity. Nutrients. 2018;10:1614.
45. Khadangi F, Azzi A. Vitamin E - the next 100 years. IUBMB Life. 2019;71:411–415.
46. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.