Back to Journals » Cancer Management and Research » Volume 14
Treatment Options for Metastatic Urothelial Carcinoma After First-Line Chemotherapy
Authors Tassinari E, Mollica V, Nuvola G, Marchetti A , Rosellini M, Massari F
Received 24 March 2022
Accepted for publication 21 May 2022
Published 13 June 2022 Volume 2022:14 Pages 1945—1960
DOI https://doi.org/10.2147/CMAR.S287904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Elisa Tassinari,1 Veronica Mollica,1 Giacomo Nuvola,1 Andrea Marchetti,1 Matteo Rosellini,1 Francesco Massari1,2
1Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 2Department of Experimental, Diagnostic and Specialty Medicine, S.Orsola-Malpighi University Hospital, University of Bologna, Bologna, Italy
Correspondence: Francesco Massari, Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Via Albertoni-15, Bologna, Italy, Email [email protected]
Abstract: Urothelial carcinoma (UC) is a frequently diagnosed tumor and an important cause of cancer deaths worldwide. Until a few years ago, despite the unquestioned role of platinum-based chemotherapy, therapeutic choices beyond the first line were limited and related to unsatisfactory outcomes. Metastatic UC has always been associated with a poor prognosis, with overall survival only slightly above a year. In the recent past, huge progress has been made in our understanding of the molecular and genomic disease characteristics, to enable stratification of patients in terms of prognosis and treatment responses. Unfortunately, we still do not have the perfect combination of clinical biomarkers to tailor the optimal treatment for each patient, despite making several efforts in this direction. The therapeutic arsenal has been augmented by immune checkpoint inhibitors (ICIs), which nowadays represent the backbone of the second-line setting. Equally revolutionary was the FDA’s approval of erdafitinib, a potent fibroblast growth factor receptor (FGFR) inhibitor, the use of which is reserved for patients whose tumor harbors specific FGF pathway alterations. Recently, the therapeutic landscape of metastatic UC has been enhanced by the introduction of novel compounds, consisting of antibody–drug conjugates (ADCs). Enfortumab vedotin is an antibody targeting nectin-4, a cell adhesion molecule highly expressed in UC, conjugated to monomethyl auristatin E (MMAE), a microtubule-disrupting agent. Sacituzumab govitecan is a humanized monoclonal antibody targeting Trop-2, a transmembrane glycoprotein, conjugated to the active metabolite of irinotecan. These two compounds have received accelerated approval by the FDA in patients pretreated with platinum-based chemotherapy and immunotherapy. Several ongoing trials are investigating the role of ICIs combined with chemotherapy, antiangiogenic drugs, or other ICIs, as well as the efficacy of PARP inhibitors and target therapies, hoping to provide information for some important unmet needs. In this review, we aim to evaluate the current potential treatment options after first-line chemotherapy.
Keywords: urothelial carcinoma, immunotherapy, immune-checkpoint inhibitors, FGFR inhibitor, antibody–drug conjugates
Introduction
The great majority of urinary tract cancers are classified as urothelial carcinoma (UC).1 The most common UCs affect the lower urinary tract, mainly the bladder and, to a lesser extent, the urethra. Otherwise, upper urinary tract neoplasms occur in the renal pelvis and ureter, accounting for only 10% of UCs.2 According to the latest statistics, bladder cancer is the tenth most frequently diagnosed tumor worldwide, with approximately 213,000 deaths during 2020. Thanks to the therapeutic arsenal available in the most developed countries, a decline in mortality rates has been described during the past few years.3 Nevertheless, metastatic urothelial carcinoma (mUC), presenting in 4% of recently diagnosed patients, still has a limited prognosis, amounting to approximately 13–15 months.4 The combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) has been considered similar, with regard to long-term overall survival (OS) and progression-free survival (PFS), to gemcitabine–cisplatin (GC); however, since MVAC shows more adverse effects, the latter schedule is still considered the first-line backbone strategy. UC has proven response rates of up to 50% to first-line chemotherapy; nevertheless, the duration of the response is limited and the prognosis is poor after progression.5 Following decades of cisplatin-based chemotherapy as the only choice for patients with metastatic disease, the first-line scenario has recently been improved by the approbation of immune checkpoint inhibitors (ICIs), as we shall examine later. Currently, ICIs also have a key role in maintenance therapy after first-line chemotherapy, with the aim of prolonging time to treatment failure and providing clinical benefits.6 In the recent past, when progressing to first-line treatment, limited options were available for clinicians, and treatment also gave unsatisfactory results in terms of median OS. Nowadays, single-agent vinflunine7 or taxanes (ie paclitaxel8 or docetaxel9), and combination regimens such as MVAC10 or gemcitabine–paclitaxel,11 have been almost entirely replaced by a large assortment of ICIs, tyrosine kinase inhibitors (TKIs), and antibody–drug conjugates (ADCs).12 In the past few years, great steps have been taken to better target the molecular machinery of UC and, consequently, to widen the variety of therapies in the advanced disease setting. In this review, we aim to specifically focus on treatment options for progression after first-line chemotherapy.
Overview of the Existing First-Line Strategies
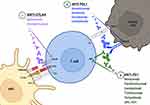
Cisplatin-based regimens have long been depicted as the cornerstone of UC first-line therapy, with an overall response rate (ORR) of 50%, a median PFS of 7 months, with one patient out of five alive at 5 years from diagnosis.5 Equally well acknowledged, although in part questioned, are the required characteristics in order to be considered “cisplatin fit”, also known as the “Galsky criteria” (ie glomerular filtration rate >60 mL/min, performance status 0–1, congestive heart failure NYHA class ≤III, and neuropathy/hearing loss grade ≤2). Unfortunately, about 30–50% of patients with mUC are cisplatin unfit, often because of their advanced age.3,13 Until recently, the only alternative supported by historical data was the combination of gemcitabine plus carboplatin, although it showed inferior outcomes compared to cisplatin-based first-line therapy.14 To fulfill this unmet need, some trials tried to explore new strategies, moving away from classical chemotherapy. The microenvironment of UC should be considered as fertile soil for the application of ICIs, in view of the high load of DNA aberrations related to the formation of several neoantigens. The presence of this huge immune stimulation elicits an antitumor T-cell-mediated immune response.15 In particular, the ability to form an immune response against the attenuated strain of Mycobacterium bovis has been associated with antitumor activity in non-muscle-invasive bladder cancer.16 Based on these principles, promising phase II trials have emerged to evaluate the role of ICI monotherapy in chemotherapy-naïve cisplatin-ineligible patients (Table 1; Figure 1).
 |
Table 1 ICI Monotherapy, FGFR Inhibitors, and ADCs That Have Received Approval from the FDA For Use in the Second-Line Setting |
In a multicenter single-arm phase II study, a human monoclonal antibody immunoglobulin G4 (IgG4) designed to target programmed death-1 (PD-1), pembrolizumab, resulted in a 24% complete or partial response, while 23% of patients had stable disease as their best response, with an acceptable tolerability.17 Long-term outcomes of the KEYNOTE-052 study demonstrate durable and remarkable results, with an ORR of 28.6%, a median duration of response of 30.1 months, and an OS of 11.3 months. In particular, as already shown in a pivotal study, patients with programmed death ligand-1 (PD-L1) expression combined positive score (CPS) ≥10 showed higher ORR (47.3%) and notable median OS (18.5 months).18
Atezolizumab, a humanized monoclonal antibody IgG1 that inhibits binding of PD-L1 to its receptors, PD-1, was studied in the phase II IMvigor210 study. Cohort 1, which enrolled patients without previous treatment for mUC, had an ORR of 23% and complete responses were seen in 11 patients (9%), with a median OS of 15.9 months regardless of PD-L1 expression.19
The FDA has limited the use of these two ICIs in the setting of metastatic disease, to patients who are cisplatin ineligible (with CPS ≥10 for pembrolizumab and PD-L1 ≥5% tumor-infiltrating cells for atezolizumab) or who are not eligible for any platinum-containing chemotherapy, regardless of PD-L1 status.20
Several therapeutic approaches are currently still under evaluation, assessing the role of combination strategies. In particular, the ICI–chemotherapeutic strategies arise from the rationale that the immune system can be stimulated by tumor neoantigens induced by the effect of chemotherapy on malignant cells.21 Worthy of note are two phase III trials, IMvigor130, focusing on atezolizumab as monotherapy or in combination with platinum/gemcitabine, and KEYNOTE-361, similar to the previous study but with pembrolizumab. Both of these trials failed to demonstrate an improvement in terms of OS using the combination of chemotherapy with the immunotherapeutic compound.22,23 A less commonly used anti-PD-1 antibody, tislelizumab, is under evaluation in a phase III double-blinded multicenter trial (NCT03967977). Plenty of trials are ongoing to assess the role of combination ICIs as potential first-line strategies. The phase III study DANUBE failed to show a statistically significant improvement in terms of OS with the combination of durvalumab, an anti-PD-L1 agent, plus tremelimumab, an IgG2 monoclonal antibody against cytotoxic T-lymphocyte antigen 4 (CTLA-4) versus standard of care chemotherapy.24 The same two ICIs are under evaluation in combination with standard of care chemotherapy in the NILE phase III study (NCT03682068). The potential role of nivolumab, a monoclonal antibody targeting PD-1, plus ipilimumab, an anti-CTLA-4 agent, is under evaluation in the CheckMate-901 phase III study (NCT03036098), in comparison with standard chemotherapy. Other encouraging combination strategies are ICIs with antiangiogenic agents; worthy of note is the phase III trial LEAP-011 (NCT03898180), analyzing the role of pembrolizumab plus lenvatinib, a multi-TKI, in the first-line setting in cisplatin-unfit patients expressing PD-L1 or in platin-ineligible patients irrespective of PD-L1 levels. The results of LEAP-011 were presented at the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers symposium, highlighting no additional benefit in terms of PFS, OS, ORR, or DOR for the combination strategy compared to pembrolizumab alone in cisplatin-ineligible patients expressing PD-L1 (CPS >10) or platinum-unfit patients regardless of PD-L1 status. The median PFS was 4.5 months for pembrolizumab and lenvatinib versus 4.0 months for pembrolizumab and placebo; analogously, the median OS did not differ significantly between the two groups (11.8 months with the combination compared to 12.9 months with pembrolizumab and placebo).25 Other pioneering approaches are ongoing to investigate the role of ICIs plus novel immunotherapies, such as PARP inhibitors or target therapies, which have been thoroughly assessed in second or later line settings, as we shall explore later.
As mentioned in the Introduction, ICIs play a role in maintenance therapy in patients with advanced UC who have not progressed with first-line platinum-containing chemotherapy. According to the results of the phase III JAVELIN Bladder 100 trial, avelumab showed significant improvements versus best supportive care, and it is now considered standard of care in treatment guidelines. In particular, the updated follow-up data further underline the benefits of avelumab in terms of median OS (23.8 months vs 15 months) and PFS (5.5 months vs 2.1 months), suggesting a prolonged use of maintenance therapy from 1 year up to 2 years.26 A phase III randomized trial of 2 years’ maintenance avelumab plus cabozantinib versus avelumab alone after first-line platinum-based chemotherapy in patients with mUC is ongoing (NCT05092958).
Second-Line Therapies: What’s Now
In the past, single-agent chemotherapies were largely used in the second-line setting, despite showing unsatisfactory benefits in terms of outcomes. Vinflunine, for instance, can only provide a median 2-month survival advantage compared to best supportive care, with a median OS of only 7 months.7 Combination therapies, such as MVAC, achieved a slightly longer OS, up to 11 months, despite important grade 3 or 4 toxicities.10 Therefore, experts in the scientific community have come together to offer effective and tolerable therapies to fulfill this unmet need.
As mentioned in Introduction section, UC appears to be an immunogenic tumor, demonstrating a higher expression of PD-L1 than other solid tumors. Furthermore, the presence of high levels of PD-L1 has been associated with poor OS and more aggressive disease.27 Unfortunately, despite the unquestioned role of PD-L1 as a prognostic indicator, the information arising from PD-L1 as a single source of data must, in the future, be integrated with other potential biomarkers, such as tumor mutational burden (TMB), microsatellite instability, or T-cell gene signatures.28
On the back of these reports, supported by encouraging results emerging from phase II trials, several anti PD-1 and anti-PD-L1 monoclonal antibodies have received approval for use in the second-line setting, after progression to first-line chemotherapy.
In the phase III, open-label, international study KEYNOTE-045, pembrolizumab showed a significant survival advantage compared with standard chemotherapy (vinflunine, paclitaxel, or docetaxel) in patients who had relapsed after prior platinum-based therapy, with a median OS of 10.3 months compared with 7.4 months in the chemotherapy group, regardless of PD-L1 expression levels. Although the PFS did not increase significantly (2.1 vs 3.3 months), the objective response rate was higher in the pembrolizumab group (21.1%) than in chemotherapy group (11.4%), with considerably fewer adverse events.29 Further evidence of the clinical advantages of pembrolizumab was shown during ASCO 2021, where the 5-year follow-up results were announced, demonstrating a 60-month OS of 14.9% with pembrolizumab versus 8.7% with standard chemotherapy.30 Gradually, pembrolizumab has become the standard of care in the second-line setting, once treatment has progressed to chemotherapy.
In 2016, atezolizumab was granted accelerated approval for mUC in the second-line setting, based on the results from the IMvigor210 trial. Unfortunately, the IMvigor211 trial, focusing specifically on atezolizumab in patients with platinum-refractory mUC overexpressing PD-L1, failed to show a statistically significant OS advantage. There may be several explanations for the divergent results of IMvigor211 and KEYNOTE-045, such as disparities in PD-L1 assessment or the different results of chemotherapy survival compared to previous studies. However, the duration of response was longer in the atezolizumab subgroup, at 15.9 months versus 8.3 months in the chemotherapy group; in addition, patients in the atezolizumab population experienced fewer adverse events.31 In an updated analysis, with a median of 33 months of follow-up, atezolizumab showed a 24-month OS rate of 23% versus 13% with chemotherapy.32 To draw conclusions, some guidelines still support the use of atezolizumab, but with a weaker recommendation than for pembrolizumab.33 While waiting for the results of the real-world phase III SAUL study, we can find interesting real-life reports from some retrospective studies. Data on 115 pretreated mUC patients corroborated the outcomes of the previous clinical trials in this setting; at a median follow-up of 23.5 months, the median duration of response was 20.4 months, with a PFS of 3.8 months and an OS of 9.8 months.34 According to two other retrospective studies, adverse pretreatment clinical characteristics were pointed out as an independent risk factors for poorer OS, in particular liver metastases, ECOG performance status ≥1, and hemoglobin level <10 mg/dL (confirming the Bellmunt criteria, only excluding time since the completion or discontinuation of previous therapy of <3 months).35,36 Furthermore, a neutrophil-to-lymphocyte ratio (NLR) >3 and GFR <60 mL/min, according to the first paper,35 and a clinical benefit from first-line chemotherapy, in the second work,36 all maintained a significant association with OS in multivariate analysis, suggesting some potentially useful stratification features for future clinical trials.
With the insights obtained from CheckMate-275, a multicenter, single-arm, phase II trial, the fully human IgG4 anti-PD-1 nivolumab monotherapy has been approved by the FDA in previously treated patients with metastatic or surgically unresectable UC. A fulfilling objective response was achieved in all subgroups of patients, despite PD-L1 expression (in particular, an ORR of 28.4%, 23.8%, and 16.1% in cases of PD-L1 expression of ≥5%, ≥1%, and <1%). Grade 3–4 treatment-related adverse events, in particular fatigue and diarrhea, occurred in less than 20% of patients.37 A recent update with a minimum follow-up of 33.7 months supports the long-lasting antitumor activity of nivolumab. In addition, a retrospective exploratory biomarker analysis reported that higher TMB was associated with improved ORR, PFS, and OS. Furthermore, the analyses of TMB together with PD-L1 expression revealed that the combination of these two biomarkers is a better predictor of PFS and OS than TMB or PD-L1 alone.38
The selective, high-affinity, monoclonal antibody IgG1 that inhibits PD-L1, durvalumab, has been under evaluation in the phase I/II 1108 study as monotherapy for patients with locally advanced or metastatic UC, who have progressed on, were ineligible for, or had refused prior chemotherapy. Durvalumab showed encouraging clinical activity, with an ORR of 17.8% and median OS of 18.2 months, with early and durable responses regardless of PD-L1 expression.39 Originally, durvalumab received FDA accelerated approval in this disease setting; however, the indication has recently been withdrawn considering the results of the DANUBE trial, which did not find a statistically significant improvement in median OS in the durvalumab monotherapy group.24
To conclude, with ICI monotherapies, we should explore the role of the IgG1-specific anti-PD-L1 (leaving the interaction between PD-1 and PD-L2 intact) monoclonal antibody avelumab, as investigated in the phase Ib JAVELIN trial. In this evaluation, median PFS was approximately 2 months and median OS was 6.5 months. It is important to emphasize that avelumab was generally well tolerated, even though 11.6% of patients manifested an autoimmune adverse event and despite one treatment-related death due to pneumonitis.40
Notwithstanding the proven role of ICI monotherapy in the second-line setting, further suggestions regarding potential therapeutic options have come from the recent efforts to categorize different UC types. The molecular classification proposed by Kamoun includes six molecular classes, according to different characteristics (such as oncogenic mechanisms, microenvironment infiltration, clinical and histological features, and, consequently, outcomes): luminal papillary, luminal non-specified, luminal unstable, stroma-rich, basal/squamous, and neuroendocrine-like.41
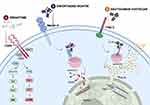
Luminal papillary tumors are mainly enriched in FGFR3 mutations and have been related to a longer median OS.41 Patients with a tumor harboring particular FGFR alterations (specific type of FGFR3 mutations or FGFR2/3 fusion, described in 15–20% of mUCs) have been reported to clinically benefit from the use of novel FGFR inhibitors. The phase II BLC2001 trial highlighted the role of erdafitinib, a small-molecule TKI, that works as a potent selective inhibitor of FGFR1–4. Erdafitinib obtained FDA approval as a second-line choice after first-line platinum-based progression, showing an ORR of 40%, a median PFS of 5.5 months, and a median OS of 13.8 months. Analyzing the toxicity profile, an adverse event of grade 3 or higher has been reported in the 46% of the population, with a special focus on retinopathy, which could suggest the need for periodic ocular check-ups.42 It is important to emphasize that, despite its approval for use in the second-line setting, clinicians usually reserve avelumab until after ICI progression. Nowadays, we can only suggest a lower sensitivity to ICIs in luminal papillary UCs, owing to the poor infiltration of the microenvironment by the immune system. These speculations have been strengthened by the registration study, where only one of the 22 patients pretreated with ICIs had a history of response to immunotherapy. More studies are still needed to better understand the most suitable sequence of therapy in patients with FGFR alterations43 (Figure 2).
For cisplatin-unfit patients, new perspectives have recently emerged. Enfortumab vedotin, which we will thoroughly explore later, in the section “Beyond the Second Line”, is an ADC targeting nectin-4. EV-201, a single-arm two-cohort trial, obtained promising results in cohort 2, composed of patients with locally advanced or metastatic UC previously treated with PD-1 or PD-L1 inhibitors only. In this setting, enfortumab vedotin showed an ORR of 52%, with a complete response in 20% of patients, and a median OS of 14.7 months, leading to the accelerated FDA approval44 (Table 2; Figure 2).
 |
Table 2 Therapeutic Approaches Under Evaluation: Phase II Studies, Second or Later Lines of Therapy |
Second-Line Therapies: What’s New
Numerous clinical trials are now ongoing to assess the role of different drugs or combination therapies in the wider mUC therapeutic scenario after first-line progression.
Several ongoing studies are exploring ICIs plus chemotherapy in the second-line setting. Atezolizumab plus docetaxel or gemcitabine–carboplatin is under evaluation in cisplatin-ineligible patients in a phase II trial (NCT03737123). In particular, the first combination is reserved for patients pretreated with carboplatin and gemcitabine with concomitant or maintenance ICI, while the latter has been proposed for patients without a prior platinum-based chemotherapy regimen. A phase II study (NCT03744793) is investigating the role of avelumab in combination with pemetrexed in pretreated patients with methylthioadenosine phosphorylase (MTAP) deficiency. Pembrolizumab is under investigation in combination with paclitaxel in platinum-refractory patients (NCT02581982) (Table 2a).
Assuming the synergistic effect due to ICI combination, in particular CTLA-4 and PD-1/PD-L1 blockade, an expansion cohort of the phase I/II CheckMate-032 showed interesting results. Patients pretreated with a platinum regimen took a combination of nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg), followed by nivolumab monotherapy (3 mg/kg), revealing an ORR of 38%, a median PFS of 4.9 months, and a median OS of 15.3 months.45 The two ICIs are under evaluation in TITAN-TCC, a phase II trial with nivolumab monotherapy with additional nivolumab/ipilimumab “boost” cycles, for both treatment-naïve and platinum-refractory patients (NCT03219775). During the recent ASCO GU symposium, results were presented for patients after prior platinum-based chemotherapy (cohort 2): a confirmed ORR was achieved in 27 of the 83 total patients (32.5%), with a median PFS of 1.9 months and a median OS of 7.6 months46 (Table 2b).
With the aim of targeting both the “hot” microenvironment and the process of neoangiogenesis, the backbone of disease progression and metastization, numerous trials have been started. Cabozantinib, a small TKI targeting VEGFR-2, MET, TYRO3, AXL, and MER, is under evaluation alone and in combination with durvalumab or nivolumab plus ipilimumab in pretreated mUC. ARCADIA, a phase II trial evaluating durvalumab plus cabozantinib in 16 platinum-pretreated patients, showed, as a preliminary result, an ORR of 37.5%, including four partial and two complete responses.47 Nivolumab plus ipilimumab in combination with cabozantinib is under investigation in a phase II study (NCT03866382). A phase II study (NCT02717156) is ongoing to assess the role of pembrolizumab plus EphB4-HSA in previously treated mUC. EphB4-HSA is a recombinant fusion protein composed of the extracellular domain of human receptor tyrosine kinase ephrin type-B receptor 4 (sEphB4), which is fused to full-length human serum albumin (HSA), to improve itself half-life. The complex works as a trap receptor for the membrane-bound ligand EphrinB2 and interferes with the binding of EphrinB2 to its native receptors, including EphB4. EphrinB2 and EphB4 are both highly expressed in UC and have been considered negative prognostic markers. According to the preliminary results presented during ESMO 2021, the median OS was 14.4 months, with a PFS of 4 months; the ORR was 38%, with a median DOR of 8.0 months. Otherwise, outcomes seemed improved in the population expressing the target, EphrinB2.48 Nivolumab and pembrolizumab are under evaluation in combination with sitravatinib, a TKI targeting VEGFR, PDGFR, KIT, RET, MET, and selected Eph family members, in an ongoing phase II trial (NCT03606174) (Table 2c).
Despite the important role of combination therapies, some clinical trials are evaluating ICI monotherapy. A recombinant, humanized, anti PD-1 monoclonal antibody, toripalimab, has been proposed for pretreated patients with advanced UCs, in the phase II trial POLARIS-03. In the intention-to-treat population, composed of 151 patients, median PFS was 2.3 months and OS was 14.4 months. Remarkably, both PD-L1-positive and TMB-high patients had better ORR than PD-L1-negative and TMB-low patients.49 Tremelimumab, an IgG2 monoclonal antibody against CTLA-4, showed an ORR of 18.8%, with complete and partial responses in two and four patients, in a cohort of 32 patients who had failed on platinum-containing first-line chemotherapy (NCT03557918). A phase II study is ongoing with the purpose of testing the safety and effectiveness of various ICIs in advanced bladder cancer when given intermittently (NCT04322643). The effects of nivolumab monotherapy are under evaluation in mUC, specifically in patients with aberrations in the ARID1A gene, and correlate with the expression level of the immune cytokine CXCL13 (NCT04953104) (Table 2d).
In the past few years, several novel immunotherapeutic approaches have been proposed, with the aim of enhancing the immune response against cancer. OX40 (or CD134) is a membrane protein expressed in CD4+, CD8+ T cells, NK cells, and neutrophils, which assumes a co-stimulatory function binding to its ligand, OX40L, placed on APCs. OX40 is responsible for a high level of production of cytokines and for T-cell survival and proliferation.50 JAVELIN Medley (NCT02554812) is a phase I/II study which is evaluating avelumab in combination with different treatment options, such as PF-04518600, an OX40 agonist (Figure 1).
CYT-107, a glycosylated recombinant human interleukin (IL)-7, is now under evaluation together with atezolizumab in patients with platinum-refractory UC (NCT03513952). In addition, QUILT-3.055 (NCT03228667) is a phase IIb study with the aim of evaluating several ICIs (nivolumab, pembrolizumab, avelumab, and atezolizumab) combined with ALT-803, an IL-15/IL-15Rα complex fused to an IgG1 Fc with improved agonism of the IL-2 and 15βγ receptor, in patients pretreated with ICIs.51 Several clinical trials are still evaluating the role of lymphocyte activation gene-3 (LAG3) and mucin-domain-containing (TIM3) inhibitors (NCT03538028 and NCT03250832), tumor vaccines in combination with ICIs (eg NCT03639714), and the efficacy of chimeric antigen receptor (CAR) T cells (NCT03185468) and macrophages (NCT04660929). Furthermore, a phase II trial is ongoing to assess the role of autologous tumor-infiltrating lymphocyte (TIL) infusion and subsequent treatment with high doses of IL-2 (NCT04383067) (Table 2e).
DNA damage repair (DDR) genes may be frequently altered in mUC and they are associated with better responses to platinum-based chemotherapy. In particular, 2–14% of mUCs showed alterations in ATM, ERCC2, and RAD51B; and 3.7–12.3% of all macs had damage to BRCA-1/2, PALB2, FANCD2, ERCC2, and ATM.52 The phase II study ATLAS (NCT03397394) failed to assess rucaparib monotherapy as a therapeutic choice in patients who had received two prior lines of therapy with or without DDR alterations. In addition, rucaparib is being investigated in combination with lucitanib, a VEGFR1–2–3, FGFR1–2, and PDGFRα–β inhibitor, or sacituzumab govitecan, an ADC that we explore further in the next section, in the phase Ib–II trial SEASTAR (NCT03992131). BISCAY, a phase Ib trial (NCT02546661), did not demonstrate a meaningful benefit with the use of olaparib in combination with durvalumab in patients pretreated with platinum-based chemotherapy. Two phase II studies (NCT03448718 and NCT03375307), assessing the role of olaparib monotherapy in mUC harboring somatic DDR alterations, are ongoing. Another phase II trial (NCT03682289) is investigating olaparib in combination with AZD6738, an orally available morpholino-pyrimidine-based inhibitor of ATM and RAD3-related kinase, in pretreated mUC (Table 2f).
Supplementary potential weapons are target therapies, alone or in combination with immunotherapies or ADCs. As we have noted in the previous section, the FGFR inhibitor erdafitinib has a definite role in the second-line setting. Other drugs aimed at the same target are currently under evaluation, such as pemigatinib, an FGFR1–3 inhibitor, in the phase II study FIGHT-201, in patients with mUC harboring FGF/FGFR alterations. During ASCO GU 2020, the results of FORT-1 were presented; a phase II/III study of rogaratinib (an FGFR1–4 inhibitor) versus chemotherapy, which found a comparable efficacy with standard chemotherapy and an acceptable safety profile in patients with mUC stratified by FGFR1/3 mRNA expression.53 Another FGFR inhibitor, ICP-192, is under evaluation in a phase II study in patients whose disease presented FGFR alterations (NCT04492293). Thanks to the encouraging results of FIERCE-22, evaluating the association of vofatamab, a monoclonal antibody against FGFR3, and pembrolizumab, showing an ORR of 29.6% and a PFS of 4.7 months, several studies have been initiated.54 In particular, erdafitinib is being tested in association with an anti-PD-1, cetrelimab (NCT03473743), while derazantinib (an FGFR1–3 inhibitor) is under evaluation with atezolizumab (NCT04045613). Another potential target is HER-2; ERBB has been found to be altered in one out of three of UCs, specifically in 9% of muscle-invasive UCs.12,52 According to Kamoun’s molecular classification, luminal unstable UCs underlie, as an oncogenic mechanism, ERBB2 alterations.41 An ADC composed of the anti-HER-2 trastuzumab and deruxtecan, a DNA topoisomerase-1 inhibitor derivative of the camptothecin analogue exatecan, is under evaluation in combination with nivolumab in a phase I/II trial (NCT03523572) in patients who have progressed on prior platinum-based chemotherapy, expressing HER-2 by IHC. Disitamab vedotin is an ADC composed of an anti-HER-2 monoclonal antibody conjugated to a microtubule-disrupting agent, monomethyl auristatin E (MMAE), and it is being tested in a phase II trial in previously treated patients with or without HER-2 overexpression (NCT03809013). Furthermore, a phase II study in patients with HER-2 overexpression is ongoing (NCT04879329). An analogue ADC, known as MRG002, is under evaluation in a phase II trial (NCT04839510). To conclude, the PI3K/AKT/mTOR pathway has been investigated as a potential therapeutic strategy, considering that 42% of UCs present alterations dependent on these genes.52 Combination strategies including immunotherapy and target therapies are ongoing, such as nivolumab and eganelisib, known as IPI-549, an oral PI3K inhibitor (NCT03980041). Monotherapies are also being tested, such as sapanisertib, an mTORC1/2 inhibitor, in a phase II study on patients with tuberous sclerosis (TSC)1 and/or TSC2 mutations (NCT03047213).
Beyond the Second Line
Until a few years ago, it seemed very hard to speculate on a therapeutic line beyond the second one. As we analyzed in previous sections, numerous efforts have been made to widen the treatment scenario in mUC. As well as single-agent chemotherapy regimens (ie vinflunine or taxanes, which have modest activity) and inclusion in ongoing clinical trials, innovative options are emerging in the third-line setting. In particular, the role of ADCs is greatly expanding, showing encouraging results in this disease setting, which has poor survival.
Enfortumab vedotin (EV) is an ADC conjugated to monomethyl auristatin E (MMAE), a microtubule-disrupting agent. The antibody targets nectin-4, a cell adhesion molecule that is highly expressed in several solid tumors, such as breast, gastric, lung, and urothelial carcinomas.55 EV-201 was a phase II single-arm two-cohort trial analyzing the role of EV in patients pretreated with a platinum chemotherapy regimen and PD-1 or PD-L1 inhibitors, in a neoadjuvant, adjuvant, or first-line setting (cohort 1) and in cisplatin-unfit patients previously treated with ICIs (cohort 2). In cohort 1, EV showed an objective response rate of 44%, with 12% of complete responses, an estimated median PFS of 5.8 months and a median OS of 11.7 months. The most common treatment-related adverse events included peripheral neuropathy, skin rash – due to skin nectin-4 expression – and, to a lesser extent, hyperglycemia. These encouraging results led to accelerated FDA approval in 2019.56 The confirmatory phase III trial, EV-301, demonstrated a median OS of 12.88 months, with a median PFS of 5.55 months versus 3.71 months with chemotherapy (standard docetaxel, paclitaxel, or vinflunine), after prior platinum chemotherapy and checkpoint inhibitor immunotherapy57 (Table 2; Figure 2).
Sacituzumab govitecan (SG) consists of a humanized monoclonal antibody IgG1k targeting trophoblast cell surface antigen-2 (Trop-2) conjugated to SN-38, the active metabolite of irinotecan. Trop-2, a transmembrane glycoprotein implicated in cell cancer growth, invasion, and spread, is highly expressed in several solid tumors, such as urothelial, lung, gastric, and colorectal cancers, as well as glioma.58 The complex Trop-2–SG, internalized by cancer cells, leads to topoisomerase I inhibition, eventually causing cell death. Otherwise, the ADC is held together by the hydrolyzable linker CL2A, which enables SN-38 to be released into the tumor microenvironment, attacking adjacent tumor cells, with a sort of bystander effect. Cohort 1 of TROPHY-U-01, a phase II multi-cohort trial, recruited 113 mUC patients who had been pretreated with platinum and immunotherapy. The ORR was of 27.4%, with a reduction of lesion target size achieved by 77% of patients; the median PFS was 5.4 months and the median OS was 10.9 months. The principal side effects, the majority of which were predictable and manageable, were neutropenia and diarrhea. In particular, patients harboring the UGT1A1 homozygous *28/*28 genotype presented an increased risk of developing neutropenia. For this reason, SG received accelerated approval from the FDA for mUC in patients who had undergone prior platinum-based chemotherapy and immunotherapy.59 At the ASCO GU 2022 symposium, the rationale and design of the phase III confirmatory study TROPiCS-04 (NCT04527991) have been presented; it is hoped that the study will corroborate the satisfactory results of the TROPHY-U-01 trial60 (Table 3; Figure 2).
 |
Table 3 Antibody–Drug Conjugates in the Third-Line Setting |
Conclusions
In the past few years, the scientific community has made huge progress in enlarging the therapeutic scenario of metastatic urothelial carcinoma, thanks to the greater knowledge on molecular and genomic disease characteristics. The encouraging results of novel clinical trials in terms of OS and PFS have changed the way in which we deal with patients, allowing us to provide several different therapeutic choices. Undoubtedly, the role of immunotherapy, together with anti-FGFR drugs in selected patients, has completely transformed the treatment algorithm once the treatment has progressed to chemotherapy, which remains a well-established first-line therapy in urothelial cancer. Stunning innovations are being seen beyond the second line, with the introduction of promising antibody–drug conjugates, such as enfortumab vedotin and sacituzumab govitecan. Several clinical trials are still ongoing to assess the role of novel target therapies, PARP inhibitors, and immunotherapies in combination with chemotherapy or antiangiogenic drugs, in the hope of progressively moving from a traditional and unselected approach to a more tailored one, based on the molecular profile of each patient (Figure 3).
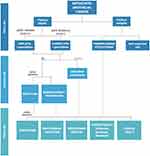 |
Figure 3 Flowchart of treatment of metastatic urothelial carcinoma. Adapted from the template “Flow Chart (6 Levels, Vertical)” by BioRender.com (2022). |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Takahara T, Murase Y, Tsuzuki T, et al. Urothelial carcinoma: variant histology, molecular subtyping, and immunophenotyping significant for treatment outcomes. Pathology. 2021;53(1):56–66. doi:10.1016/j.pathol.2020.09.004
2. Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–122. doi:10.1016/j.eururo.2017.07.036
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
4. Lenis AT, Lec MP, Chamie K, et al. Bladder cancer: a review. JAMA. 2020;324(19):1980–1991. doi:10.1001/jama.2020.17598
5. Von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi:10.1200/JCO.2005.07.757
6. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi:10.1056/NEJMoa2002788
7. Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–4461. doi:10.1200/JCO.2008.20.5534
8. Vaughn DJ, Manola J, Dreicer R, et al. Phase II study of paclitaxel plus carboplatin in patients with advanced carcinoma of the urothelium and renal dysfunction (E2896): a trial of the Eastern Cooperative Oncology Group. Cancer. 2002;95(5):1022–1027. doi:10.1002/cncr.10782
9. McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15(5):1853–1857. doi:10.1200/JCO.1997.15.5.1853
10. Han KS, Joung JY, Kim TS, et al. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. Br J Cancer. 2008;98(1):86–90. doi:10.1038/sj.bjc.6604113
11. Albers P, Park S-I, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol. 2011;22(2):288–294. doi:10.1093/annonc/mdq398
12. Mollica V, Rizzo A, Montironi R, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers. 2020;12(6):1449. doi:10.3390/cancers12061449
13. Galsky MD, Hahn NM, Rosenberg JE, et al. Defining “cisplatin ineligible” patients with metastatic bladder cancer. J Clin Oncol. 2011;29(7_suppl):238. doi:10.1200/JCO.2011.34.8433
14. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191–199. doi:10.1200/JCO.2011.37.3571
15. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi:10.1038/s41588-018-0312-8
16. Farina MS, Lundgren KT, Bellmunt J, et al. Immunotherapy in Urothelial Cancer: recent Results and Future Perspectives. Drugs. 2017;77(10):1077–1089. doi:10.1007/s40265-017-0748-7
17. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, Phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi:10.1016/S1470-2045(17)30616-2
18. Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020;38(23):2658–2666. doi:10.1200/JCO.19.01213
19. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi:10.1016/S0140-6736(16)32455-2
20. Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. doi:10.3390/cancers12030738
21. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi:10.1158/2326-6066.CIR-15-0064
22. Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi:10.1016/S0140-6736(20)30230-0
23. Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi:10.1016/S1470-2045(21)00152-2
24. Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (Danube): a randomised, open-label, multicentre, phase 3 trial [published correction appears in Lancet Oncol 2021; 22: e5]. Lancet Oncol. 2020;21(12):1574–1588. doi:10.1016/S1470-2045(20)30541-6
25. Christopher JDW. First-line pembrolizumab with or without lenvatinib in patients with advanced urothelial carcinoma (LEAP-011): a Phase 3, randomized, double-blind study.
26. Powles T, Park SH, Voog E, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): long-term follow-up results from the JAVELIN bladder 100 trial. J Clin Oncol. 2022;40(6_suppl):487. doi:10.1200/JCO.2022.40.6_suppl.487
27. Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–1182. PMID: 17186290. doi:10.1007/s00262-006-0266-z
28. Lopez-Beltran A, Cimadamore A, Blanca A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13(1):131. doi:10.3390/cancers13010131
29. Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. PMID: 28212060; PMCID: PMC5635424. doi:10.1056/NEJMoa1613683
30. Van der Heijden MS, Loriot Y, Durán I, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the Phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80(1):7–11. PMID: 33902955. doi:10.1016/j.eururo.2021.03.024
31. Bellmunt J, Necchi A, De Wit R, et al. Pembrolizumab (pembro) versus investigator’s choice of paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC): 5-year follow-up from the phase 3 KEYNOTE-045 trial. J Clin Oncol. 2021;39(15_suppl):4532. doi:10.1200/JCO.2021.39.15_suppl.4532
32. Powles T, Durán I, Van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. Erratum in: Lancet. 2018 Oct 20;392(10156):1402. PMID: 29268948. doi:10.1016/S0140-6736(17)33297-X
33. Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol. 2019;76(1):73–81. PMID: 30910346. doi:10.1016/j.eururo.2019.03.015
34. Tural D, Ölmez ÖF, Sümbül AT, et al. Atezolizumab in patients with metastatic urothelial carcinoma who have progressed after first-line chemotherapy: results of real-life experiences. Eur Urol Focus. 2021;7(5):1061–1066. doi:10.1016/j.euf.2020.09.010
35. Tural D, Ölmez ÖF, Sümbül AT, et al. Prognostic factors in patients with metastatic urothelial carcinoma who have treated with Atezolizumab. Int J Clin Oncol. 2021;26(8):1506–1513. doi:10.1007/s10147-021-01936-6
36. Tural D, Selçukbiricik F, Ölmez ÖF, et al. Response to first-line chemotherapy regimen is associated with efficacy of ımmune checkpoint blockade therapies in patients with metastatic urothelial carcinoma. Int J Clin Oncol. 2022;27(3):585–591. doi:10.1007/s10147-021-02072-x
37. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. PMID: 28131785. doi:10.1016/S1470-2045(17)30065-7
38. Galsky MD, Saci A, Szabo PM, et al. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from CheckMate 275. Clin Cancer Res. 2020;26(19):5120–5128. PMID: 32532789; PMCID: PMC8166422. doi:10.1158/1078-0432.CCR-19-4162
39. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a Phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. PMID: 28817753; PMCID: PMC5824288. doi:10.1001/jamaoncol.2017.2411
40. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51–64. Erratum in: Lancet Oncol. 2018;19(7):e335. PMID: 29217288; PMCID: PMC7984727. doi:10.1016/S1470-2045(17)30900-2
41. Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77(4):420–433. PMID: 31563503; PMCID: PMC7690647. doi:10.1016/j.eururo.2019.09.006
42. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. PMID: 31340094. doi:10.1056/NEJMoa1817323
43. Stecca C, Abdeljalil O, Sridhar SS. Metastatic urothelial cancer: a rapidly changing treatment landscape. Ther Adv Med Oncol. 2021;13:17588359211047352. PMID: 34616491; PMCID: PMC8488509. doi:10.1177/17588359211047352
44. Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):872–882. Erratum in: Lancet Oncol.P21;22(6):e239. PMID: 33991512. doi:10.1016/S1470-2045(21)00094-2
45. Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: checkMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol. 2019;37(19):1608–1616. Erratum in: J Clin Oncol. 2019 Aug 10;37(23):2094. PMID: 31100038; PMCID: PMC6879315. doi:10.1200/JCO.19.00538
46. MO Grimm, BJ Schmitz-Dräger, U Zimmermann, et al. Tailored immunotherapy approach with nivolumab in advanced transitional cell carcinoma (TITAN-TCC).
47. Marandino L, Raggi D, Calareso G, et al. Cabozantinib plus durvalumab in patients with Advanced urothelial carcinoma after platinum chemotherapy: safety and preliminary activity of the open-label, single-arm, Phase 2 ARCADIA trial. Clin Genitourin Cancer. 2021;19(5):457–465. PMID: 34006499. doi:10.1016/j.clgc.2021.04.001
48. Sadeghi S, Quinn DI, Dorffet T, et al. Phase II trial of pembrolizumab (P) in combination with sEphB4-HSA (B4) in previously treated metastatic urothelial carcinoma (mUC). Ann Oncol. 2021;32(5):S678. doi:10.1016/j.annonc.2021.08.047
49. Sheng X, Chen H, Hu B, et al. Safety, efficacy, and biomarker analysis of toripalimab in patients with previously treated advanced urothelial carcinoma: results from a multicenter Phase II trial POLARIS-03. Clin Cancer Res. 2022;28(3):489–497. PMID: 34740921. doi:10.1158/1078-0432.CCR-21-2210
50. Cebada J, Perez-Santos M, Bandala C, et al. OX40 agonists for cancer treatment: a patent review. Expert Opin Ther Pat. 2021;31(1):81–90. doi:10.1080/13543776.2021.1825688
51. Rhode PR, Egan JO, Xu W, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016;4(1):49–60. PMID: 26511282; PMCID: PMC4703482. doi:10.1158/2326-6066.CIR-15-0093-T
52. Mollica V, Maggio I, Lopez-Beltran A, et al. Combination therapy in advanced urothelial cancer: the role of PARP, HER-2 and mTOR inhibitors. Expert Rev Anticancer Ther. 2020;20(9):755–763. doi:10.1080/14737140.2020.1807334
53. Quinn DI, Petrylak DP, Bellmunt J, et al. FORT-1: phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J Clin Oncol. 2020;38(6_suppl):489. doi:10.1200/JCO.2020.38.6_suppl.489
54. Siefker-Radtke AO, Lugowska I, Tupikowski K, et al. Clinical activity of vofatamab (V), an FGFR3 selective antibody in combination with pembrolizumab (P) in metastatic urothelial carcinoma (mUC), updated interim analysis of FIERCE-22. Ann Oncol. 2019;30(suppl_5):v356–v402. doi:10.1093/annonc/mdz249.016
55. Bouleftour W, Guillot A, Magné N. The anti-nectin 4: a promising tumor cells target. A systematic review [published online ahead of print, 2022 Feb 7]. Mol Cancer Ther. 2022:493–501. doi:10.1158/1535-7163.MCT-21-0846
56. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–2600. PMID: 31356140; PMCID: PMC6784850. doi:10.1200/JCO.19.01140
57. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. PMID: 33577729; PMCID: PMC8450892. doi:10.1056/NEJMoa2035807
58. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–29006. doi:10.18632/oncotarget.25615
59. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a Phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–2485. doi:10.1200/JCO.20.03489
60. C Vulsteke, P Grivas, ST Tagawa, et al. TROPiCS-04: study of Sacituzumab govitecan in patients with locally advanced unresectable or metastatic urothelial cancer that has progressed after prior platinum and checkpoint inhibitor therapy.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.