Back to Journals » Journal of Inflammation Research » Volume 16
The Pivotal Role of Nrf2 Signal Axis in Intervertebral Disc Degeneration
Authors Pan C, Hou W, Deng X , Liu J, Chi R, Shang X, Xu T, Hao X
Received 28 July 2023
Accepted for publication 21 November 2023
Published 5 December 2023 Volume 2023:16 Pages 5819—5833
DOI https://doi.org/10.2147/JIR.S432575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Chunran Pan,* Wenjie Hou,* Xiaofeng Deng, Jiawei Liu, Ruimin Chi, Xingru Shang, Tao Xu, Xiaoxia Hao
Department of Rehabilitation, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoxia Hao, Department of Rehabilitation, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095#, Jie-Fang Avenue, Qiaokou District, Wuhan, 430030, People’s Republic of China, Tel +86 1-597-296-0593, Fax +86- 27-83662640, Email [email protected]
Abstract: Intervertebral disc degeneration (IDD) is considered as a dominant contributor to low back pain (LBP), causing severe pain, limited range of lumbar motion, physical dysfunction, and restriction of social activity. However, the specific pathological mechanisms underlying IDD remain elusive, and effective strategies to delay the pathogenesis of IDD are still unclear and limited. In recent years, some studies have found that nuclear factor erythroid 2-related factor 2 (Nrf2), an important antioxidant transcription factor, may play crucial roles in the pathogenesis and progression of age-related diseases including IDD. Nrf2 can maintain redox homeostasis and protecting nucleus pulposus (NP) cells against oxidative stress, inflammatory response, extracellular matrix (ECM) catabolism, cell senescence and cell death involving in the progression of IDD. In this review, we aim to systematically describe the vital roles and pathological mechanism of Nrf2 signaling axis in the pathogenesis of IDD, which may put forward potential therapeutic strategies for the prevention and treatment of IDD by targeting Nrf2.
Keywords: Nrf2, intervertebral disc degeneration, nucleus pulposus cells, oxidative stress
Introduction
Intervertebral disc degeneration (IDD) is the most common and prevalent musculoskeletal disorder and one of the major causes of lower back pain (LBP), accounting for nearly 40% of the causes of LBP, resulting in severe socioeconomic burden worldwide.1 The exact pathogenesis relevant to IDD progression is largely unknown, and the existent clinical treatments for IDD are limited to surgery or conservative approaches with the purpose of relieving clinical symptoms.2 Therefore, there is an urgent need to address the pathological mechanism and successful treatment of IDD.
The intervertebral disc (IVD) is primarily composed of three tissue types: peripheral annulus fibrosus (AF), the internal nucleus pulposus (NP) and the upper and lower cartilaginous endplates.3 Compared with other two components, NP tissue is crucial for maintaining IVD physiological function by regulating the dynamic balance between extracellular matrix (ECM) synthesis and degradation.4 Dysregulation of NP cells results in their reduced ability to synthesize ECM components and increased capacity to secrete ECM degradative molecules.5 Additionally, inflammation, apoptosis, cell senescence and ECM degradation are termed as the hallmarks of IDD and are known to be interconnected and interdependent with each other contributing to the complicated pathological process of IVD degeneration.6
It is generally accepted that oxidative stress is another typical mediator in the initiation and progression of IDD.7 Redox homeostasis predominantly referred to keep the balance of reactive oxygen species (ROS) production and antioxidant system scavenging activity, which is crucial for maintaining physiological processes.8 Accumulating studies have reported that aged and degenerated discs exhibit decreased antioxidant activity and elevated concentrations of ROS during IDD development.9 Excessive accumulation of ROS can induce oxidative stress and cause damage to the regular function of NP cells, which triggers inflammatory responses and accelerates the senescence and apoptosis of NP cells, ultimately leading to ECM degradation.10
Moreover, ROS was regarded as messengers to activate various signaling pathways, including mitogen activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB), and nuclear factor erythroid 2-related factor 2 (Nrf2).11 Nrf2, which is involved in the regulation of the cellular redox homeostasis against oxidative stress and generating beneficial effects on anti-inflammatory and antioxidant response, plays an indispensable role in relieving joint diseases and preventing the progress of IDD.12,13 The expression of Nrf2 progressively decreased in human NP tissue samples of patients with increased IVD degeneration.14 Interestingly, therapeutic approach targeting Nrf2 could alleviate oxidative stress-induced apoptosis and ECM decomposition of human NP cells, protecting against IDD progression.15
In this review, we attempt to introduce the biological characteristics and essential role of Nrf2 in IDD and provide new evidence, insights, and potential strategies for the prevention and treatment of IDD progression.
The Nrf2 Signaling Pathway
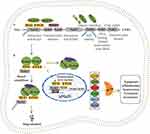
Nrf2 is a pivotal redox-sensitive transcription factor, is expressed in all cell types, which regulates antioxidant defense system by activating a great deal of cytoprotective genes including the regulation of glutathione (GSH) and thioredoxin (TXN) biosynthesis and utilization, as well as enzymes involved in nicotinamide adenine dinucleotide phosphate (NADPH) regeneration, heme and iron metabolism, and xenobiotic detoxification, such as glutathione S-transferase (GST), NAD(P)H:quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1).16 In addition to oxidative and xenobiotic responses, Nrf2 has been viewed as an emerging regulator of other cellular processes, such as mitochondrial bioenergetics, autophagy, unfolded protein response, intermediary metabolism as well as stem cell quiescence.17
Nrf2 belongs to the Cap “n” Collar (CNC) subfamily and contains seven functional homology domains, arranging as follows from N- to C-terminus: Neh2, Neh4, Neh5, Neh7, Neh6, Neh1, Neh3.17 Neh2 is crucial for the interaction between Nrf2 and its negative regulator Kelch-like ECH associated protein (Keap1) associated with the highlighted amino acid motifs DLG and ETGE. Neh4 and Neh5 react as transactivation domains, which play a part in binding to transcriptional co-activator cAMP-response-element-binding protein-binding protein (CBP).18 The retinoid X receptor α (RXRα) suppresses Nrf2 transcriptional activity via interacting with Neh7 domain. Neh6 is related to the negative regulation of Nrf2 stability. NEH1 allows Nrf2 to be heterodimerized with small myofascial fibrosarcoma (MAF) protein, which is necessary for DNA binding. Neh3 also acts as transactivation domain to recruit the chromo-ATPase/helicase DNA-binding protein 6 (CHD6).19
Under normal conditions, Nrf2 exists in the cytoplasm, segregates by Keap1, a redox-sensitive Cul3 E3 ubiquitin ligase adaptor protein, which is responsible for the ubiquitylation and degradation of Nrf2 to inhibit the transcriptional activity.20 Under oxidative stress conditions, Nrf2 dissociates from Keap1 and then translocates into nucleus, heterodimerizes with small Maf protein family (MafF, MafG, and MafK).21 The Nrf2-sMaf complex binds to the antioxidant response element (ARE) and activates the transcription of its target genes.22 In this context, the regulation of Nrf2 transcription depends on Keap1. Recently, emerging evidence has showed further Nrf2 regulation mechanism that is in a Keap1-independent manner.23
The serine-rich Neh6 domain of Nrf2 contains two conservative peptide motifs (DSGIS and DSAPGS), which are recognized by β-transducing repeat-containing protein (β-TrCP), involving in Keap1-independent Nrf2 negative regulation.24 β-TrCP, a substrate receptor, binds more effectively to the Neh6 domain after glycogen synthase kinase-3b (Gsk-3b)-mediated phosphorylation of the DSGIS motif and facilitates the collection of Skp1-Cul1-F-box protein (SCF) ubiquitin ligase complex, which targets at the ubiquitination and subsequent proteasomal degradation of Nrf225 (Figure 1).
In addition, accumulating evidence has revealed other non-classical Nrf2 regulatory pathway including the process of p62-dependent Nrf2 activation in which p62 isolates Keap1 to autophagic degradation that eventually leads to the liberation of Nrf2 and the transactivation of ARE-driven genes as well as the crucial role of micro-RNAs (miRNAs) in the regulation of Nrf2 activity.26 Liao et al found that the interaction between long non-coding RNA MT1DP and miR-365 induces increased apoptosis of NP cells and damaged mitochondrial function by inhibiting Nrf2 pathway.27
The Roles of Nrf2 in IVD Degradation
Nrf2 and Inflammation
It is generally accepted that increased production of inflammatory cytokines such as interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α), which motivate the generation of a series of other pro-inflammatory cytokines and catabolic enzymes, such as nitric oxide (NO), cyclooxygenase-2 (COX-2), a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5), prostaglandin E2 (PGE2) and matrix metalloproteinase-13 (MMP-13), leading to IDD progresses.28 A large number of experiments on Nrf2-knockout mice have demonstrated its pivotal role in the regulation of inflammation and activation of pro-inflammatory genes such as COX-2 and inducible nitric oxide synthase (iNOS).29 NF-ĸB, a protein complex, which is responsible for DNA transcription. Abnormal regulation of NF-ĸB has been related to transcriptional upregulation of pro-inflammatory mediators such as IL-6, TNF-α, iNOS and IL-1β. As reported, activation of Nrf2 pathway could inhibit the overproduction of pro-inflammatory mediators as well as suppressing the activation of NF-ĸB signaling pathway.30 HO-1 is rate-limiting enzyme catalyzing the degradation of heme into carbon monoxide (CO) and free iron, which has prominent anti-inflammatory properties. Elevation of HO-1 expression mediated by activated Nrf2 acts protective effects in regulating inflammatory conditions.31 Xie et al showed that activating the Nrf2/HO-1 signaling axis leading to the inhibition of NF-κB pathway could protect NP cells against IL-1β-induced inflammatory response and catabolism, which was beneficial to prevention and therapy of IVD degeneration.3 In other words, the reduction or deletion of Nrf2 and HO-1 genes may be key factors leading to IDD.32 Another study revealed that activation of Nrf2-ARE signaling pathway could suppress MAPK (P38 and JNK) pathway, contributing to ameliorate IL-1β-treated inflammation in NP cells.33 Mechanically, Nrf2 binds to ARE and promotes the transcription of antioxidant genes such as HO-1 and NQO1 via translocating into the nucleus.
Taken together, these findings suggest that development of novel therapies targeted on Nrf2 for anti-inflammation activity could be advantageous of clinical management of IDD.
Nrf2 and ECM Metabolism
To the best of our knowledge, ECM degradation is one typical characteristic of IVD degeneration due to disturbing the balance of ECM anabolic and catabolic metabolism. During the process, the degradation of major components of ECM in NP including collagen-II (Col2), proteoglycans (PGs) and glycosaminoglycan (GAG) was accelerated, resulting from up-regulation catabolic proteinases, such as matrix metalloproteinase 3 (MMP3), MMP13 and ADMATS5, while their biosynthesis is decreased.34 Therefore, maintenance of ECM homeostasis plays a crucial part in preventing and treating IVD degeneration. Chen et al observed that a moderate dose of fluid shear stress (FSS) could regulate ECM homeostasis through increased expressions of Col2, aggrecan and sulfated glycosaminoglycan (sGAG) and decreased expressions of MMP13 and ADMATS5 in rat NP cells.35 It is interesting to note that Nrf2/HO-1 pathway acts as a protective regulator involved not only in the progression of joint destruction but also in IVD degeneration.36 For instance, it has been shown that induction of HO-1 attenuates the increased ECM catabolism and ameliorates the reduced ECM anabolic activities in human NP cells under IL-1β stimulation conditions.37 In summary, these findings put forward novel interest in targeting the Nrf2/HO-1 pathway as a trigger point to regulate relevant anti-ECM catabolism mechanisms involved in the degeneration of IVD as well as the possibilities of this signal path for the development of therapeutic strategies.
Nrf2 and Oxidative Stress
Numerous studies reinforce the notion that the presence of oxidative stress is regarded as a key intermediator resulting in pathological degeneration of IDD. Oxidative stress occurs when the balance between ROS production and scavenging is disrupted.38 A large amount of ROS is produced due to mitochondrion dysfunction during oxidative stress response to various exogenous stimulation in disc cells including hydrogen peroxide (H2O2) and nitric oxide (NO).39 Previous studies have shown that H2O2 may lead to IVD degeneration via oxidative stress damage, inflammatory response and apoptosis of NP cells.40 Furthermore, the accumulation of ROS not only accelerates the degradation of peripheral matrix proteins but also disturbs the microenvironment of healthy intervertebral disc.41 Under regular conditions, ROS modulates the function and stability of cellular proteins such as nucleic acids, carbohydrates and lipids. However, the increased accumulation and decreased elimination of these species and disruption of the antioxidant/oxidant balance ultimately leading to oxidative stress and pathological changes of diseases.42 In recent decades, the activation of Keap1/Nrf2/ARE signaling pathway is the most noticeable discovery involving in the response to oxidative stress, which make sure that ROS is appropriately scavenged.22 Upon exposure to oxidative stress, Nrf2 rapidly dissociates from Keap1 and translocates into nucleus, where it interacts with ARE and accelerates the transcription of antioxidant genes to regulate DNA repair, xenobiotic metabolism and redox homeostasis.43 In addition, the phosphorylation and nuclear translocation of Nrf2 may promote the expression of HO-1 by binding to ARE to protect NP cells from oxidative stress injury induced by high glucose.44 Taken together, these studies reflect that oxidative stress has an adverse effect on the pathogenesis of IDD, and the therapeutic potential of targeting the Nrf2 pathways is deserved to highlight for the management of IDD.
Nrf2 and Cell Senescence
Cellular senescence is characterized by cell cycle arrest and secreting various inflammatory cytokines, chemokines and matrix proteases, which collectively are known as the senescence-associated secretory phenotype (SASP).45 As reported, NP cell senescence is a crucial contributor to the initiation and progression of IVD.46 Previous study demonstrated that inflammatory cytokine TNF-α promoted premature senescence of NP cells via PI3K/Akt signaling pathway, as indicated by decreased cell proliferation, increased senescence-associated beta-galactosidase (SA-β-gal) staining and the up-regulated expression of the senescence marker p16 and p53.47
Obviously, protecting NP cells against senescence is conducive to the amelioration of IDD. Che et al exhibited that the deficiency of p16 could attenuate IVD by promoting cell cycle and inhibiting SASP, cell senescence, and oxidative stress.48 Aged and degenerated IVDs have increased oxidative stress and NP cell senescence is associated with increased ROS level in IDD.49 Therefore, molecular signaling modulation related to ROS could be targeted to control IVD senescence and degeneration. To date, research has shown that the activation of Nrf2 signaling pathway enhanced cellular antioxidant capacity and inhibited cellular senescence, thereby exerting protective effects against aging diseases.50 A recent study by Dimozi et al demonstrated that prolonged exposure to H2O2 induces premature senescence in human NP cells and nuclear translocation of Nrf2 as a response to oxidative stress.38
These researches revealed that the exploration of antagonistic cell senescence by regulating Nrf2 signaling pathway may be a potential strategy for the treatment of IDD.
Nrf2 and Autophagy and Mitophagy
Autophagy is mainly regarded as a process for the clearance of impaired organelles, misfolded proteins and intracellular pathogens that were wrapped in autophagosomes and degraded by lysosomes. Autophagy-mediated degradation plays an important role in maintaining cellular homeostasis and development and protecting organisms against oxidative or proteotoxic stress.51 Given its vital impacts, there is no doubt that damaged autophagic pathway is associated with the pathogenesis of diverse diseases such as cancer, cardiomyopathy, diabetes, osteoarthritis and IDD.52 A latest study has shown that Nrf2/Keap1 is a key pathway to regulate the expression of antioxidant proteins, and autophagy can promote Nrf2 expression and nuclear translocation and activate Nrf2/Keap1 signaling pathway. As a result, the Nrf2/Keap1 complex is disrupted and Nrf2 translocated to the nucleus, where it activates the transcription of genes encoding antioxidant enzymes, thereby affecting ROS clearance and cartilage endplate senescence.53 Consistent with the above, Nrf2 is involved in the autophagic process in response to detrimental influence of H2O2-induced oxidative stress via Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration.54 Upon exposure to oxidative stresses, p62 works as an autophagy adaptor protein, sequestrated Keap1 into aggregates or autophagosomes, which harms the ubiquitylation of Nrf2, resulting in activating the Nrf2 signaling pathway.55 Furthermore, damaged mitochondria can be degraded by a specific type of autophagy called mitophagy, and Nrf2 plays a part in maintaining mitochondrial homeostasis through modulating mitochondrial function and metabolism such as mitochondrial dynamics and mitophagy.56 Recent evidences have shown that Parkin-mediated mitophagy for selectively removing dysfunctional mitochondria and Nrf2-mediated antioxidant system are critical to improve NP cells and endplate chondrocyte survival under pathological conditions.57 Recently, a study has shown that Nrf2 is a regulator of Sirtuin 3 (SIRT3) expression, which maintains mitochondrial homeostasis by promoting mitochondrial dynamics, antioxidation and mitophagy, and deacetylation of manganese superoxide dismutase (MnSOD).58 Hu et al proposed that Nrf2/Sirt3 signaling could activate mitophagy and restore the disruption of autophagic flux induced by oxidative stress, thereby protecting NP cells from apoptosis and ECM degradation.59 Taken together, these studies suggest that activating Nrf2 signaling to accelerate autophagy and mitophagy in NP cells is a promising strategy to treat IDD.
Nrf2 and Cell Death (Apoptosis, Pyroptosis, Ferroptosis)
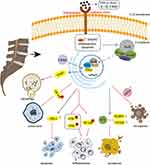
It is well known that excessive apoptosis of NP cells is regarded as the hallmarks of IVD degeneration.60 In addition to the widely accepted apoptosis, other forms of cell death are newly discovered in the progress of IDD, such as pyroptosis and ferroptosis, which are obviously distinct from apoptosis according to the cellular morphological and biochemical characteristics.7 Apoptosis is a type of programmed cell death via eliminating damaged cells by phagocytes, which is critical for the development and homeostasis of organism.61 It is mainly caused by excessive ROS via increasing mitochondrial outer membrane permeability and pro-apoptotic cytochrome c release.62 It has been reported that excessive and sustained endoplasmic reticulum (ER) stress leads to disc ECM degradation and NP cell apoptosis.63 Nrf2 activation inhibits oxidative stress-mediated ER stress and mitochondrial dysfunction.64 Wang et al proposed that Nrf2/HO-1 signaling pathway is involved in regulating lipopolysaccharide (LPS)-induced NP cell dysfunction, including apoptosis caused by oxidative stress, inflammation, and ER stress.65 Pyroptosis is a form of inflammatory cell death which is mediated by activated caspase 1, characterized by cell osmotic pressure changes and cell membrane rupture.66 According to the report, the relationship was between the negative regulatory effect of Nrf2 and H2O2-induced NP cell pyroptosis through the expression of NLR family pyrin domain containing 3 (NLRP3) and PYD and CARD domain containing (PYCARD) inflammasome.67 Studies have found that NLRP3 mediates pyroptosis and inflammation in H2O2-induced IVD degeneration. However, ferroptosis is a non-apoptotic cell death distinguished by mitochondrial shrinkage, enhanced mitochondrial membrane density, and accumulation of lipid peroxides. Nrf2 has been shown to participate in the development of IDD by reducing NP cell viability.68,69 In recent years, it has been found that enhancing glutathione peroxidase 4 (GPX4) expression can alleviate human NP cell degeneration, while Nrf2 regulates endplate cell calcification and ECM homeostasis and inhibits endplate cell ferroptosis by upregulating GPX4 expression70. As mentioned above, Nrf2 is known as a protective regulator, and it is not surprising that the activation of Nrf2 plays a critical role in mediating cellular death, including apoptosis, pyroptosis and ferroptosis.71
Therapeutic Strategies by Targeting Nrf2
Therapeutic Strategies by Targeting Nrf2, Inflammation, and ECM Metabolism
In recent years, numerous studies have demonstrated that Dimethyl fumarate (DMF) can activate Nrf2 effectively and promote the activation of its downstream antioxidant stress pathway.72 Zhu et al also revealed that DMF shown promising effects in the prevention of IDD by promoting the expression of Nrf2 and HO-1, significantly reducing the expression of inflammatory factors, increasing SOD activity, and reducing IL-1β-induced nuclear endoplasmic reticulum stress.73 Moreover, evidence has suggested that moracin can antagonize LPS-mediated inflammatory mediators such as IL-1β, TNF-α and IL-6. Interestingly, treating with Nrf2-specific siRNA, the anti-inflammatory effects were significantly reversed. Moreover, they found that moracin treatment could remarkably reverse the increased expression of MMP-3 and MMP-13 levels and decreased expression of collagen-I, Col2 and aggrecan.74
Furthermore, Wang et al demonstrated that Polydatin (PD) suppresses TNF‐α‐-induced ECM destruction via activating Nrf2/HO‐1 signaling pathway in rat NP cells to attenuate IVD degeneration.75 Consistent with the above, Cyanidin-3-glucoside (C3G) prevents HG-induced ROS production in human NP cells and reversing apoptosis and degradation of extracellular matrix through regulating the Nrf2/HO-1 pathway. Besides, they also found that C3G could increase the proteoglycan and Col2 content and inhibit MMP3 and MMP13 expression in human disc cells.76
Therapeutic Strategies by Targeting Nrf2 and Cell Senescence
Currently, it has been found that activation of Nrf2 signaling pathway may be an effective target for delaying cell senescence. Quercetin (QUE), a natural senolytic compound, could reduce the expression of senescence markers.77,78 Shao et al discovered that QUE promoted ECM homeostasis and inhibited SASP factors expression and senescence phenotype in NP cells. Mechanistically, QUE ameliorated the progression of IDD via activating Nrf2/NF-κB axis.79 PD, a resveratrol glucoside, possessed multiple pharmacological properties including anti-oxidation, anti-inflammation and anti-tumour. Wang et al found that PD prevented TNF-α-induced NP cell senescence by alleviating ROS-mediated mitochondrial dysfunction via promoting Nrf2 nucleus translocation and activating Nrf2/HO-1 signaling pathway.75 Consistent with the above, a previous work reported that kinsenoside (Kin) could suppress tert-butyl hydroperoxide (TBHP)-induced NP cell senescence under oxidative stress by activating of AKT-ERK1/2-Nrf2 signaling pathway to delay the progression of IDD.80 In summary, triggering Nrf2 activation to inhibit senescence of NP cells is a potential therapeutic method for IVD.
Therapeutic Strategies by Targeting Nrf2 and Apoptosis
Icariin, which is an isopentenyl flavonol glycoside isolated from epimedium, has been proved to have anti-inflammatory and antioxidant effects on NP cells.81 A recent study reported that icariin could activate Nrf2 signaling pathway, upregulate the protein expression of nuclear respiratory factor-1 and the mitochondrial transcription factor, promoting mitochondrial biogenesis to protect against H2O2-induced mitochondria-mediated human NP cell apoptosis.12 A recent study by Tian et al revealed that bardoxolone methyl (BARD) protected NP cells from compression-induced mitochondrial apoptosis and ECM degradation. The authors found that BARD could promote Nrf2 protein nuclear translocation and Nrf2 target protein overexpression.82 In addition, Nrf2 can maintain redox balance in NP cells by reducing ROS production from the source by maintaining mitochondrial homeostasis.14 Interestingly, another study reported that Luteoloside, a flavonoid glycoside extracted from the plant luteolin, was able to maintain cell morphology and inhibit inflammation and apoptosis characterized by the reduced expression of cleaved caspase 3 in IL-1β-treated NP cells. In addition, the study also demonstrated the protective effects of luteoloside in NP cells through activating Nrf2/HO-1 signaling axis.83 Selenium (Se), a vital component of antioxidant enzymes, is indispensable for maintaining redox homeostasis and promoting cell survival. It could suppress TBHP-induced oxidative stress and mitochondrial fission, rescued the imbalance of mitochondrial dynamics and inhibited apoptosis of NP cells to ameliorate IDD. Moreover, the effect of Se on protecting mitochondrial function was attributed to the activation of Nrf2 pathway.84 Se reversed the excessive apoptosis of NP cells induced by TBHP via reducing the excessive production of intracellular ROS and the increase of malondialdehyde (MDA) level, as well as upregulating the expression of superoxide dismutase 2 (SOD2).85 In addition, with the development of research, an increasing number of molecules have been shown to protect IVD cells from mitochondrial dysfunction and apoptosis by activating Nrf2 signaling, including VO-OHpic,86 Andrographolide,87 hyperoside,88 Amobarbital9 and Procyanidin B2.89
Therapeutic Strategies by Targeting Nrf2 and Pyroptosis
As a programmed necrosis different from classical apoptosis, pyroptosis can mediate the activation and release of inflammatory factors IL-1β and IL-18 by forming pores in the plasma membrane, which leads to inflammatory cascades and ECM degradation.66 Increasing evidence indicates that a series of pyroptosis-occurring elements such as caspase-1, Gasdermin-D (GSDMD), and NLRP3 inflammasome were found to increase or activate in the pathological progression of IDD.90 Existing evidence has revealed that application with melatonin significantly upregulated the expression of Nrf2 and downregulated the level of pyroptosis-related proteins such as NLRP3, cleaved caspase-1, gasdermin D, IL-18, and IL-1β to protect NP cells against ROS-induced pyroptosis.91 Milk fat globules-epidermal growth factor (EGF) factor 8 (MFG-E8) is an endogenous multifunctional glycoprotein, which is beneficial for anti-inflammatory, antioxidant, and modulation of NLRP3 inflammasome. Ma et al found that Nrf2/TXNIP/NLRP3 axis plays a crucial role in MFG-E8-mediated suppression of H2O2-induced oxidative stress, mitochondrial dysfunction, and NLRP3 inflammasome activation and protection of NP cells from pyroptosis and ECM degradation.92 Interestingly, consistent with the above, Xu et al showed that platelet-rich plasma (PRP)-derived exosomes miR-141-3p could reverse H2O2-induced NP cell pyroptosis by promoting the dissociation of Nrf2 from Keap1/Nrf2 complex and its further translocation contributing to achieve anti-oxidant biological functions and exert protective effects to slow down IVD translocation.93
Overall, these findings suggested that the regulation of Nrf2 in NP cell pyroptosis plays a crucial part in IDD treatment.
Therapeutic Strategies by Targeting Nrf2 and Ferroptosis
After explaining the effect of Nrf2 on apoptosis and pyroptosis of NP cells, ferroptosis has to be mentioned. As a form of iron-dependent necrotic cell death, ferroptosis is characterized by iron ion-catalyzed disorder of cellular lipid metabolism and aggravates the degeneration of nucleus pulposus.94 Yu et al found that bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles (EVs) carrying circ_0072464 could promote matrix synthesis and proliferation of NP cells via upregulating the expression of miR-431-mediated Nrf2 to inhibit IL-1β-induced NP cells ferroptosis. Collectively, BMSC-EVs alleviated intervertebral disc lesions through the circ_0072464/miR-431/Nrf2 axis.95 Hesperetin-7-o-rutinoside (HRD) is a flavanone glycoside extracted from lime peel powder. It belongs to the subgroup of citrus flavonoids because of its anti-inflammation and anti-oxidation properties.96 Previous studies have confirmed that HRD can alleviate oxidative stress and mitochondrial dysfunction through the Nrf2-ARE pathway.97 Recently, Zhu et al confirmed that HRD may protect NP cells from degeneration by mitigating oxidative stress-dependent ferroptosis via enhancing the expression of Nrf2 and suppressing NF-κB pathway.98 Astaxanthin (Ast), a natural lipid-soluble carotenoid, possesses antioxidant, anti-inflammatory, and anti-aging capacities. Additional supplement with Ast could protect vertebral cartilage endplate degeneration against TBHP-induced cartilage endplate chondrocytes ferroptosis including restoring the downregulated expression of GPX4, ferritin heavy chain (FTH1) and solute carrier family 7 member 11 (SLC7A11) via activating Nrf-2/HO-1 pathway.99
Taken together, it is demonstrated that targeting Nrf2 signaling axis inhibits NP cell ferroptosis, which may be an effective therapeutic approach for IDD intervention and treatment (Figure 2). In addition to some of the molecular mechanisms described above, there are many mechanisms and related signaling pathways elaborating the role of Nrf2 activation in IDD therapies, see Table 1.
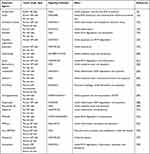 |
Table 1 Regulation of Nrf2 Signaling as Potential Therapeutic Options for IDD |
Therapeutic Strategies without Targeting Nrf2
Given the limited known researches on the momentous role of Nrf2-targeted therapeutic strategies for alleviating IDD, complementary findings pave way for the treatment and prevention of IDD. Wang et al found that phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1 (PINK1), a mitochondrial-targeted serine/threonine kinase, protects against mitochondrial dysfunction by activating PINK1/Parkin mediated mitophagy to clear damaged mitochondrial and alleviate H2O2-induced NP cell senescence.100 In addition, according to published studies, the expression of proliferator-activated receptor gamma (PPARγ) is significantly decreased in degenerative disc tissue. However, its activator pioglitazone could significantly inhibit the activation of the NF-κB pathway induced by IL-17 or IL-17 combined with TNF-α, reduce the levels of metalloproteinases, and preserve the expression of key matrix components such as aggrecan and Col2.101 Interestingly, more studies have provided new insight into the beneficial effect of bone marrow mesenchymal stem cells (BMSCs) transplantation in the prevention and treatment of IDD.102 A recent study also demonstrated that the TNF-α-stimulated gene 6 protein (TSG-6) secreted by BMSCs displays notable effect to inhibit the activation of the TLR2/NF-κB inflammatory pathway. Mechanistically, BMSC reduced the expression of MMPS (such as MMP-3 and MMP-13) and simultaneously increased the expression of Col2 and aggrecan in IL-1β-treated NP cells by enhancing TSG-6 expression.103 Consistent with the above, Zhang et al demonstrated that MSCs-exosomes could inhibit LPS-induced pyroptosis in NP cells. They found that administration of exosomal miR-3 downregulated NLRP410, resulting in decreased expression of caspase-1 and GSDMD, thereby weakening pyroptosis in NP cells to alleviate IDD.104 Furthermore, it has been reported that some other important biologically active components also ameliorate IDD without targeting Nrf2 signal, these compounds included oxymatrine liposomes,105 Caspase 3 siRNA,106 Adenovirus-mediated growth and differentiation factor-5 (Ad-GDF5),107 O-linked β-N-acetylglucosaminylation (O-GlcNAcylation),108 Gamma-oryzanol,109 ascorbic acid,110 prolactin,111 see Table 2.
 |
Table 2 Potential Therapeutic Options for IDD without Targeting Nrf2 |
In summary, there are still many studies that provide new insights into IDD treatment through different molecular mechanisms and pathways. It is well known that Nrf2 is considered a central hub modulating a variety of physiological and pathological processes, and the interaction between Nrf2 and further signal path and molecules remains an urged need to be clearly investigated in favour of providing more feasible method for the clinical treatment of IVD.
Conclusion
In this review, we synthesize and conduct deep understanding of the key mechanisms of Nrf2 in regulating the pathophysiological phenotypes of IDD. Inflammatory response, imbalance of ECM metabolism, oxidative stress-induced mitochondrial dysfunction, cell senescence and apoptosis, pyroptosis and ferroptosis of NP cells are the key characteristics of IDD. In general, the activation of Nrf2 or Nrf2-related signal axis can decrease the expression of inflammatory cytokines, inhibit ECM degradation, overexpression of ROS, cell senescence and NP cell death and promote the expression of antioxidant genes, matrix synthesis and mitophagy, which play a protective role in delaying the pathogenesis of IDD. This leads to the possibility that activation of this signaling pathway may open up new therapeutic approaches to support targeting Nrf2 for prevention and treatment of IDD. Although this review has proven the beneficial effects of Nrf2 for preventing IDD development in vitro and in vivo models, there is a lack of clinical application. Therefore, further systematic studies are demanded to clarify complicated mechanisms involved in IDD therapy depending on Nrf2 or the interaction with additional key molecule.
Abbreviations
IDD, Intervertebral disc degeneration; LBP, Lower back pain; Nrf2, Nuclear factor erythroid 2-related factor 2; NP, Nucleus pulposus; ECM, Extracellular matrix; IVD, Intervertebral disc; AF, Annulus fibrosus; ROS, Reactive oxygen species; MAPK, Mitogen-activated protein kinase; NF-κB, Nuclear factor kappa-B; GSH, Glutathione; TXN, Thioredoxin; NADPH, Nicotinamide adenine dinucleotide phosphate; GST, Glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1; HO-1, Heme oxygenase-1; CNC, Cap “n” Collar; Keap1, Kelch-like ECH associated protein 1; CBP, cAMP-response-element-binding protein; RXRα, Retinoid X receptor α; MAF, Myofascial fibrosarcoma; CHD6, Chromo-ATPase/helicase DNA-binding protein 6; ARE, Antioxidant response element; β-TrCP, β-transducing repeat-containing protein; Gsk-3b, Glycogen synthase kinase-3b; SCF, Skp1-Cul1-F-box; miRNAs, Micro-RNAs; IL-1β, Interleukin 1 beta; TNF-α, Tumor necrosis factor alpha; NO, Nitric oxide; COX-2, Cyclooxygenase-2; ADAMTS-5, A disintegrin and metalloproteinase with thrombospondin motifs-5; PGE2, Prostaglandin E2; MMP-13, Matrix metalloproteinase-13; iNOS, inducible nitric oxide synthase; CO, carbon monoxide; Col2, Collagen-II; PGs, Proteoglycans; GAG, Glycosaminoglycan; MMP3, Matrix metalloproteinase 3; FSS, Fluid shear stress; sGAG, sulfated glycosaminoglycan; H2O2, Hydrogen peroxide; NO, Nitric oxide; SASP, Senescence-associated secretory phenotype; SA-β-gal, Senescence-associated beta-galactosidase; SIRT3, Sirtuin3; MnSOD, Manganese superoxide dismutase; ER, Endoplasmic reticulum; LPS, Lipopolysaccharide; NLRP3, NLR family pyrin domain containing 3; PYCARD, PYD and CARD domain containing; GPX4, Glutathione peroxidase 4; DMF, Dimethyl fumarate; PD, Polydatin; C3G, Cyanidin-3-glucoside; QUE, Quercetin; PD, Polydatin; Kin, Kinsenoside; TBHP, Tert-butyl hydroperoxide; BARD, Bardoxolone methyl; Se, Selenium; MDA, Malondialdehyde; SOD2, Superoxide dismutase 2; GSDMD, Gasdermin-D; MFG-E8, Milk fat globules-epidermal growth factor (EGF) factor 8; PRP, Platelet-rich plasma; BMSCs, Bone marrow mesenchymal stem cells; EVs, Extracellular vesicles; HRD, Hesperetin-7-o-rutinoside; Ast, Astaxanthin; FTH1, Ferritin heavy chain 1; SLC7A11, Solute carrier family 7 member 11; PTEN, Phosphatase and tensin homolog; PINK1, (PTEN)-induced putative kinase protein 1; PPARγ, Proliferator-activated receptor gamma; TSG-6, TNF-α-stimulated gene 6 protein; Ad-GDF5, Adenovirus-mediated growth and differentiation factor-5; O-GlcNAcylation, O-linked β-N-cetylglucosaminylation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Urits I, Burshtein A, Sharma M, et al. Low back pain, a comprehensive review: pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep. 2019;23(3):23. doi:10.1007/s11916-019-0757-1
2. Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD. Treatment of chronic low back pain - new approaches on the horizon. J Pain Res. 2017;10:1111–1123. doi:10.2147/JPR.S132769
3. Xie C, Ma H, Shi Y, et al. Cardamonin protects nucleus pulposus cells against IL-1β-induced inflammation and catabolism via Nrf2/NF-κB axis. Food Funct. 2021;12(6):2703–2714. doi:10.1039/D0FO03353G
4. Liu W, Xia P, Feng J, et al. MicroRNA-132 upregulation promotes matrix degradation in intervertebral disc degeneration. Exp Cell Res. 2017;359(1):39–49. doi:10.1016/j.yexcr.2017.08.011
5. Li Z, Li X, Chen C, Chan MTV, Wu WKK, Shen J. Melatonin inhibits nucleus pulposus (NP) cell proliferation and extracellular matrix (ECM) remodeling via the melatonin membrane receptors mediated PI3K-Akt pathway. J Pineal Res. 2017;63(3). doi:10.1111/jpi.12435
6. Roh EJ, Darai A, Kyung JW, et al. Genetic therapy for intervertebral disc degeneration. Int J Mol Sci. 2021;22(4):1579. doi:10.3390/ijms22041579
7. Yang RZ, Xu WN, Zheng HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. 2021;236(4):2725–2739. doi:10.1002/jcp.30039
8. Nishimura Y, Kanda Y, Sone H, Aoyama H, Cipak Gasparovic A. Oxidative stress as a common key event in developmental neurotoxicity. Oxid Med Cell Longev. 2021;2021:6685204. doi:10.1155/2021/6685204
9. Seol D, Coleman MC, Martin JA, et al. Targeting oxidative stress with amobarbital to prevent intervertebral disc degeneration: part I. in vitro and ex vivo studies. Spine J. 2021;21(6):1021–1030. doi:10.1016/j.spinee.2021.02.008
10. Cao G, Yang S, Cao J, et al. The role of oxidative stress in intervertebral disc degeneration. Oxid Med Cell Longev. 2022;2022:2166817. doi:10.1155/2022/2166817
11. Ge J, Zhou Q, Cheng X, et al. The protein tyrosine kinase inhibitor, genistein, delays intervertebral disc degeneration in rats by inhibiting the p38 pathway-mediated inflammatory response. Aging. 2020;12(3):2246–2260. doi:10.18632/aging.102743
12. Hua W, Li S, Luo R, et al. Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. Biochim Biophys Acta Mol Basis Dis. 2020;1866(1):165575. doi:10.1016/j.bbadis.2019.165575
13. Xiang Q, Zhao Y, Lin J, Jiang S, Li W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: molecular insights. Exp Mol Med. 2022;54(8):1067–1075. doi:10.1038/s12276-022-00829-6
14. Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10(7):510. doi:10.1038/s41419-019-1701-3
15. Lu Y, Zhou L, He S, Ren HL, Zhou N, Hu ZM. Lycopene alleviates disc degeneration under oxidative stress through the Nrf2 signaling pathway. Mol Cell Probes. 2020;51:101559. doi:10.1016/j.mcp.2020.101559
16. Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43. doi:10.1016/j.ccell.2018.03.022
17. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signaling. 2018;29(17):1727–1745. doi:10.1089/ars.2017.7342
18. Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6(10):857–868. doi:10.1046/j.1365-2443.2001.00469.x
19. McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279(30):31556–31567. doi:10.1074/jbc.M403061200
20. Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A. 2005;102(29):10070–10075. doi:10.1073/pnas.0502402102
21. Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294(1–2):1–12. doi:10.1016/S0378-1119(02)00788-6
22. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. doi:10.1016/j.tibs.2014.02.002
23. Chatterjee N, Tian M, Spirohn K, Boutros M, Bohmann D, Thummel CS. Keap1-independent regulation of Nrf2 activity by protein acetylation and a BET bromodomain protein. PLoS Genet. 2016;12(5):e1006072. doi:10.1371/journal.pgen.1006072
24. Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32(32):3765–3781. doi:10.1038/onc.2012.388
25. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–1133. doi:10.1128/MCB.01204-10
26. Shah NM, Rushworth SA, Murray MY, Bowles KM, MacEwan DJ. Understanding the role of NRF2-regulated miRNAs in human malignancies. Oncotarget. 2013;4(8):1130–1142. doi:10.18632/oncotarget.1181
27. Liao ZW, Fan ZW, Huang Y, et al. Long non-coding RNA MT1DP interacts with miR-365 and induces apoptosis of nucleus pulposus cells by repressing NRF-2-induced anti-oxidation in lumbar disc herniation. Ann Transl Med. 2021;9(2):151. doi:10.21037/atm-20-8123
28. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am Vol. 2006;88(Suppl 2):10–14. doi:10.2106/JBJS.F.00019
29. Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58(5):588–598. doi:10.1002/glia.20947
30. Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
31. Tang H, Gao L, Mao J, et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 2016;21(2):239–249. doi:10.1007/s12192-015-0654-4
32. Zhang CY, Hu XC, Zhang GZ, Liu MQ, Chen HW, Kang XW. Role of Nrf2 and HO-1 in intervertebral disc degeneration. Connect Tissue Res. 2022;63(6):559–576. doi:10.1080/03008207.2022.2089565
33. Fang W, Zhou X, Wang J, et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. 2018;65:539–549. doi:10.1016/j.intimp.2018.10.024
34. Krupkova O, Hlavna M, Amir Tahmasseb J, et al. An inflammatory nucleus pulposus tissue culture model to test molecular regenerative therapies: validation with epigallocatechin 3-gallate. Int J Mol Sci. 2016;17(10):1640. doi:10.3390/ijms17101640
35. Chen S, Qin L, Wu X, et al. Moderate fluid shear stress regulates heme oxygenase-1 expression to promote autophagy and ECM homeostasis in the nucleus pulposus cells. Front Cell Dev Biol. 2020;8:127. doi:10.3389/fcell.2020.00127
36. Wang H, Jiang Z, Pang Z, Zhou T, Gu Y. Acacetin alleviates inflammation and matrix degradation in nucleus pulposus cells and ameliorates intervertebral disc degeneration in vivo. Drug Des Devel Ther. 2020;14:4801–4813. doi:10.2147/DDDT.S274812
37. Hu B, Shi C, Xu C, et al. Heme oxygenase-1 attenuates IL-1β induced alteration of anabolic and catabolic activities in intervertebral disc degeneration. Sci Rep. 2016;6(1):21190. doi:10.1038/srep21190
38. Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cells Mater. 2015;30:89–102; discussion 103. doi:10.22203/eCM.v030a07
39. Hou G, Lu H, Chen M, Yao H, Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr. 2014;59(3):665–669. doi:10.1016/j.archger.2014.07.002
40. Yang L, Rong Z, Zeng M, et al. Pyrroloquinoline quinone protects nucleus pulposus cells from hydrogen peroxide-induced apoptosis by inhibiting the mitochondria-mediated pathway. European Spine J. 2015;24(8):1702–1710. doi:10.1007/s00586-014-3630-2
41. Feng C, Yang M, Lan M, et al. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid Med Cell Longev. 2017;2017:5601593. doi:10.1155/2017/5601593
42. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signalling. 2012;24(5):981–990. doi:10.1016/j.cellsig.2012.01.008
43. Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17(24):3145–3156. doi:10.1038/sj.onc.1202237
44. Zhang CX, Wang T, Ma JF, Liu Y, Zhou ZG, Wang DC. Protective effect of CDDO-ethyl amide against high-glucose-induced oxidative injury via the Nrf2/HO-1 pathway. Spine J. 2017;17(7):1017–1025. doi:10.1016/j.spinee.2017.03.015
45. He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi:10.1016/j.cell.2017.05.015
46. Patil P, Dong Q, Wang D, et al. Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18(3):e12927. doi:10.1111/acel.12927
47. Li P, Gan Y, Xu Y, et al. The inflammatory cytokine TNF-α promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Sci Rep. 2017;7:42938. doi:10.1038/srep42938
48. Che H, Li J, Li Y, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. eLife. 2020;9. doi:10.7554/eLife.52570
49. Silwal P, Nguyen-Thai AM, Mohammad HA, et al. Cellular senescence in intervertebral disc aging and degeneration: molecular mechanisms and potential therapeutic opportunities. Biomolecules. 2023;13(4):686. doi:10.3390/biom13040686
50. Guo H, Chen J, Yu H, et al. Activation of Nrf2/ARE pathway by anisodamine (654-2) for inhibition of cellular aging and alleviation of radiation-induced lung injury. Int Immunopharmacol. 2023;124(Pt A):110864. doi:10.1016/j.intimp.2023.110864
51. Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi:10.4161/auto.19496
52. Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575–581. doi:10.1136/annrheumdis-2011-200557
53. Zuo R, Wang Y, Li J, et al. Rapamycin induced autophagy inhibits inflammation-mediated endplate degeneration by enhancing Nrf2/Keap1 signaling of cartilage endplate stem cells. Stem Cells. 2019;37(6):828–840. doi:10.1002/stem.2999
54. Kapuy O, Papp D, Vellai T, Bánhegyi G, Korcsmáros T. Systems-level feedbacks of NRF2 controlling autophagy upon oxidative stress response. Antioxidants. 2018;7(3). doi:10.3390/antiox7030039
55. Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi:10.1111/j.1365-2443.2010.01473.x
56. Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179–188. doi:10.1016/j.freeradbiomed.2015.04.036
57. Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X. Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci. 2020;243:117244. doi:10.1016/j.lfs.2019.117244
58. Wang J, Nisar M, Huang C, et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp Mol Med 2018;50(11):1–14.
59. Hu S, Zhang C, Qian T, et al. Promoting Nrf2/Sirt3-dependent mitophagy suppresses apoptosis in nucleus pulposus cells and protects against intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021:6694964. doi:10.1155/2021/6694964
60. Liu Y, Li JM, Hu YG. Transplantation of gene-modified nucleus pulposus cells reverses rabbit intervertebral disc degeneration. Chinese Med J. 2011;124(16):2431–2437.
61. Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082–2092. doi:10.1080/15548627.2020.1822097
62. Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signaling. 2009;11(11):2777–2789. doi:10.1089/ars.2009.2804
63. Krupkova O, Sadowska A, Kameda T, et al. p38 MAPK facilitates crosstalk between endoplasmic reticulum stress and IL-6 release in the intervertebral disc. Front Immunol. 2018;9:1706. doi:10.3389/fimmu.2018.01706
64. Yang ZB, Chen WW, Chen HP, Cai SX, Lin JD, Qiu LZ. MiR-155 aggravated septic liver injury by oxidative stress-mediated ER stress and mitochondrial dysfunction via targeting Nrf-2. Exp Mol Pathol. 2018;105(3):387–394. doi:10.1016/j.yexmp.2018.09.003
65. Wang R, Luo D, Li Z, Han H. Dimethyl fumarate ameliorates nucleus pulposus cell dysfunction through activating the Nrf2/HO-1 pathway in intervertebral disc degeneration. Comput Math Methods Med 2021;2021:6021763. doi:10.1155/2021/6021763
66. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
67. Bai Z, Liu W, He D, et al. Protective effects of autophagy and NFE2L2 on reactive oxygen species-induced pyroptosis of human nucleus pulposus cells. Aging. 2020;12(8):7534–7548. doi:10.18632/aging.103109
68. Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi:10.3389/fphys.2019.00139
69. Xiao L, Xu X, Zhang F, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi:10.1016/j.redox.2016.12.022
70. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi:10.1016/j.cell.2017.09.021
71. Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi:10.1016/j.redox.2019.101107
72. Oey O, Rao P, Luciuk M, et al. Effect of dimethyl fumarate on renal disease progression in a genetic ortholog of nephronophthisis. Exp Biol Med. 2018;243(5):428–436. doi:10.1177/1535370218759313
73. Zhu H, Chen G, Wang Y, et al. Dimethyl fumarate protects nucleus pulposus cells from inflammation and oxidative stress and delays the intervertebral disc degeneration. Exp Ther Med. 2020;20(6):269. doi:10.3892/etm.2020.9399
74. Gu R, Huang Z, Liu H, et al. Moracin attenuates LPS-induced inflammation in nucleus pulposus cells via Nrf2/HO-1 and NF-κB/TGF-β pathway. Biosci Rep 2019;39(12). doi:10.1042/BSR20191673
75. Wang J, Huang C, Lin Z, et al. Polydatin suppresses nucleus pulposus cell senescence, promotes matrix homeostasis and attenuates intervertebral disc degeneration in rats. J Cell Mol Med. 2018;22(11):5720–5731. doi:10.1111/jcmm.13848
76. Bai X, Lian Y, Hu C, et al. Cyanidin-3-glucoside protects against high glucose-induced injury in human nucleus pulposus cells by regulating the Nrf2/HO-1 signaling. J Appl Toxicol. 2022;42(7):1137–1145. doi:10.1002/jat.4281
77. Sun Y, Coppé JP, Lam EW. Cellular senescence: the sought or the unwanted? Trends Mol Med. 2018;24(10):871–885. doi:10.1016/j.molmed.2018.08.002
78. Kim SR, Jiang K, Ogrodnik M, et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 2019;213:112–123. doi:10.1016/j.trsl.2019.07.005
79. Shao Z, Wang B, Shi Y, et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage. 2021;29(3):413–422. doi:10.1016/j.joca.2020.11.006
80. Wang Y, Zuo R, Wang Z, et al. Kinsenoside ameliorates intervertebral disc degeneration through the activation of AKT-ERK1/2-Nrf2 signaling pathway. Aging. 2019;11(18):7961–7977. doi:10.18632/aging.102302
81. Hua W, Zhang Y, Wu X, et al. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr Pharm Des. 2018;23(39):6071–6078. doi:10.2174/1381612823666170615112158
82. Tian Y, Duan J, Cao Y, Zhou H, Diwan AD, Tu J. Bardoxolone methyl ameliorates compression-induced oxidative stress damage of nucleus pulposus cells and intervertebral disc degeneration ex vivo. Front Bioeng Biotechnol. 2021;9:814040. doi:10.3389/fbioe.2021.814040
83. Lin J, Chen J, Zhang Z, et al. Luteoloside inhibits IL-1β-induced apoptosis and catabolism in nucleus pulposus cells and ameliorates intervertebral disk degeneration. Front Pharmacol. 2019;10:868. doi:10.3389/fphar.2019.00868
84. Shalihat A, Hasanah AN, Lesmana R, Budiman A, Gozali D. The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: a literature review. Biomed Pharmacother. 2021;134:111125. doi:10.1016/j.biopha.2020.111125
85. Wang P, Zhang S, Liu W, et al. Selenium attenuates TBHP-induced apoptosis of nucleus pulposus cells by suppressing mitochondrial fission through activating nuclear factor erythroid 2-related factor 2. Oxid Med Cell Longev. 2022;2022:7531788. doi:10.1155/2022/7531788
86. Cui X, Liu X, Kong P, et al. PTEN inhibitor VO-OHpic protects endplate chondrocytes against apoptosis and calcification via activating Nrf-2 signaling pathway. Aging. 2023;15(6):2275–2292. doi:10.18632/aging.204612
87. Zhang C, Lu Z, Lyu C, Zhang S, Wang D. Andrographolide inhibits static mechanical pressure-induced intervertebral disc degeneration via the MAPK/Nrf2/HO-1 pathway. Drug Des Devel Ther. 2023;17:535–550. doi:10.2147/DDDT.S392535
88. Xie T, Yuan J, Mei L, Li P, Pan R. Hyperoside ameliorates TNF‑α‑induced inflammation, ECM degradation and ER stress‑mediated apoptosis via the SIRT1/NF‑κB and Nrf2/ARE signaling pathways in vitro. Mol Med Rep. 2022;26(2). doi:10.3892/mmr.2022.12776
89. Zou YP, Zhang QC, Zhang QY, Jiang LB, Li XL. Procyanidin B2 alleviates oxidative stress-induced nucleus pulposus cells apoptosis through upregulating Nrf2 via PI3K-Akt pathway. J Orthop Res. 2023;41(7):1555–1564. doi:10.1002/jor.25492
90. Chao-Yang G, Peng C, Hai-Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29(6):793–801. doi:10.1016/j.joca.2021.02.204
91. Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8:10. doi:10.1038/s41413-020-0087-2
92. Ma H, Xie C, Chen Z, et al. MFG-E8 alleviates intervertebral disc degeneration by suppressing pyroptosis and extracellular matrix degradation in nucleus pulposus cells via Nrf2/TXNIP/NLRP3 axis. Cell Death Discovery. 2022;8(1):209. doi:10.1038/s41420-022-01002-8
93. Xu J, Xie G, Yang W, Wang W, Zuo Z, Wang W. Platelet-rich plasma attenuates intervertebral disc degeneration via delivering miR-141-3p-containing exosomes. Cell Cycle. 2021;20(15):1487–1499. doi:10.1080/15384101.2021.1949839
94. Zhang X, Huang Z, Xie Z, et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med. 2020;160:552–565. doi:10.1016/j.freeradbiomed.2020.08.029
95. Yu X, Xu H, Liu Q, et al. circ_0072464 shuttled by bone mesenchymal stem cell-secreted extracellular vesicles inhibits nucleus pulposus cell ferroptosis to relieve intervertebral disc degeneration. Oxid Med Cell Longev. 2022;2022:2948090. doi:10.1155/2022/2948090
96. Qi W, Lin C, Fan K, et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. doi:10.1016/j.cbi.2019.04.002
97. Li J, Wang T, Liu P, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12(9):3898–3918. doi:10.1039/D0FO02736G
98. Zhu J, Sun R, Yan C, et al. Hesperidin mitigates oxidative stress-induced ferroptosis in nucleus pulposus cells via Nrf2/NF-κB axis to protect intervertebral disc from degeneration. Cell Cycle. 2023;22(10):1196–1214. doi:10.1080/15384101.2023.2200291
99. Yang G, Liu X, Jing X, et al. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway. Int Immunopharmacol. 2023;119:110159. doi:10.1016/j.intimp.2023.110159
100. Wang Y, Shen J, Chen Y, et al. PINK1 protects against oxidative stress induced senescence of human nucleus pulposus cells via regulating mitophagy. Biochem Biophys Res Commun. 2018;504(2):406–414. doi:10.1016/j.bbrc.2018.06.031
101. Liu Y, Qu Y, Liu L, et al. PPAR-γ agonist pioglitazone protects against IL-17 induced intervertebral disc inflammation and degeneration via suppression of NF-κB signaling pathway. Int Immunopharmacol. 2019;72:138–147. doi:10.1016/j.intimp.2019.04.012
102. Fassett DR, Kurd MF, Vaccaro AR. Biologic solutions for degenerative disk disease. J Spinal Disord. 2009;22(4):297–308. doi:10.1097/BSD.0b013e31816d5f64
103. Yang H, Tian W, Wang S, et al. TSG-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-κB signaling pathway. Lab Invest. 2018;98(6):755–772. doi:10.1038/s41374-018-0036-5
104. Zhang J, Zhang J, Zhang Y, et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020;24(20):11742–11754. doi:10.1111/jcmm.15784
105. Wang H, Ding Y, Zhang W, et al. Oxymatrine liposomes for intervertebral disc treatment: formulation, in vitro and vivo assessments. Drug Des Devel Ther. 2020;14:921–931.
106. Sudo H, Minami A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 2011;63(6):1648–1657. doi:10.1002/art.30251
107. Liang H, Ma SY, Feng G, Shen FH, Joshua Li X. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10(1):32–41. doi:10.1016/j.spinee.2009.10.006
108. Luo R, Li G, Zhang W, et al. O-GlcNAc transferase regulates intervertebral disc degeneration by targeting FAM134B-mediated ER-phagy. Exp Mol Med. 2022;54(9):1472–1485. doi:10.1038/s12276-022-00844-7
109. Xu H, Dai ZH, He GL, et al. Gamma-oryzanol alleviates intervertebral disc degeneration development by intercepting the IL-1β/NLRP3 inflammasome positive cycle. Phytomedicine. 2022;102:154176. doi:10.1016/j.phymed.2022.154176
110. Yi YY, Zhang SB, Chen H, Xu HW, Wang SJ. Ascorbic acid promotes nucleus pulposus cell regeneration by regulating proliferation during intervertebral disc degeneration. J Nutr Biochem. 2022;108:109099. doi:10.1016/j.jnutbio.2022.109099
111. Wu X, Liu Y, Guo X, et al. Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF-κB pathway in rats. Cell Death Dis. 2018;9(2):98. doi:10.1038/s41419-017-0151-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.