Back to Journals » Journal of Inflammation Research » Volume 16
Dental Pulp Stem Cells Ameliorate Elastase-Induced Pulmonary Emphysema by Regulating Inflammation and Oxidative Stress
Received 12 January 2023
Accepted for publication 17 March 2023
Published 8 April 2023 Volume 2023:16 Pages 1497—1508
DOI https://doi.org/10.2147/JIR.S402794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiaoli Gao, Zhiqiang Liu, Zuomin Wang
Department of Stomatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China
Correspondence: Zuomin Wang; Zhiqiang Liu, Department of Stomatology, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86 10 85231492, Email [email protected]; [email protected]
Background: Dental pulp stem cells (DPSCs) are considered excellent candidates for stem cell-based tissue regeneration. In this study, we aimed to evaluate the therapeutic effect of DPSCs in a mouse chronic obstructive pulmonary disease (COPD) model and to explore whether DPSCs reduce lung inflammation and oxidative stress by regulating the nuclear factor erythroid‐2 related factor‐2 (Nrf2) signaling pathway.
Methods: DPSCs were isolated from dental pulp tissue by the tissue block method. Emphysema of C57BL/6 mice was induced by endotracheal administration of porcine pancreatic elastase (PPE). Then, the DPSCs were injected into the lungs through the trachea, and after 3 weeks of stem cell treatment, various efficacy tests were performed. The AniRes2005 animal lung function analytic system was used to detect lung function. Hematoxylin-eosin staining (H&E) and Victoria blue staining was used to assess emphysema severity. The animal tissues were detected by Western blot, RT‒qPCR, ELISA and oxidative stress related detection.
Results: In experimental COPD models, DPSCs transplantation improved lung function, body weight, and emphysema-like changes better than bone marrow mesenchyml stem cells (BM-MSCs). Compared with the COPD group, the levels of IL-1β, TNF-α and IL-6 in lung tissue and bronchoalveolar lavage fluid (BALF) were decreased after transplantation of DPSCs. DPSCs may be associated with lower malondialdehyde (MDA) levels, and higher catalase (CAT) and glutathione (GSH) levels. Western blot results showed that the expression of Nrf2 and its downstream factors increased after transplantation of DPSCs.
Conclusion: The current study showed that DPSCs had good performance in the treatment of a mouse COPD model and could be a promising option for stem cell therapy. DPSCs may play antioxidant and anti-inflammatory roles in COPD by activating the Nrf2 signaling pathway.
Keywords: chronic obstructive pulmonary disease, dental pulp stem cells, Nrf2, inflammation, oxidative stress
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease, and it covers a diverse group of diseases with common functional features, such as chronic bronchitis, chronic respiratory failure, and emphysema.1,2 Affecting nearly 400 million people, the World Health Organization predicts that COPD will be the third leading cause of death worldwide by 2030. Although progress has been made in treating symptoms and preventing acute exacerbations, little progress has been made in improving disease progression or affecting mortality.3
COPD is a heterogeneous disease associated with irreversible airway obstruction, destruction of alveoli, and chronic inflammation.4 In recent years, a hypothesis about the pathogenesis of chronic obstructive pulmonary disease is that the increase in reactive oxygen species (ROS) (directly caused by smoking and indirectly caused by the increase in reactive oxygen species released by inflammatory cells) may not be fully balanced by the lung antioxidant system, leading to oxidative stress.5,6 Excessive oxidants may lead to increased expression of pro-inflammatory genes and protein release, inactivation of antiproteases, and oxidative tissue damage in COPD.7 Therefore, treatments with antioxidant and anti-inflammatory properties may be beneficial in preventing or slowing the progression of COPD. Over the past few years, there has been a growing interest in the use of antioxidants as drug treatments for airway diseases.8
Mesenchymal stromal cells (MSCs) are cells of nonhematopoietic origin that have the ability to differentiate into multiple lineages of mesenchyme.9 Due to its potent and broad immunomodulatory activities, bacterial clearance, tissue regeneration and proangiogenic and antifibrotic properties, it offers a novel and promising therapeutic option, and its therapeutic effect has been confirmed in various types of lung disease, including chronic obstructive pulmonary disease.10
While all MSCs have similar general properties, different sources of MSCs have an impact on growth factors, cytokines, extracellular vesicles and secreted bioactive factors in regenerative environments, thus affecting clinical outcomes.11 Bone marrow is the traditional way of harvesting mesenchymal stem cells; however, the highly invasive process of obtaining bone marrow from donors and the need for multiple bone marrow aspirations to obtain a sufficient number of transplanted cells make bone marrow mesenchymal stem cells (BM-MSCs) transplantation a very unsatisfactory approach in clinical practice.11 Since their first isolation in 2000, dental pulp stem cells (DPSCs) have attracted wide attention due to their easy accessibility, lack of ethical issues, low immunogenicity, and multidirectional differentiation potential.12–14 Moreover, compared with traditional sources of mesenchymal stem cells, DPSCs have stronger proliferative, anti-inflammatory and antifibrotic abilities.15,16 Currently, DPSCs and their derivatives have shown beneficial effects in a variety of disease models in different tissues and organs and are considered excellent candidates for stem cell-based tissue regeneration.17–19 At present, there are no studies on the application of DPSCs in the treatment of COPD.
Nuclear factor erythroid 2-associated factor 2 (Nrf2) is a key transcription factor that protects the lungs from oxidative damage and inflammation.20,21 Bousnaki et al used proteomics to analyze the anti-inflammatory, antioxidant, and angiogenesis potential of DPSCs secretome under different conditions and concluded that it can be considered a promising therapeutic tool for the treatment of diseases involving oxidative stress and inflammation.22 DPSC secretes common anti-inflammatory cytokines, such as matrix metalloproteinase-3 (MMP-3) and IL-10, and DPSCs secretome contains several proteins with antioxidant properties, such as superoxide dismutase, catalase, and heme oxygenase (HO1).22 Moreover, the secretion of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) by DPSC can enhance the proliferation and migration of endothelial cells and induce angiogenesis.23
Therefore, the purpose of this study was to evaluate the therapeutic effect of DPSCs in a mouse COPD model and to explore whether DPSCs reduce lung inflammation and oxidative stress by regulating the Nrf2 signaling pathway.
Methods
The Isolation and Culture of DPSCs
The study approved by the institutional ethical Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University. All study donors provided written informed consent, in accordance with the Declaration of Helsinki. We isolated DPSCs from the discarded third molars of patients (18–22 years old), and DPSCs were isolated as previously described.12 In short, pulp tissue was cut into 0.5 mm^3 pieces, and the tissue was seeded into 60 mm culture dishes randomly and covered with alpha Modified Eagle Medium (α-MEM) (Gibco BRL, USA) with 20% foetal bovine serum (FBS) (HyClone, Logan, UT, USA) and 1% 100 IU/mL penicillin/streptomycin (Gibco BRL, USA). The dishes were incubated at 37°C with 5% CO2, and added culture medium to 4mL after 24 hours. Then the culture medium was changed every three days. Cell growth was monitored periodically with an inverted phase contrast microscope (Olympus, Japan), when the cells grew to 80~90% confluence, they were subcultured by trypsinastion (Gibco BRL, USA), and the third-generation pulp stem cells were harvested and prepared for characterization.
The Characterization of DPSCs
Cell surface markers, including CD73, CD90, CD105, CD45, CD34 and HLA-DR were identified by flow cytometry. All fluorescently labeled antibodies were purchased from eBioscience. Third-generation DPSCs were digested with trypsin and washed with PBS 1–2 times. The DPSCs suspension was incubated with the antibody and its allotype control at 4°C for 30 min. Cell surface markers were then detected by an Attune™ Nxt Acoustic Focusing Flow Cytometer system (Thermo Fisher Scientific, Cleveland, OH, USA).
Identification of multidirectional differentiation ability: When transplanted to the third generation, the complete medium was replaced with osteogenic differentiation medium (α-MEM, 10% FBS, 1% penicillin/streptomycin, 1% β-sodium glycershate (Sigma‒Aldrich, MO, USA), 1% vitamin C (Sigma‒Aldrich, MO, USA), 0.2% dexamethasone (Sigma‒Aldrich, MO, USA)), or lipogenic differentiation medium (Oricell, Guangzhou, China). Then, lipid formation was identified by angelic oil red O staining at day 7, and calcium formation was identified by alizarin red staining at day 21.
Source and Culture of BM-MSCs
Clinical grade human bone marrow mesenchymal stem cells were provided by ScienCell Research Laboratories. The cells were maintained in alpha Modified Eagle Medium (α-MEM) (Gibco BRL, USA) with 10% foetal bovine serum (HyClone, Logan, UT, USA) and 1% 100 IU/mL penicillin/streptomycin (Gibco BRL, USA).
Animal Model Preparation
Specific pathogen-free-grade C57BL/6 mice (male, 180–200 g, 8 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All animal procedures were approved by the ethical standards of the Animal Ethics Committee of Capital Medical University, and were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals published by the US NIH (NIH publication No. 85-23, revised 2011)” and Basel Declaration. The mice were randomly assigned to different groups with at least six mice in each group. The COPD model was induced by intratracheal injection (IT) of 0.01 (U/g body weight) PPE (Sigma‒Aldrich, MO, USA).24 After 2 weeks, mice were randomly selected for intratracheal injection of 5*10^5 fifth generation BM-MSCs in 50 µL PBS (COPD/BM-MSCs group), 5*10^5 fifth generation DPSCs in 50 µL PBS (COPD/DPSCs group), or 50 µL PBS (COPD group). The mice were sacrificed at the fifth week (Figure 1A).
Lung Function Tests
Mice were intraperitoneally injected with 2% pentobarbital (75 mg/kg) and subjected to tracheotomy. Then, the mice were placed in a plexiglass whole-body plethysmograph in a supine position. Expiratory velocity is monitored using a tube attached to the trachea in a pressure sensor, and changes in lung volume are assessed based on changes in pressure in the plethysmographic chamber. The AniRes2005 animal lung function analytic system (Beijing Bestlab High-Tech, China) was used to detect lung function, and the function parameters were automatically measured, including the ratio of forced expiratory volume in 0.1 s to forced vital capacity (FEV0.1/FVC), resistance of lung (RL), and respireatory dynamic compliance (Cydn).
Histological Assessment
Mouse lung tissues were placed in 4% paraformaldehyde, embedded in paraffin, cut into 4-µm sections. Then the lung tissue sections were subjected to hematoxylin and eosin (H&E) and Victoria blue staining. Image-Pro Plus 6.0 software was used to calculate the proportion of alveolar space area and mean linear intercept (MLI) of lung tissue according to the previously described method.25,26 ImageJ software (NIH, USA) was used to calculate the content of elastic fibers around small airways and in lung parenchyma.
Airway Inflammation Assay
The right lung was ligated, and the left lung was repeatedly lavaged with 400 µL PBS through endotracheal intubation to obtain bronchoalveolar lavage fluid (BALF). After centrifugation (1000 rpm, 10 minutes), the supernatant was tested for inflammatory factors (TNF-α and IL-1β) by commercial ELISA kits (Solarbio, Beijing, China) according to the manufacturers’ instructions.
Real-Time qPCR
Total RNA was extracted from lung tissues using an RNAeasy™ Animal RNA Isolation Kit with a Spin Column (Beyotime Biotechnology, Shanghai, China) and reverse-transcribed to cDNA using a FastKing cDNA First Strand Synthesis Kit (Tiangen, Beijing, China). Real-time qPCR was performed using AceQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and an ABI 7500 system (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s instructions. The primers were as follows: IL-6, 5′-CCACTTCACAAGTCGGAGGCTTA-3′ (Forward), 5′-GCAAGTGCATCATCGTTGTTCATAC-3′ (Reverse); IL-1β, 5′-CGCAGCAGCACATCAACAAGAGC-3′ (Forward), 5′-TGTCCTCATCCTGGAAGGTCCACG-3′ (Reverse); TNF-α, 5′- AAAGGGGATTATGGCTCAGG-3′ (Forward), 5′-CTCCCTTTGCAGAACTCAGG-3′ (Reverse); GAPDH, 5′-AGAAGGTGGTGAAGCAGGCATC-3′ (Forward), 5′-CGAAGGTGGAAGAGTGGGAGTTG-3′ (Reverse); NFE2L2, 5′-CTTTAGTCAGCGACAGAAGGAC-3′ (Forward), 5′-AGGCATCTTGTTTGGGAATGTG-3′ (Reverse); and Hmox1, 5′-AGGTACACATCCAAGCCGAGA-3′ (Forward), 5′-CATCACCAGCTTAAAGCCTTCT-3′ (Reverse).
Immunohistochemical Analysis
The paraffin sections were dewaxed and rehydrated, followed by antigen retrieval, inactivation of endogenous peroxidase and non-specific block. Then, sections were incubated with anti-NRF2 antibodies (1:300 dilution; Proteintech) at 4°C for 12 h and incubated with horseradish peroxidase-conjugated secondary antibodies (ZSGB-BIO, Beijing, China) at 37°C for 50 min. DAB (ZSGB-BIO, Beijing, China) was used to visualize nrf2 expression, and the positive result was brownish yellow. Hematoxylin was used for nuclear staining.
Western Blot
Lung tissues were lysed in RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing protease and phosphatase inhibitors. Protein extracts were separated by 10% SDS‒PAGE and transferred to 0.22 μm polyvinylidene fluoride membranes at 240 mA at constant flow. After blocking the membrane at room temperature for 1 hour, the membranes were incubated overnight at 4 °C with the following primary antibodies: anti-Nrf2 (1:1500, Proteintech), anti-NQO1 (1:5000, Proteintech), anti-HO-1 (1:1500, Proteintech), anti-SOD2 (1:5000, Proteintech), and anti-tubulin (1:6000, Proteintech). Then, the membranes were washed with TBST 3 times and incubated with HRP-conjugated secondary antibodies (1:5000, Proteintech) for 1.5 hours at room temperature in 1:1000 diluent. Proteins were visualized with an ECL detection kit, and protein band intensity was analyzed by ImageJ software (NIH, USA).
MDA, CAT and GSH Level Measurements
The contents of malondialdehyde (MDA), catalase (CAT), and glutathione (GSH) were measured using a kit purchased from Nanjing Jiancheng Bioengineering Institute, according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 version 7.0, and values are expressed as the mean ± standard deviation. One-way ANOVA followed by Tukey’s test was used to compare the different groups. When results were not normally distributed, Kruskal–Wallis test was used, followed by Dunn’s test. P value <0.05 was considered significant.
Results
Isolation and Characterization of DPSCs
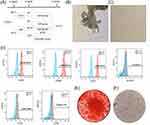
After 5–8 days, cells penetrated out from pulp tissue pieces (Figure 1B), and primary DPSCs were long spindle or polygonal with different morphologies. By the third passage, DPSCs displayed a homogeneous, fibroblast-like morphology (Figure 1C). Flow cytometry analysis demonstrated that the third-generation DPSCs expressed high levels of CD73 (99.2%), CD90 (99.7%), and CD105 (98.2%) but almost did not express CD45 (0.17%), CD34 (0.045%) and HLA-DR (0%) (Figure 1D). To identify the multidirectional differentiation ability of DPSCs, osteogenic differentiation culture and lipogenic differentiation culture were performed on the third generation of DPSCS. The results of oil red staining and alizarin red staining showed that DPSCs could differentiate into adipocytes and osteocytes (Figure 1E and F). Thus, these results confirmed that the cells cultured in our study are dental pulp stem cells, which can be used in subsequent experiments.
DPSCs Transplantation Improves Body Weight in a Mouse COPD Model
To investigate the effects of DPSCs on the reversal of emphysematous tissue damage induced by PPE, we transferred DPSCs and BM-MSCs into mice 2 weeks after intratracheal elastase infusion.
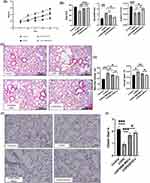
Figure 2A shows the body weight changes of mice during the experimental period. The body weight of mice in the four groups increased, among which the COPD group had the least weight gain, and the DPSCs/COPD group and BM-MSCs/COPD group had less weight gain than the control group.
DPSCs Transplantation Improves Lung Function in a Mouse COPD Model
To further investigate the functional effects of DPSCs transplantation, lung function tests were performed at the fifth week. The results (Figure 2B) showed that the DPSCs transplantation groups had significantly improved respiratory dynamic compliance (Cydn) and lung resistance (RL) compared with the COPD group, while BM-MSCs transplantation group had no significant statistical difference compared with COPD group. Both DPSCs group and BM-MSCs group showed significant improvement in FEV0.1/FVC compared with COPD group.
DPSCs Transplantation Improves Emphysematous Changes in a Mouse COPD Model
Mice were sacrificed after 3 weeks of stem cell treatment, and HE staining showed that in the COPD group, the alveolar space was enlarged, the alveolar number was decreased, and some alveolar septa were thinner or even fractured to the pulmonary bulla. Compared with the COPD group, the BM-MSCs or DPSCs transplantation group had significantly fewer emphysematous changes (Figure 2C). Then, the expansion of alveolar space was quantified by measuring the proportion of alveolar space area and the mean linear intercept (MLI) of alveolar space using the Image-Pro Plus program. The results showed that the mean linear intercept of the BM-MSCs or DAPCs transplantation group was lower than that of the COPD group (P<0.05), but there was no significant difference between the two groups (Figure 2D). The proportion of alveolar space in the BM-MSCs and DAPCs treatment groups was lower than that in the COPD group, and the reduction was more obvious in the DAPCs/COPD group (P<0.05) (Figure 2D).
Victoria Blue staining was used to show the amount of elastic fibers in lung tissue. The elastic fiber is blue, nucleus is red and the results showed that elastic fibers decreased in COPD group, while stem cell transplantation therapy restored elastic fibers in lung tissue and around small airways, and the content of elastic fibers in DPSCs group was higher than that in BM-MSCs group (P<0.05) (Figure 2E and F).
DPSCs Transplantation Reduces the Level of Inflammation in a Mouse COPD Model
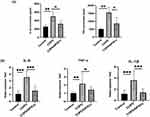
To assess the effect of DPSCs transplantation on the inflammatory process in PPE-induced pulmonary emphysema mice, we examined the mRNA expression levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α. Figure 3B suggests that the mRNA expression levels of IL-1β, TNF-α and IL-6 in the lung tissues of the COPD group were significantly higher than those of the control group, but DPSCs transplantation reversed the expression of inflammatory genes in PPE-induced pulmonary emphysema mice. Furthermore, ELISA analysis confirmed that the levels of IL-1β and TNF-α in BULF decreased significantly 3 weeks after DPSCs transplantation (Figure 3A) (all p < 0.05).
DPSCs Transplantation Reduces Oxidative Stress in a Mouse COPD Model
Oxidative stress drives the pathology of COPD;20 therefore, we studied the levels of oxidative damage indicators, including MDA, CAT and GSH. We found that MDA, an indicator of oxidative damage, was significantly increased in the COPD group, and DPSCs transplantation was able to reduce the level of MDA. In addition, CSH and CAT, as antioxidant substances, decreased in the COPD group but significantly increased in the DPSCs/COPD group (Figure 4). These results indicate that DPSCs have an antioxidant effect.
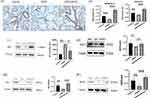
Effects of DPSCs on the Expression of Nrf2 and Its Downstream Factors in Lung Tissue
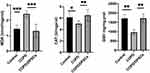
Nuclear factor erythrocyte 2-related factor 2 (Nrf2) is an important transcription factor that regulates inflammation and oxidative stress.27 Immunohistochemical results showed that nrf2 expression in the alveolar and bronchial epithelium in COPD/DPSCs group was significantly higher than that in the COPD group (Figure 5A) (P<0.05). Furthermore, we measured the mRNA levels of Nrf2 and its downstream target HO-1 by RT-qPCR (Figure 5B). The results showed that the mRNA levels of Nrf2 and HO-1 increased in lung tissues after DAPCs transplantation. In addition, we evaluated the protein expression of total Nrf2 and HO-1, NQO1, and SOD2 by Western blotting. We found that these proteins were downregulated in the COPD group but upregulated in the DPSCs/COPD group, and the difference between the two groups was statistically significant (P<0.05) (Figure 5C–F).
Discussion
In this study, we successfully extracted dental pulp stem cells from dental pulp tissue and transplanted them into PPE-induced emphysema mouse models by intratracheal injection. By comparing the efficacy of dental pulp stem cells and bone marrow mesenchymal stem cells in the treatment of COPD from the perspective of lung function, body weight, and histology, we found that dental pulp stem cells had a better performance in the treatment of COPD. To the best of our knowledge, this is the first study to evaluate the potential therapeutic effect of dental pulp stem cells on COPD.
A good animal model should have a lung anatomy similar to that of humans. Unfortunately, all known animal models meet only some of these criteria. In this study, we used endotracheal injection of porcine pancreatic elastase to induce a COPD model, the main advantage of which is that emphysema can be rapidly induced with a single treatment using an inexpensive reagent, making it cheaper and easier to administer than 6 months of smoke exposure. In addition, disease severity can be controlled by the selection of enzyme doses, which are more likely to produce severe emphysema than smoke-induced emphysema. Therefore, it can help detect abnormalities in lung function, especially in evaluating the effectiveness of inhibitors or interventions.28 In this study, both the pulmonary function test and pathological evaluation verified the successful establishment of a mouse model of COPD.
Apart from the source of the cells, another key factor in stem cell therapy is the optimal transplant route. Intravenous injection of mesenchymal stem cells is commonly used in preclinical studies of experimental emphysema, but pulmonary capillary embolism is an inevitable result.29 In contrast, endotracheal transplantation is easier to perform, and it has been found to reduce alveolar hyperinflation more than intravenous administration.30
Our HE staining results confirmed that DPSCs can improve elastase-induced emphysema, even when they are injected 2 weeks after injury. Specifically, the decrease in alveolar space and MLI indicated that stem cells can reduce the destruction of alveolar structures in damaged lungs. In addition, elastic fibers have an important effect on the loss of structural integrity of alveolar walls. Enzymatic and oxidative breakdown of elastic fibers impairs tissue recoil and increases residual lung capacity and pressure in the airspaces.31 In this study, we found that the transplantation of DPSCs and BM-MSCs can increase the content of elastic fibers in lung tissue, which is consistent with the study of Antunes et al30. COPD not only causes emphysema changes in the lungs but also leads to airflow obstruction. Similar to human spirometry, devices have been developed for testing lung function in mice.32 FEV0.1/FVC, its actual significance is the average flow rate within 0.1 s. Dynamic lung compliance refers to the change in lung volume caused by the change in unit pressure when the air flow is not blocked. The resistance of the lung is the ratio between the pressure difference needed to maintain a certain flow rate and the flow rate. We found that FEV0.1/FVC and Cydn were significantly decreased and RL was significantly increased in elastase-induced emphysema mice, as reported previously.32 Current results show that DPSCs improve the respiratory flow rate within 0.1 s of emphysema, dynamic lung compliance and resistance of the lung better than BM-MSCs. The above results indicate that DPSCs can not only improve COPD histologically but also improve lung function.
At present, the mechanism of stem cell transplantation in treating COPD remains unclear. Previous studies have found that stem cells can alleviate lung injury in a variety of ways, such as paracrine signaling, immune regulation, and differentiation into alveolar epithelial cells.33,34 In this study, we confirmed that DPCSs can reduce inflammation and oxidative stress in COPD. The results of this study showed that DPSCs can reduce IL-1β, TNF-α and IL-6 levels in lung tissue, which may be related to their anti-inflammatory properties. Malondialdehyde (MDA), a byproduct of polyunsaturated fatty acid peroxidation, may be a reliable marker of oxidative stress in various diseases.35 Glutathione (GSH) and catalase (CAT) protect cells from the effects of hydrogen peroxide and play an important role in the cellular adaptive response to oxidative stress tolerance.36 We found that DPSCs can upregulate the level of CAT and GSH, downregulate the level of MDA and have a protective effect on PPE-induced oxidative stress.
The Nrf2 signaling pathway maintains a balanced intracellular redox process. As a master transcription factor, it regulates the expression of various antioxidant genes, including NQO1, SOD2, and HO-1. Animal and human studies have shown that Nrf2/Keap-1 and its target genes protect against inflammation and oxidative stress caused by cigarette smoke.37 Our animal experiments suggest that DPSCs may inhibit inflammation and oxidative stress by increasing the expression of Nrf2 and its downstream factors.
Conclusion
The local application of DPSCs in the lungs of experimental emphysema mice can effectively improve the oxidative stress and inflammation state of lung tissue and alleviate the COPD-like performance of mice, indicating that DPSCs have good potential application in the clinical treatment of COPD.
Abbreviations
DPSCs, dental pulp stem cells; COPD, chronic obstructive pulmonary disease; Nrf2, nuclear factor erythroid‐2 related factor‐2; PPE, porcine pancreatic elastase; H&E, hematoxylin-eosin staining; BALF, bronchoalveolar lavage fluid; MDA, malondialdehyde; CAT, catalase; GSH, glutathione; ROS, reactive oxygen species; MSCs, mesenchymal stromal cells; BM-MSCs, bone marrow mesenchymal stem cells; FBS, foetal bovine serum; α-MEM, alpha Modified Eagle Medium; FEV0.1/FVC, the ratio of forced expiratory volume in 0.1 s to forced vital capacity; RL, resistance of lung; Cydn, respireatory dynamic compliance; MLI, mean linear intercept.
Ethics Approval and Consent to Participate
All animal procedures were approved by the ethical standards of the Animal Ethics Committee of Capital Medical University and were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals published by the US NIH (NIH publication No. 85-23, revised 2011)” and Basel Declaration. All human procedures were approved by the institutional ethical Committee of Beijing Chaoyang Hospital Affiliated with Capital Medical University. All study donors provided written informed consent, in accordance with the Declaration of Helsinki.
Author Contributions
Zuomin Wang conceived and designed the study, and reviewed the article critically. Xiaoli Gao performed the experiment and wrote the manuscript. Zhiqiang Liu analyzed and interpreted the data, and substantially revised the article. All authors give final approval to the journal to which the article will be submitted and agreed to all versions of the article. All authors agree to take responsibility for the content of the article.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221. doi:10.1183/09059180.00003609
2. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
3. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;10082:389.
4. Kaur M, Chandel J, Malik J, Naura AS. Particulate matter in COPD pathogenesis: an overview. Inflamm Res. 2022;71(7–8):797–815. doi:10.1007/s00011-022-01594-y
5. Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi:10.2147/COPD.S10770
6. Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–242. doi:10.1183/09031936.06.00053805
7. Drost EM, Skwarski KM, Sauleda J, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60(4):293–300. doi:10.1136/thx.2004.027946
8. Wang C, Zhou J, Wang J, et al. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct Target Ther. 2020;5(1):248. doi:10.1038/s41392-020-00345-x
9. McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217–231. doi:10.22203/eCM.v034a14
10. Chen X, Wang F, Huang Z, et al. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: an update and concise review. Int J Med Sci. 2021;18(13):2849–2870. doi:10.7150/ijms.59218
11. Costela-Ruiz VJ, Melguizo-Rodriguez L, Bellotti C, et al. Different sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. 2022;23(11):6356. doi:10.3390/ijms23116356
12. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi:10.1073/pnas.240309797
13. Potdar PD, Jethmalani YD. Human dental pulp stem cells: applications in future regenerative medicine. World J Stem Cells. 2015;7(5):839–851. doi:10.4252/wjsc.v7.i5.839
14. Zhang SY, Ren JY, Yang B. Priming strategies for controlling stem cell fate: applications and challenges in dental tissue regeneration. World J Stem Cells. 2021;13(11):1625–1646. doi:10.4252/wjsc.v13.i11.1625
15. Sui B, Wu D, Xiang L, et al. Dental pulp stem cells: from discovery to clinical application. J Endod. 2020;46(9):S46–S55. doi:10.1016/j.joen.2020.06.027
16. Ren H, Sang Y, Zhang F, et al. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int. 2016;2016:3516574. doi:10.1155/2016/3516574
17. Verma K, Bains R, Bains VK, et al. Therapeutic potential of dental pulp stem cells in regenerative medicine: an overview. Dent Res J. 2014;11(3):302–308.
18. Makino E, Nakamura N, Miyabe M, et al. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019;10(5):1199–1208. doi:10.1111/jdi.13045
19. Li S, Luo L, He Y, et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54(8):e13093. doi:10.1111/cpr.13093
20. Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi:10.1378/chest.12-2664
21. Park SW, Lee AY, Lim JO, et al. Loranthus tanakae Franch. & Sav. suppresses inflammatory response in cigarette smoke condensate exposed bronchial epithelial cells and mice. Antioxidants. 2022;11(10). doi:10.3390/antiox11101885
22. Bousnaki M, Bakopoulou A, Pich A, Papachristou E, Kritis A, Koidis P. Mapping the secretome of dental pulp stem cells under variable microenvironmental conditions. Stem Cell Rev Rep. 2022;18(4):1372–1407.
23. Bronckaers A, Hilkens P, Fanton Y, et al. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8(8):e71104. doi:10.1371/journal.pone.0071104
24. Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19(1):196. doi:10.1038/mt.2010.192
25. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967;95(5):752–764. doi:10.1164/arrd.1967.95.5.752
26. Chen M, Huang Z, Bi H, et al. Effects of bone marrow‑derived mesenchymal stem cell transplantation on chronic obstructive pulmonary disease/obstructive sleep apnea overlap syndrome in rats. Mol Med Rep. 2019;20(5):4665–4673. doi:10.3892/mmr.2019.10714
27. Qian Y, Yan L, Wei M, Song P, Wang L. Seeds of Ginkgo biloba L. inhibit oxidative stress and inflammation induced by cigarette smoke in COPD rats through the Nrf2 pathway. J Ethnopharmacol. 2023;301:115758. doi:10.1016/j.jep.2022.115758
28. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L1–L15. doi:10.1152/ajplung.90200.2008
29. von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–1578. doi:10.1002/stem.1118
30. Antunes MA, Abreu SC, Cruz FF, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15(1):118. doi:10.1186/s12931-014-0118-x
31. Mehraban S, Gu G, Ma S, Liu X, Turino G, Cantor J. The proinflammatory activity of structurally altered elastic fibers. J Respir Cell Mol Biol. 2020;63(5):699–706. doi:10.1165/rcmb.2020-0064OC
32. Devos FC, Maaske A, Robichaud A, et al. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir Res. 2017;18(1):123.
33. Gu W, Song L, Li XM, et al. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733. doi:10.1038/srep08733
34. Fukui E, Funaki S, Kimura K, et al. Adipose tissue-derived stem cells have the ability to differentiate into alveolar epithelial cells and ameliorate lung injury caused by elastase-induced emphysema in mice. Stem Cells Int. 2019;2019:5179172. doi:10.1155/2019/5179172
35. Antus B, Harnasi G, Drozdovszky O, Barta I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology. 2014;19(1):74–79. doi:10.1111/resp.12155
36. Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1–3):83–104. doi:10.1016/S0300-483X(00)00306-1
37. Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17(7):363–371. doi:10.1016/j.molmed.2011.02.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.