Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Advances in Antibody-Drug Conjugates in the Treatment of HER2-Positive Breast Cancer
Authors Yu Y , Wang J, Liao D, Zhang D, Li X, Jia Y, Kong F
Received 5 August 2022
Accepted for publication 17 November 2022
Published 9 December 2022 Volume 2022:14 Pages 417—432
DOI https://doi.org/10.2147/BCTT.S384830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Yongchao Yu,1– 3,* Jin Wang,1– 3,* Dongying Liao,1– 3 Dou Zhang,1– 3 Xiaojiang Li,1,2 Yingjie Jia,1,2 Fanming Kong1– 3
1Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, People’s Republic of China; 3Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fanming Kong, Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China, Tel +862227986525, Email [email protected]
Abstract: Although targeted drugs improved the therapeutic effect of HER2-positive breast cancer, the long-term prognosis was still poor. In this regard, the research and development of antibody-drug conjugates (ADCs) came into being and made a lot of progress. ADCs had the characteristics of both chemotherapeutic agents and targeted agents by combining chemotherapeutic agents and targeted agents through a linker. It not only had a strong anti-tumor effect on HER2-positive breast cancer, but also had certain anti-tumor effects on HER2-low and even HER2-negative patients. In addition, the clinical researches of ADCs combined with immune checkpoint inhibitors (ICIs) therapy had also made a great breakthrough. This review aimed to summarize the clinical progress of ADCs, in particular the two drugs approved by the US Food and Drug Administration (FDA) for HER2-positive metastatic breast cancer as well as to summarize the current status of ADCs in combination with ICIs.
Keywords: breast cancer, HER2-positive, antibody-drug conjugates, immunotherapy, advances
Introduction
Breast cancer (BC) had become the most common malignant tumor in women worldwide, accounting for approximately 11.7% of new cancer cases in 2020, which seriously endangered women’s health and became the main cause of female death.1,2 HER2 (human epidermal growth factor receptor-2) is an important biomarker of breast cancer and a member of the ERBB family of tyrosine kinase receptors. It is a transmembrane protein with tyrosine protein kinase activity, which consists of three parts: extracellular ligand binding domain, single strand transmembrane domain and intracellular protein tyrosine kinase domain.3 When HER-2 binds to the ligand, it activates tyrosine kinase activity mainly by inducing receptor dimerization and autophosphorylation of cytoplasmic tyrosine kinase. The types of variation in HER2 include overexpression, mutation and amplification.4 At present, the only cancers involved in routine detection and treatment of HER2 targets are gastric cancer and breast cancer. The HER2 criteria used were the same, that is, combined with HER2 protein overexpression and gene amplification to determine HER2 positive.5 In short, if the immunohistochemistry (IHC) result is 3+, it is judged as positive; if it is 0 or 1+, it is judged as negative; if the IHC result is 2+, it is determined by detecting HER2 gene amplification using in situ hybridization (ISH) technique. The so-called HER2 low expression specifically refers to the subtypes IHC1+ and IHC2+/ISH-. Among them, the HER2-positive patients accounted for 20–25% of the total and were considered a poor prognostic factor in the past. However, with the evolution of anti-HER2 treatment drugs, the treatment pattern of HER2-positive advanced breast cancer was constantly rewritten.6–8 Among them, monoclonal antibody drugs such as trastuzumab, pertuzumab and inituximab mainly target the extracellular binding domain of HER2 and block the heterodimerization of HER2 with other HER receptors by binding HER2, thus slowing down the growth of tumor.9 Small molecular tyrosine kinase inhibitors (TKI), including Afatinib, Neratinib, Pyrotinib, Lapatinib and Tucatinib, target the intracellular tyrosine kinase region of HER2 to block signal transduction by inhibiting phosphorylation.10 Despite achieving the breakthrough, nearly all patients with HER2-positive metastatic breast cancer (MBC) progressed eventually on anti-HER2 therapy due to the resistance.11 For the patients with low-level expression of HER2 which is nearly 50% of BC patients, targeted drugs were not effective.12 As a result, currently, the treatment of HER2-positive BC is still a difficult problem and a great challenge all over the world.
One strategy is to apply antibody-drug conjugates (ADCs), improving the therapeutic index by combining the targeted specificity of monoclonal antibodies with the anti-tumor ability of cytotoxic drugs. ADCs are composed of three elements, respectively, a monoclonal antibody to identify tumor cells and internalize the entire ADC complex via endocytosis, a cytotoxic agent (also called “payload”) to kill tumor cells, and a linker to attach the cytotoxic agent to the antibody. The above three parts are vital for designing an effective ADC. Another factor influencing the therapeutic index of an ADC is the drug-to-antibody ratio (DAR), specifically the average number of payloads linked to each antibody. Too small means the treatment may less effective, and too many may make side effects harder to tolerate.
The incidence of HER-2 overexpression was the highest in breast cancer, and decreased successively in gastric cancer and colon cancer.13 This allowed some ADCs to treat other cancers besides BC. For example, T-DM1 was effective in BC, colorectal cancer, gastric cancer, non-small cell lung cancer, and so on, but it had been approved only for the treatment of HER2+ breast cancer. With a particular emphasis placed on the two FDA-recommended drugs, this study will mainly provide the latest advances of ADCs in the treatment of HER2-positive BC, offering an overview of ADC combined immunotherapy.
ADCs Approved by FDA for HER2-Positive BC
Trastuzumab Emtansine (T-DM1)
T-DM1 was a classic ADC, which was the first ADC approved by the FDA for the treatment of HER2-positive MBC in 2013, specifically for the treatment of patients who had received trastuzumab and a taxane. The payload is DM1, which is a derivative of maytansine. Maytansine is a potent inhibitor of tubulin polymerization, which is stable and can be adequately soluble in the aqueous milieu of the antibody. The antibody is trastuzumab. Per antibody molecule links with 3.5 maytansinoid molecules on average, and the conversion of trastuzumab into an ADC significantly enhanced its cell-killing power.14 Trastuzumab and DM1 are covalently linked via a stable thioether linker (N maleimidomethyl) cyclohexane-1-carboxylate, which is considered to limit the exposure of normal tissue to DM1, thus restricting its toxicity.15 The cytotoxicity of T-DM1 may vary with the concentration of DM1 accumulated in cancer cells. High intracellular concentration will lead to rapid apoptosis, lower concentration will lead to cell transport damage and mitotic disaster, and the lowest concentration will lead to poor response to T-DM1.16
Approval of this drug was based on the date of the phase III EMILIA study which investigated the efficacy of T-DM1 versus capecitabine and lapatinib in patients with HER2-positive advanced breast cancer (ABC). T-DM1 was more effective, showing an improvement in progression-free survival (PFS) (9.6 vs 6.4 months; HR 0.65, 95% CI 0.55 to 0.77) and median overall survival (OS) (29.9 vs 25.9 months; HR 0.75, 95% CI 0.64 to 0.88).17
TH3RESA was a phase III study that evaluated the efficacy and safety of T-DM1 in patients with progressive HER2-positive BC after trastuzumab-based and lapatinib-based therapy for advanced disease and previously treated with a taxane. T-DM1 significantly improved PFS and objective response, with a favorable safety profile in comparison with the physician’s choice (median PFS: 6.2 vs 3.3 months). The study demonstrated that even after several lines of previous therapy (median of four previous regimens), the use of a more effective HER2-directed therapy can contribute to meaningful clinical benefits.18 It was the study MARIANNE that supported T-DM1 to be the first-line treatment for patients with HER2-positive MBC who were deemed unsuitable for taxane-based therapy. The MARIANNE was a phase III study, which was designed to assess the efficacy and safety of T-DM1 and T-DM1 plus pertuzumab compared with trastuzumab plus taxane in those with HER2-positive ABC, and no prior therapy for advanced disease (n=1095 patients). T-DM1 showed non-inferior, but not superior, efficacy and better tolerability when compared with a taxane plus trastuzumab for first-line treatment. Median PFS was 13.7 months in the trastuzumab plus taxane group, 14.1 months in the T-DM1 group, and 15.2 months in the T-DM1 plus pertuzumab group.19
The treatment of HER2-positive MBC after initial HER2-directed therapy, T-DM1 continued to be a significant medical need by the study of TDM4374g and TDM4258g. Moreover, the trials also showed that the therapeutic effect of T-DM1 was related to HER2 expression levels. Like the results of TDM4374g, the objective response rate (ORR) per independent central review (IRF) in patients with at least median HER2 expression was 42.9% (95% CI 26.3 to 60.6) with median PFS being 8.0 months (95% CI 5.4 months to N/E). ORR in patients with less than median HER2 expression was 38.2% (95% CI 22.2 to 56.4), and median PFS was 6.2 months (95% CI 3.9 to 12.3).20,21
The risk of recurrence or death of invasive BC was 50% lower with T-DM1 than with trastuzumab for those with residual invasive early breast cancer (EBC) after neoadjuvant chemotherapy plus HER2-targeted therapy recently based on the results of KATHERINE, a phase III study. A lower rate of invasive disease occurred in the T-DM1 group than in the trastuzumab group (12.2% vs 22.2%). The percentage of patients in the T-DM1 group who were estimated to be free of invasive disease at 3 years was 88.3% and 77.0% in the trastuzumab group. Invasive disease-free survival was significantly higher among those who received T-DM1 than among those who received trastuzumab (HR 0.50, 95% CI 0.39 to 0.64, P<0.001).22
T-DM1 is efficient and well-tolerated while some challenges remain. In the USA, T-DM1 carried black box warnings for hepatotoxicity, cardiac toxicity, and embryo-fetal toxicity.23 The lack of payload bystander effects limits utility in tumors with heterogeneous HER2 expression. Resistance can be caused due to defects in intracellular trafficking and increased expression of drug transporters MDR1 and MRP1.24
Trastuzumab Deruxtecan (T-DXd; DS-8201; Enhertu; AZD4552; Fam-Trastuzumab Deruxtecan-Nxki)
T-DXd was the second ADC approved by FDA to treat patients with HER2-positive, unresectable or metastatic BC following two or more prior anti-HER2-based regimens in December 2019 and was officially approved as a second-line drug on May 4, 2022. The payload of T-DXd is DXd, which is a derivative of DX-8951. DX-8951 is a more potent topoisomerase I inhibitor than SN-38, which can bind to topoisomerase I-DNA cleavable complexes and stabilize them, resulting in the induction of double-strand DNA breaks and apoptosis.25,26 At the same time, because DXd is a topoisomerase I inhibitor, which is different from the mechanism of chemotherapy drugs commonly used in the treatment of breast cancer, cross-drug resistance can be effectively avoided. The antibody is a humanized HER2-targeted antibody that has the same amino structure as trastuzumab. Each antibody binds to eight DXd molecules, so T-DXd has a higher DAR than T-DM1, thus allowing it to deliver more payload molecules to targeted tumor cells. The novel enzyme-cleavable linker remains stable in plasma, which is cleaved by proteases known as lysosomal cathepsins once in the cell, causing the release of the cytotoxic drug.27 After optimization, the hydrophobicity of T-DXd connectors is greatly reduced and the stability is enhanced. Basic studies showed that after 21 days of treatment, the drug release rate of T-DXd in human plasma was only 2.1%, while that of T-DM1 was 18.4% after 4 days. In addition, the connectors are specifically cleaved by lysosomal enzymes highly expressed in tumor cells, such as cathepsin B and L, ensuring the stability of T-DXd in systemic circulation and limited systemic toxicity.28,29 And the half-life of DXd is short (based on animal data, it is about 1.37h in systemic circulation), which helps to minimize the miss toxicity of T-DXd.
The phase I DS8201-A J101 study and the phase II DESTINY-Breast01 study provided strong validation of the breakthrough efficacy of DS-8201 in the late-line treatment of HER2-positive breast cancer. However, the application of DS-8201 was not limited to the back line, and its performance in the field of second-line treatment was even more commendable. DS8201-A-J101 was the first-in-human trial of T-DXd. T-DXd showed preliminary anti-tumor activity in patients with HER2-positive and previously treated with T-DM1. The study showed that the confirmed ORR was 59.5% (95% CI 49.7 to 68.7).30,31 The phase II trial of DESTINY-Breast01 showed that T-DXd was significant and sustained in patients with HER2-positive MBC who progressed on or after T-DM1. With a median duration of follow-up of 20.5 months, patients treated with T-DXd achieved a confirmed ORR (by ICR) of 61.4% (95% CI 54.0 to 68.5) with a median duration of response (DOR) of 20.8 months (95% CI 15.0 to NE).32,33 Based on the earlier results from the trial, T-DXd had been approved for posterior line therapy in patients with HER2-positive BC by the FDA.34 T-DXd replaced T-DM1 as the preferred second-line drug to treat HER2-positive MBC according to DESTINY-Breast03. DESTINY-Breast03 was a phase III trial that assessed the efficacy and safety of T-DXd vs T-DM1 in patients with HER2-positive MBC previously treated with trastuzumab and taxane and met the primary endpoint. T-DXd had a superior PFS and ORR. The PFS assessed by the researchers achieved an unprecedented breakthrough, with a median PFS of 25.1 months, more than three times that of the T-DM1 group. The percentage of those who were alive without disease progression at 12 months was 75.8% for T-DXd and 34.1% for T-DM1.35 For overall patients, confirmed ORR with T-DXd was 79.7% and 34.2% with T-DM1. Subgroup analysis showed that all subgroups of patients with HR status, pertuzumab treated, baseline visceral disease, previous treatment lines, and brain metastasis could obtain more significant PFS from the late second-line treatment of T-DXd than that of the T-DM1 group. And T-DM1 had a manageable safety profile.36 Under this background, T-DXd continues to expand its territory. DESTINY-Breast09 research is moving towards first-line therapy. DESTINY-Breast11 study challenges the neoadjuvant therapy of PH dual-target combined chemotherapy. DESTINY-Breast05 study launches an impact on the adjuvant therapy stage of high-risk HER2-positive breast cancer patients after neoadjuvant therapy. There is much to look forward to.
Because of the cleavage of connectors and the high membrane permeability of DXd, DS-8201 not only has cytotoxicity against targeted tumor cells, but also plays a strong bystander effect on nearby tumor cells.28 Based on this, DS-8201 may be effective in the treatment of heterogeneous tumors, as well as in tumor cells with low expression of HER2. On August 5th, T-DXd was approved by the FDA for the treatment of ABC with low expression of HER2, which became the first targeted therapy for BC with low expression of HER2 in the world. DESTINY-Breast04 was a randomized controlled Phase 3 study that established the first targeted therapy for breast cancer with low HER2 expression.37 The study included breast cancer patients with low HER2 expression who received first-or second-line treatment, and who were randomly assigned to receive T-DXd or physician-selected chemotherapy. The main endpoint was PFS in patients with positive hormone receptor (HR). In the general population (regardless of HR status), T-DXd significantly prolonged PFS (9.9 vs 5.1 months; HR 0.5, P < 0.001) and OS (23.4 vs 16.8 months; HR 0.64, P = 0.0010) compared with the physician-selected chemotherapy (TPC), and the significant benefits of PFS (10.1 vs 5.4 months; HR 0.51, P < 0.001) and OS (23.4 vs 16.8 months; HR 0.64, P=0.0010) could also be seen in HR+ patients. Patients with HR were in an exploratory study population, with a smaller sample size, but we could also saw benefits from PFS (8.5 vs 2.9 months; HR 0.46) and OS (18.2 vs 8.3 months; HR 0.48). A Ib trial (NCT03523572) was conducted to investigate the efficacy of T-DXd in combination with an anti-PD-1 antibody (nivolumab) in patients with HER2-expressing MBC or advanced urothelial cancer. Interim results for the BC cohorts had been presented and revealed efficacy. Respectively, the confirmed ORR was 59.4% and 37.5% in the HER2-positive and HER2-low cohorts.38 Durvalumab (a humanized monoclonal IgG1 antibody against PD-L1) plus T-DXd also showed promising early safety and efficacy in triple-negative breast cancer (TNBC) by a study (NCT03742102) and the confirmed ORR was 100% (4/4).39 The reason for its efficacy to treat HER2-low BC may be the bystander effect. DXd is cell membrane permeable, and thus it may enter nearby cells, even those without strong HER2 expression.40 However, the lower limit of low expression of HER2 is still controversial. The DAISY study explored the efficacy of DS8201 in BC with different HER2 expression status. The results suggested that about 30% of patients with HER2 IHC0 could still benefit from DS8201 treatment. Therefore, some questions are worth thinking about: whether patients with lower HER2 expression can also benefit from ADC drug therapy? Can patients with lower HER2 expression also be classified as people with low HER2 expression?
Side effects of T-DXd were more commonly associated with adverse reactions to cytotoxic chemotherapy, such as cytopenia, nausea, vomiting, diarrhea, and hair loss.41 A high incidence of interstitial lung disease was observed in early trials while this appears to be manageable in most patients treated with glucocorticoids.41 In addition, drug resistance of T-DXd was also inevitable At present, the exploratory analysis of DAISY studies on T-DXd suggested that the primary drug resistance may be related to the hemizygotic deletion mutation of ERBB2 gene on chromosome 17, and the SLX4 mutation may induce secondary drug resistance in T-DXd. At the same time, the decrease of HER2 expression level was also observed in patients with T-DXd drug resistance, but the specific mechanism of drug resistance needed further clinical verification. Other trials that study the synergy in combination with other therapies are ongoing, such as DESTINY-Breast07/08/09, BEGONIA, and HER2CLIMB-04.
The updated data and ongoing trials of the T-DXd are shown in Tables 1 and 2, respectively.
 |
Table 1 Trials of T-DXd That Have Updated Date |
 |
Table 2 Trials of T-DXd That is on Going |
Other ADCs Under Development
There are many HER2-targeted ADCs under development. This review will discuss some ADCs, which have different compositions.
Trastuzumab Duocarmazine (SYD985)
SYD985 is the second-generation ADC and has obtained fast-track designation from FDA to treat patients with HER2-positive MBC who have progressed during or after at least two different HER2-target treatment regimens. The antibody is trastuzumab, binding to a potent duocarmycin payload via a cleavable linker (VC-Seco-DUBA).42 Wim Dokter et al predicted that its membrane-permeable nature and the cleavable linker in SYD985 allow for significant bystander effects and may lead to the successful treatment of tumors where not all tumor cells express high levels of HER2.43 A phase I trial has shown efficacy on HER2-positive, HER2-low, and triple-negative MBC whose ORR were 33%, 27%, and 40% respectively.44 SYD985 displays an unusual activity on models resistant to T-DM1, suggesting that it is an efficient drug to overcome resistance to T-DM1.45 TULIP is a phase III trial involving 437 patients with HER2-positive MBC who had received at least two regimens or T-DM1 for MBC. SYD985 was more effective than the physician’s choice, with the median PFS for the SYD985 cohort being 7.0 months (95% CI 5.4–7.2), and the physician’s choice was 4.9 months (95% CI 4.0–5.5).46
XMT-1522
An ADC XMT-1522 has a humanized anti-HER2 antibody (HT-19) conjugated to 12–15 molecules of the payload AF-HPA (an auristatin derivative). XMT-1522 is more potent than T-DM1 in vitro, showing early signs of anti-tumor activity in a phase I trial to treat patients with HER2-expressing breast, lung, and gastric tumors.47,48 The disease control rate (DCR) was 83% (5/6) for patients at a dose of 16 or 21.3 mg/m2, and 25% (3/12) in patients treated at doses less than 16 mg/m2, providing that drug efficacy may be related to dose.48 In addition, in preclinical studies, XMT-1522 was effective on T-DM1 resistant models of breast cancer and gastric cancer.49
A166
A166 is an ADC composed of monomethyl auristatin F derivative conjugated to trastuzumab via a stable protease-cleavable valine citrulline linker. The first-in-human study of A166 demonstrated its efficacy as DCR was 59% at the dose levels of 3.6 mg/kg and 4.8 mg/kg.50 This study is expected to be completed in December 2022. A study in China revealed that tumor response was evaluable in 6 of 19 patients at the same doses.51
RC48-Adc
RC48-ADC refers to a novel ADC conjugating a monomethyl auristatin E derivative (MMAE) with a humanized anti-HER2 antibody. A dose-escalation phase I study to treat HER-positive MBC revealed that, at doses≥1.5 mg/kg, partial response (PR) was 57.1% (8/14) and stable disease (SD) was 28.6% (4/14). ORR was 72.7% for 11 trastuzumab-pretreated patients.52 A phase Ib trial evaluated RC48-ADC in patients with HER2-positive MBC. The DCR was 96.7% (29/30) and the clinical benefit rate (CBR) was 46.7% (14/30). It was more effective in patients who had not been treated with trastuzumab with a higher ORR (57.1% vs 33.3%). Moreover, no grade ≥4 AE was observed.53 A phase II trial is performed to compare with capecitabine/lapatinib in HER2-positive BC (NCT03500380). Meanwhile, a phase III trial is also planned in HER2-low BC (NCT04400695).
MM-302
MM-302 is a HER2-targeted antibody-liposomal doxorubicin conjugate. Although efficacy was shown in phase I trial,54 the phase II trial named HERMIONE failed to find any clinical benefit of MM-302 plus trastuzumab versus TPC (chemotherapy of physician’s choice plus trastuzumab) with HER2-positive MBC.55
MEDI4276
The payload in MEDI4276 (an ADC) is MMETA, a tubulin-based microtubule inhibitor, which via the maleimidocaproyl linker to a HER2-bispecific antibody targeting two different epitopes on HER2. MEDI4276 showed cytotoxic effects on HER2-positive tumor cells in vitro containing T-DM1 resistant cells,56 and another first-in-human, phase I study also confirmed that. There was one complete response (0.5 mg/kg) and two partial responses (0.6 and 0.75 mg/kg). All had prior treatment with trastuzumab, pertuzumab, and T-DM1 for BMC.57
PF-06804103
PF-06804103 is an ADC with anti-HER2 immunoglobulin G1, which is conjugated with a cleavable linker to the cytotoxic agent auristatin microtubule inhibitor Aur0101. A phase I trial in HER2-positive BC and gastric cancer exhibited anti-tumor activity, and preliminary ORR in the patients (≥3mg/kg) was 52.4% (11/21).58
ARX788
ARX788 is an ADC with a humanized HER2 targeting mAb conjugated to a cytotoxic tubulin inhibitor, Amberstatin (AS269).59 A phase I study of ARX788 generated a preliminary anti-tumor effect and safety in Chinese patients undergoing HER2-positive MBC. The ORR was 31% (13/42) and 42% (5/12) at a dose of 1.3 mg/kg. In addition, treatment-related serious adverse events were not observed.60 And ARX-788 is investigated in an ongoing phase I trial (NCT03255070).61
ALT-P7 (HM2/MMAE)
ALT-P7 is an ADC, in which the trastuzumab bio better HM2 is conjugated to monomethyl auristatin E (MMAE). The first efficacy results from a phase I trial of 27 patients with HER2-positive MBC were presented with a DCR of 77.3% (17/22) with the median PFS being 6.2 months (95% CI 2.5–9.9) at doses from 2.4 to 4.8mg/kg.62
DHES0815A (Anti-HER2/PBD-MA)
In the ADC DHES0815A, a monoclonal HER2 targeting antibody conjugates to pyrrolobenzodiazepine monoamide (PBD-MA). A Phase I study of DHES0815A in patients with HER2-positive BC has a primary completion date while no published data is available.61
BAT8001
BAT8001 is a novel HER2-targeting ADC composed of a trastuzumab biosimilar conjugated to the drug-linker Batansine. In a phase I study, BAT8001 showed anti-tumor activity in HER2-positive BC with a reported ORR of 41.4% (95% CI 23.5–61.1) with DCR being 82.8% (95% CI 64.2–94.2).63
The payloads, antibodies, and associated clinical trials for the above-mentioned drugs are documented in Table 3.
 | 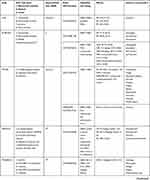 |  |
Table 3 The Approved and Investigational Anti-HER2 ADCs |
ADCs Combine with Immunotherapy
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of immunogenic cancers by enabling the priming and infiltration of T-cells into the tumor microenvironment, promoting cytotoxic signalling pathways and affecting tumor cytolysis.64 However, BC tumors had been considered immunologically quiescent historically, having intrinsic resistance to ICIs. A key strategy to overcome resistance to ICIs in BC is to develop combination immunotherapy that eliminates immunosuppressive cells and increases infiltration and activation of T-cells, thereby transforming the tumor from immune unresponsive to immune responsive.65 And the activity of an ADC can be further enhanced by adding an ICI. Chemotherapy causes the death of cancer cells, which promotes cancer cell antigen release and presentation. HER2-targeted drugs can kill tumor cells by the immune-mediated mechanism of antibody-dependent cellular cytotoxicity (ADCC). The two points support the potential of ADCs combined with immune checkpoint therapy in treating HER2-positive breast cancer.
A preclinical study showed that the combination of T-DM1 with anti-CTLA-4/PD-1 immunotherapy was considered effective in treating HER2-positive BC due to increased T-cell infiltration in tumor tissue, proliferation and signalling in the mice tumor model.66 In most relevant clinical trials, eligible patients had MBC previously treated with trastuzumab and taxane. An immunotherapy combination strategy added PD1/PD-L1 inhibitors in combination with T-DM1. A phase Ib trial (NCT03032107) enrolled a similar cohort of 20 patients and showed that T-DM1 plus the PD-1 inhibitor pembrolizumab was safe and tolerable. ORR was 20% with a median PFS of 9.6 months, and there were no grade 4 or above adverse events.67 In a phase Ib trial called GO29831 (NCT02605915), increased in PD-L1-expressing tumor-infiltrating immune cells were observed in both HER2+ EBC and MBC with the combination of atezolizumab (a PD-L1 inhibitor) and T-DM1.68 However, a phase II study named KATE2 (NCT02924883) demonstrated that T-DM1 plus atezolizumab did not significantly improve PFS, which increased the incidence of adverse events. In the PD-L1-positive subgroup, the atezolizumab group had more PFS events than the placebo group (27 vs 18). This suggested that the benefit of the combination of PD-L1 inhibitors may be limited to PD-L1-positive patients.69 KATE3 (NCT04740918) was a phase III trial, which investigated the efficacy and safety of T-DM1 in combination with atezolizumab or placebo in patients undergoing HER2-positive and PD-L1-positive MBC.70 Another immunotherapy combination strategy added the CD137-specific agonistic antibody utomilumab in combination with T-DM1 or trastuzumab. A phase IB/II clinical trial is ongoing and has not yet reported results (NCT03364348). There are also trials about T-DXd combined immunotherapy. A Ib trial (NCT03523572) was conducted to investigate the efficacy of T-DXd in combination with an anti-PD-1 antibody (nivolumab) in patients with HER2-expressing MBC or advanced urothelial cancer. Interim results for the BC cohorts have been presented and revealed efficacy. Respectively, the confirmed ORR was 59.4% and 37.5% in the HER2-positive and HER2-low cohorts.38 In addition, a trial (NCT04042701) in combination with pembrolizumab (a PD-1 inhibitor) is ongoing.71
Other ADCs that have not been approved by the FDA also have studies to explore the efficacy of the combination with immunotherapy. For instance, RC48-ADC combined with PD-1/PD-L1 immune checkpoint inhibition (pembrolizumab/atezolizumab) significantly enhanced HER2-positive tumor suppression and antitumor immunity in PD-1 transgenic mice.72 Apart from that, immune-stimulating antibody conjugates (ISACs) are also under development.73
Summary and Prospect
In the field of breast cancer, ADC had changed the treatment landscape of HER2-positive although it was previously thought to be incurable. ADCs were relatively new class of anticancer biologics with highly targeted properties and had at least three advantages. Firstly, they broaden the indications. They have been developed for the treatment of HER2-positive BC, trastuzumab sensitive and resistant. It is of note that some ADCs exert therapeutic effects on HER2-low and even TNBC. Secondly, ADC combines the advantages of antibodies and cytotoxic drugs to make it more effective by specifically recognizing tumor antigens. Meanwhile, the accumulation of the conjugated drug in tumor cells can be achieved. Chemotherapy drugs need to be combined with targeted drugs to be most effective, and targeted drugs can prevent chemotherapy resistance. Thirdly, this target-dependent activation allows selective cytotoxicity to cancer cells, thereby significantly lowering systemic side effects. ADCs combined with immunotherapy provide a new idea for the treatment of HER2-positive BC. More than 100 ADCs are currently in various stages of development. ADC is generally well tolerated, but each ADC has adverse effects that require special attention, such as T-DM1-related thrombocytopenia and T-DXD-related ILD. Antigenic selection, mechanism of drug action, chemical properties of linkers and coupling sites are important determinants of ADC-related adverse reactions.The rapid development of biotechnology has promoted the optimal selection of cytotoxins and linkers, and the effectiveness of the new generation of ADCs has greatly increased, but the risk/benefit ratio is still a factor that must be measured in clinical decisions.It is believed that ADCs will exert a further role in the treatment of BC in the future. We hope that this review gives useful information to physicians in the area.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81403220), the National Key Research and Development (R&D) Plan (No.2018YFC1707400), and the Tianjin Health and Family Planning-high Level Talent Selection and Training Project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Maomao C, He L, Dianqin S, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19:8.
3. Majumder A, Sandhu M, Banerji D, Steri V, Olshen A, Moasser MM. The role of HER2 and HER3 in HER2-amplified cancers beyond breast cancers. Sci Rep. 2021;11(1):9091. doi:10.1038/s41598-021-88683-w
4. Ruiz-Saenz A, Dreyer C, Campbell MR, Steri V, Gulizia N, Moasser MM. HER2 Amplification in Tumors Activates PI3K/Akt Signaling Independent of HER3. Cancer Res. 2018;78(13):3645–3658. doi:10.1158/0008-5472.CAN-18-0430
5. Shui R, Liang X, Li X, et al. Hormone Receptor and Human Epidermal Growth Factor Receptor 2 Detection in Invasive Breast Carcinoma: a Retrospective Study of 12,467 Patients From 19 Chinese Representative Clinical Centers. Clin Breast Cancer. 2020;20(1):e65–e74. doi:10.1016/j.clbc.2019.07.013
6. Qin T, Yuan Z, Peng R, et al. HER2-positive breast cancer patients receiving trastuzumab treatment obtain prognosis comparable with that of HER2-negative breast cancer patients. Onco Targets Ther. 2013;6:341–347. doi:10.2147/OTT.S40851
7. Ishii K, Morii N, Yamashiro H. Pertuzumab in the treatment of HER2-positive breast cancer: an evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019;14:51–70. doi:10.2147/CE.S217848
8. Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. doi:10.1200/JCO.2008.19.9844
9. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi:10.1016/S0140-6736(16)32616-2
10. Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–360. doi:10.1016/S1470-2045(20)30702-6
11. Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol. 2019;11:1758835919833519. doi:10.1177/1758835919833519
12. Nielsen KV, Jørgensen JT, Schønau A, Oster A. Human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(9):1330. doi:10.5858/2007-131-1330a-HEGFRT
13. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
14. Lambert JM, Chari RV. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57(16):6949–6964.
15. Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28(16):2698–2704. doi:10.1200/JCO.2009.26.2071
16. Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Research. 2014;16(2):209. doi:10.1186/bcr3621
17. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi:10.1016/S1470-2045(17)30312-1
18. Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi:10.1016/S1470-2045(14)70178-0
19. Perez EA, Barrios C, Eiermann W, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: primary Results From the Phase III MARIANNE Study. J Clin Oncol. 2017;35(2):141–148. doi:10.1200/JCO.2016.67.4887
20. Krop IE, LoRusso P, Miller KD, et al. A Phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30(26):3234–3241. doi:10.1200/JCO.2011.40.5902
21. Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):398–405. doi:10.1200/JCO.2010.29.5865
22. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. doi:10.1056/NEJMoa1814017
23. Raedler LA. Kadcyla (Ado-trastuzumab Emtansine): first Antibody-Drug Conjugate Approved for the Treatment of HER2-Positive Metastatic Breast Cancer. J Med. 2022;1:545.
24. Pegram MD, Miles D, Tsui CK, Zong Y. HER2-Overexpressing/Amplified Breast Cancer as a Testing Ground for Antibody-Drug Conjugate Drug Development in Solid Tumors. Clin Cancer Res. 2020;26(4):775–786. doi:10.1158/1078-0432.CCR-18-1976
25. Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6(10):789–802. doi:10.1038/nrc1977
26. Perez J, Garrigós L, Gion M, et al. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer and beyond. Expert Opin Biol Ther. 2021;21(7):811–824. doi:10.1080/14712598.2021.1890710
27. Xu Z, Guo D, Jiang Z, et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur J Med Chem. 2019;183:111682. doi:10.1016/j.ejmech.2019.111682
28. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi:10.1158/1078-0432.CCR-15-2822
29. Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The Latest Research and Development into the Antibody–Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo). 2019;67(3):173–185. doi:10.1248/cpb.c18-00744
30. Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, Phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi:10.1016/S1470-2045(19)30097-X
31. Modi S, Park H, Murthy RK, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: results From a Phase Ib Study. J Clin Oncol. 2020;38(17):1887–1896. doi:10.1200/JCO.19.02318
32. Saura Manich C, Modi S, Krop I, et al. 279P Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2021;32:S485–S486. doi:10.1016/j.annonc.2021.08.562
33. Modi S, Saura C, Yamashita T, et al. Abstract PD3–06: Updated results from DESTINY-breast01, a Phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer. Cancer Res. 2021;81(4_Supplement):PD3-06-PD03–06.
34. Mode D, Stockholm LC. AstraZeneca: enhertu continues to demonstrate durable responses with new data from DESTINY-Breast01 in HER2-positive metastatic breast cancer. Cancer Res. 2021;81(4_Supplement):PD3-07-PD03–07.
35. Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022;386(12):1143–1154. doi:10.1056/NEJMoa2115022
36. Hurvitz S, Kim S-B, Chung W-P, et al. Abstract GS3-01: trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res. 2022;82(4_Supplement):GS3-01-GS03–01. doi:10.1158/1538-7445.SABCS21-GS3-01
37. Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387(1):9–20. doi:10.1056/NEJMoa2203690
38. Hamilton E, Shapiro CL, Petrylak D, et al. Abstract PD3-07: trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021;81(4_Supplement):PD3-07-PD03–07.
39. Schmid P, Im S-A, Armstrong A, et al. BEGONIA: phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J Clin Oncol. 2021;39(15_suppl):1023. doi:10.1200/JCO.2021.39.15_suppl.1023
40. Linehan AS, Fitzpatrick OM, Morris PG. Profile of Trastuzumab Deruxtecan in the Management of Patients with HER2-Positive Unresectable or Metastatic Breast Cancer: an Evidence-Based Review. Breast Cancer. 2021;13:151–159. doi:10.2147/BCTT.S245024
41. Anders CK, LeBoeuf NR, Bashoura L, Faiz SA, Shariff AI, Thomas A, What’s the Price? Toxicities of Targeted Therapies in Breast Cancer Care. Am Soc Clin Oncol Educ Book. 2020;40:55–70. doi:10.1200/EDBK_279465
42. Elgersma RC, Coumans RGE, Huijbregts T, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: toward Selection of HER2-Targeting Antibody–Drug Conjugate SYD985. Mol Pharm. 2015;12(6):1813–1835. doi:10.1021/mp500781a
43. Dokter W, Ubink R, van der Lee M, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13(11):2618–2629. doi:10.1158/1535-7163.MCT-14-0040-T
44. Saura C, Thistlethwaite F, Banerji U, et al. A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. J Clin Oncol. 2018;36(15_suppl):1014. doi:10.1200/JCO.2018.36.15_suppl.1014
45. Nadal-Serrano M, Morancho B, Escrivá-de-Romaní S, et al. The Second Generation Antibody-Drug Conjugate SYD985 Overcomes Resistances to T-DM1. Cancers. 2020;12:3. doi:10.3390/cancers12030670
46. Saura Manich C, O’Shaughnessy J, Aftimos PG, et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S1288. doi:10.1016/j.annonc.2021.08.2088
47. Bergstrom D, Bodyak N, Park P, et al. Abstract P4-14-28: XMT-1522 induces tumor regressions in pre-clinical models representing HER2-positive and HER2 low-expressing breast cancer. Cancer Res. 2016;76(4_Supplement):P4-14-28-P14-14–28. doi:10.1158/1538-7445.SABCS15-P4-14-28
48. Hamilton EP, Barve MA, Bardia A, et al. Phase 1 dose escalation of XMT-1522, a novel HER2-targeting antibody-drug conjugate (ADC), in patients (pts) with HER2-expressing breast, lung and gastric tumors. J Clin Oncol. 2018;36(15_suppl):2546. doi:10.1200/JCO.2018.36.15_suppl.2546
49. Le Joncour V, Martins A, Puhka M, et al. A Novel Anti-HER2 Antibody-Drug Conjugate XMT-1522 for HER2-Positive Breast and Gastric Cancers Resistant to Trastuzumab Emtansine. Mol Cancer Ther. 2019;18(10):1721–1730. doi:10.1158/1535-7163.MCT-19-0207
50. Liu Y, Lian W, Zhao X, et al. A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are HER2-positive or HER2-amplified who did not respond or stopped responding to approved therapies. J Clin Oncol. 2020;38(15_suppl):1049. doi:10.1200/JCO.2020.38.15_suppl.1049
51. Liu Y, Lian W, Zhao X, et al. A phase I study of safety and pharmacokinetics of A166, a novel selective inhibitor of human epidermal growth factor receptor-2 in Chinese patients with advanced solid tumors. J Clin Oncol. 2020;38(15_suppl):e13007. doi:10.1200/JCO.2020.38.15_suppl.e13007
52. Wang J, Xu B, Wang W, Fang J. An open-label, dose-escalation phase I study to evaluate RC48-ADC, a novel antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2018;36(15_suppl):1030. doi:10.1200/JCO.2018.36.15_suppl.1030
53. Xu B, Wang J, Zhang Q, et al. An open-label, multicenter, phase Ib study to evaluate RC48-ADC in patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2018;36(15_suppl):1028. doi:10.1200/JCO.2018.36.15_suppl.1028
54. LoRusso P, Krop I, Miller K, et al. Abstract CT234: a phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015;75(15_Supplement):CT234. doi:10.1158/1538-7445.AM2015-CT234
55. Miller K, Cortes J, Hurvitz SA, et al. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer. 2016;16:352. doi:10.1186/s12885-016-2385-z
56. Li J, Toader D, Perry SR, et al. Abstract 2970: MEDI4276, a HER2-targeting antibody tubulysin conjugate, displays potent in vitro and in vivo activity in preclinical studies. Cancer Res. 2016;76(14_Supplement):2970. doi:10.1158/1538-7445.AM2016-2970
57. Pegram MD, Hamilton EP, Tan AR, et al. First-in-Human, Phase 1 Dose-Escalation Study of Biparatopic Anti-HER2 Antibody–Drug Conjugate MEDI4276 in Patients with HER2-positive Advanced Breast or Gastric Cancer. Mol Cancer Ther. 2021;20(8):1442–1453. doi:10.1158/1535-7163.MCT-20-0014
58. Meric-Bernstam F, Calvo E, Moreno V, et al. A phase I dose escalation study evaluating the safety and tolerability of a novel anti-HER2 antibody-drug conjugate (PF-06804103) in patients with HER2-positive solid tumors. J Clin Oncol. 2020;38(15_suppl):1039. doi:10.1200/JCO.2020.38.15_suppl.1039
59. Barok M, Le Joncour V, Martins A, et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett. 2020;473:156–163. doi:10.1016/j.canlet.2019.12.037
60. Hu X, Zhang J, Ji D, et al. Abstract P1-18-16: a phase 1 study of ARX788, a HER2-targeting antibody-drug conjugate, in patients with metastatic HER2-positive breast cancer. Cancer Res. 2020;80(4_Supplement):P1-18-16-P11-18–16.
61. Rinnerthaler G, Gampenrieder SP, Greil R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int J Mol Sci. 2019;20:5. doi:10.3390/ijms20051115
62. Park YH, Ahn HK, Kim J-Y, et al. First-in-human phase I study of ALT-P7, a HER2-targeting antibody-drug conjugate in patients with HER2-positive advanced breast cancer. J Clin Oncol. 2020;38(15_suppl):3551. doi:10.1200/JCO.2020.38.15_suppl.3551
63. Hong R, Xia W, Wang L, et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: an open-label, dose-escalation, phase I study. Cancer Commun. 2021;41(2):171–182. doi:10.1002/cac2.12135
64. Chen Daniel S, Mellman I. Oncology Meets Immunology: the Cancer-Immunity Cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
65. Gatti-Mays ME, Balko JM, Gameiro SR, et al. If we build it they will come: targeting the immune response to breast cancer. Npj Breast Cancer. 2019;5(1):37. doi:10.1038/s41523-019-0133-7
66. Müller P, Kreuzaler M, Khan T, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315):315ra188. doi:10.1126/scitranslmed.aac4925
67. Waks AG, Keenan T, Li T, et al. A phase Ib study of pembrolizumab (pembro) plus trastuzumab emtansine (T-DM1) for metastatic HER2+ breast cancer (MBC). J Clin Oncol. 2020;38(15_suppl):1046. doi:10.1200/JCO.2020.38.15_suppl.1046
68. Hamilton EP, Kaklamani V, Falkson C, et al. Abstract PD1-05: atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer: safety and biomarker outcomes from a multi-cohort Phase Ib study. Cancer Res. 2020;80(4_Supplement):PD1-05-PD01–05.
69. Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–1295. doi:10.1016/S1470-2045(20)30465-4
70. Loi S, Schneeweiss A, Song E, et al. 329TiP KATE3: a phase III study of trastuzumab emtansine (T-DM1) in combination with atezolizumab or placebo in patients with previously treated HER2-positive and PD-L1–positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S509. doi:10.1016/j.annonc.2021.08.612
71. Borghaei H, Besse B, Bardia A, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with pembrolizumab in patients with advanced/metastatic breast or non-small cell lung cancer (NSCLC): a phase Ib, multicenter, study. J Clin Oncol. 2020;38(15_suppl):TPS1100.
72. Huang L, Wang R, Xie K, et al. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res Treat. 2022;191(1):51–61. doi:10.1007/s10549-021-06384-4
73. Ackerman SE, Pearson CI, Gregorio JD, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nature Cancer. 2021;2(1):18–33. doi:10.1038/s43018-020-00136-x
74. Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826.
75. Hamilton E, Shapiro CL, Petrylak D, et al. Abstract PD3-07: trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Cancer Research. 2021;81(4_Supplement):PD3-07-PD3–07. doi:10.1158/1538-7445.SABCS20-PD3-07
76. Manich CS, Modi S, Krop I, et al. 279P trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2021;32:S485–S486.
77. André F, Shahidi J, Lee C, Wang K, Krop IE. Abstract OT1-07-04:[Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-Breast02). Cancer Res. 2020;80(4_Supplement):OT1. AACR.
78. Lézard L. ENHERTU® Significantly Improved Progression-Free Survival in DESTINY-Breast03 Head-to-Head Trial Versus Trastuzumab Emtansine (T-DM1) in Patients with HER2 Positive Metastatic Breast Cancer. Cancer Res;2021;1:463.
79. Cortés J, Kim S, Chung W, et al. LBA1 Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32:S1287–S1288. doi:10.1016/j.annonc.2021.08.2087
80. Modi S, Ohtani S, Lee C, Wang Y, Saxena K, Cameron DA. Abstract OT1-07-02: a phase 3, multicenter, randomized, open-label trial of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice in HER2-low breast cancer (DESTINY-Breast04). Cancer Research. 2020;80(4_Supplement):OT1-07-02-OT1-07–02. doi:10.1158/1538-7445.SABCS19-OT1-07-02
81. Geyer CE, Untch M, Prat A, et al. Abstract OT-03-01: trastuzumab deruxtecan (T-DXd; DS-8201) vs trastuzumab emtansine (T-DM1) in high-risk patients with HER2-positive, residual invasive early breast cancer after neoadjuvant therapy: a randomized, phase 3 trial (DESTINY-Breast05). Cancer Research. 2021;81(4_Supplement):OT-03-01-OT-03–01. doi:10.1158/1538-7445.SABCS20-OT-03-01
82. Hurvitz SA, Peddi PF, Tetef ML, et al. TRIO-US B-12 TALENT: Phase II Neoadjuvant Trial Evaluating Trastuzumab Deruxtecan with or Without Anastrozole for HER2-Low,HR+ Early Stage Breast Cancer. Wolters Kluwer Health; 2021.
83. Bardia A, Barrios C, Dent R, et al. Abstract OT-03-09: trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: a randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021;81(4_Supplement):OT.
84. Andre F, Hamilton EP, Loi S, et al. Trastuzumab Deruxtecan (T-Dxd) Combinations in Patients with HER2-Positive Advanced or Metastatic Breast Cancer: A Phase 1b/2, Open-Label, Multicenter, Dose-Finding and Dose-Expansion Study (DESTINY-Breast07). Wolters Kluwer Health; 2021.
85. Jhaveri K, Hamilton E, Loi S, et al. Abstract OT-03-05: trastuzumab deruxtecan (T-DXd; DS-8201) in combination with other anticancer agents in patients with HER2-low metastatic breast cancer: a phase 1b, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast08). Cancer Res. 2021;81(4_Supplement):OT.
86. Tolaney S, Barroso-Sousa R, Jiang Z, et al. 328TiP Phase III study of trastuzumab deruxtecan (T-DXd) with or without pertuzumab vs a taxane, trastuzumab and pertuzumab in first-line (1L), human epidermal growth factor receptor 2–positive (HER2+) metastatic breast cancer (mBC): DESTINY-Breast09. Ann Oncol. 2021;32:S507–S508. doi:10.1016/j.annonc.2021.08.611
87. Bartsch R, Berghoff A, Furtner J, et al. 280P Intracranial activity of trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients with active brain metastases: results from the first stage of the phase II TUXEDO-1 trial. Ann Oncol. 2021;32:S486. doi:10.1016/j.annonc.2021.08.563
88. Batista MV, Pérez-Garcia J, Cussac AL, et al. 330TiP Trastuzumab deruxtecan (T-DXd; DS-8201) in HER2-positive (HER2+) and HER2-low expressing (HER-LE) metastatic breast cancer (MBC) with brain metastases (BM) and/or leptomeningeal carcinomatosis (LMC): DEBBRAH. Ann Oncol. 2021;32:S509–S510. doi:10.1016/j.annonc.2021.08.613
89. Krop IE, Ramos J, Zhang C, Hamilton EP. HER2CLIMB-04: Phase 2 Open Label Trial of Tucatinib Plus Trastuzumab Deruxtecan in Patients with HER2+ Unresectable Locally Advanced or Metastatic Breast Cancer with and without Brain Metastases (Trial in Progress). Wolters Kluwer Health; 2021.
90. Borghaei H, Besse B, Bardia A, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with pembrolizumab in patients with advanced/metastatic breast or non-small cell lung cancer (NSCLC): a phase Ib, multicenter, study. Am Soc Clin Oncol. 2020;38(15_suppl):TPS1100–TPS1100. doi:10.1200/JCO.2020.38.15_suppl.TPS1100
91. Prat A, Gavilá J, Pernas S, et al. Abstract OT-03-07: solti-1804 HER2-PREDICT: a biomarker research study of DS8201-A-U301-U302 and-U303 trials. Cancer Research. 2021;81(4_Supplement):OT-03-07-OT-03–07. doi:10.1158/1538-7445.SABCS20-OT-03-07
92. Shen B-Q, Bumbaca D, Saad O, et al. Catabolic Fate and Pharmacokinetic Characterization of Trastuzumab Emtansine (T-DM1): an Emphasis on Preclinical and Clinical Catabolism. Curr Drug Metab. 2012;13(7):901–910. doi:10.2174/138920012802138598
93. Krop IE, Saura C, Yamashita T, et al. Abstract GS1-03: [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) in subjects with HER2-positive metastatic breast cancer previously treated with T-DM1: a phase 2, multicenter, open-label study (DESTINY-Breast01). Cancer Res. 2020;80(4_Supplement):GS1-03-GS01–03. doi:10.1158/1538-7445.SABCS19-GS1-03
94. Faria M, Peay M, Lam B, et al. Multiplex LC-MS/MS Assays for Clinical Bioanalysis of MEDI4276, an Antibody-Drug Conjugate of Tubulysin Analogue Attached via Cleavable Linker to a Biparatopic Humanized Antibody against HER-2. Antibodies. 2019;8(1):11. doi:10.3390/antib8010011
95. Graziani EI, Sung M, Ma D, et al. PF-06804103, A Site-specific Anti-HER2 Antibody–Drug Conjugate for the Treatment of HER2-expressing Breast, Gastric, and Lung Cancers. Mol Cancer Ther. 2020;19(10):2068–2078. doi:10.1158/1535-7163.MCT-20-0237
96. Nagaraja Shastri P, Zhu J, Skidmore L, et al. Nonclinical Development of Next-generation Site-specific HER2-targeting Antibody–drug Conjugate (ARX788) for Breast Cancer Treatment. Mol Cancer Ther. 2020;19(9):1822–1832. doi:10.1158/1535-7163.MCT-19-0692
97. Krop I, Hamilton E, Jung KH, et al. Abstract P2-13-25: a phase I dose-escalation study of DHES0815A, a HER2-targeting antibody-drug conjugate with a DNA monoalkylator payload, in patients with HER2-positive breast cancer. Cancer Res. 2022;82(4_Supplement):P2-13-25-P12-13–25. doi:10.1158/1538-7445.SABCS21-P2-13-25
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
