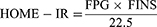Back to Journals » International Journal of Women's Health » Volume 15
The Relationship Between Insulin Resistance and Obesity and Serum Anti-Mullerian Hormone Level in Chinese Women with Polycystic Ovary Syndrome: A Retrospective, Single-Center Cohort Study
Authors Zhao H, Zhou D, Liu C, Zhang L
Received 15 October 2022
Accepted for publication 18 January 2023
Published 5 February 2023 Volume 2023:15 Pages 151—166
DOI https://doi.org/10.2147/IJWH.S393594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Han Zhao,1 Dexin Zhou,2 Cong Liu,1 Le Zhang1
1Department of Endocrinology, Shengjing Hospital, China Medical University, Shenyang, Liaoning, People’s Republic of China; 2Department of Endocrinology, Dalian Third People´s Hospital, Dalian, Liaoning, People’s Republic of China
Correspondence: Cong Liu; Le Zhang, Department of Endocrinology, Shengjing Hospital, China Medical University, Shenyang, Liaoning, People’s Republic of China, Email [email protected]; [email protected]
Background: Anti-Mullerian hormone (AMH) is vital in the pathophysiological process of polycystic ovary syndrome (PCOS). The exact relationship between obesity and insulin resistance (IR) with AMH levels remains unclear.
Methods: A retrospective, single-center cohort study of 220 women with PCOS who underwent physical, endocrine, and metabolic assessments were performed. Patients were grouped by age, body mass indices (BMI), Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), and different phenotypes. Pearson correlation analysis assessed the correlation between AMH and HOMA-IR, BMI, and other PCOS indicators, and multiple linear regression analysis was performed to determine factors influencing AMH.
Results: In 220 patients with PCOS, serum AMH levels decreased with age and were significantly higher in the IR group than in the non-IR group (P < 0.01). AMH increased significantly in anovulatory patients with hyperandrogenemia and/or polycystic ovary, with no significant difference between obese and non-obese individuals. AMH levels correlated positively with luteinizing hormone (LH), LH/follicular stimulating hormone (FSH), testosterone, fasting insulin (FINS), and HOMA-IR levels; negatively with age and BMI levels (P < 0.05) and weakly with fasting plasma glucose in the classical PCOS phenotype (r=0.148, P < 0.05). Regression analysis showed that age, testosterone, FINS, LH, LH/FSH, and BMI influenced AMH levels (P < 0.05).
Conclusion: Chinese women with PCOS-IR showed associations with greater AMH levels. AMH levels correlated positively with HOMA-IR levels and negatively with BMI. AMH combined with BMI and HOMA-IR levels may help determine PCOS severity.
Keywords: polycystic ovary syndrome, anti-Mullerian hormone, insulin resistance, obesity, metabolic abnormality
Background
Polycystic ovary syndrome (PCOS) is a common reproductive, endocrine, and metabolic disease in women of reproductive age, with a prevalence rate of approximately 2.2–5.6% in China.1–3 It is mainly characterized by hyperandrogenism (HA), chronic anovulation, and polycystic ovaries and is accompanied by metabolic abnormalities such as insulin resistance (IR) and obesity.4,5 Since PCOS was first reported in 1935, several diagnostic criteria for PCOS have emerged, including the Rotterdam criteria, Androgen Excess Society (AES) criteria, and National Institute of Child Health and Human Development (NICHD) criteria.6–8 In 2018, the Chinese Endocrine Association issued Chinese guidelines for diagnosing and treating PCOS9 (Table 1). The critical value of ovulation disorder in diagnosing and treating PCOS was emphasized. Indeed, PCOS is the leading cause of menstrual abnormalities and infertility in Chinese women of reproductive age, and ovulation disorders and high androgen performance are the main problems first diagnosed in women with PCOS in clinical practice.10,11
 |
Table 1 PCOS Phenotypes According to Diagnostic Criteria Applied.5–8 |
Anti-Mullerian hormone (AMH) is a transforming growth factor superfamily member. It is produced and secreted by granulosa cells in the antral and small antral follicles of the ovary and is a reliable indicator of ovarian reserve.12–15 AMH has an essential role in the pathophysiological process of PCOS by regulating follicular development and is closely related to the severity of PCOS.16 Furthermore, serum AMH levels are independent of the menstrual cycle and are more sensitive and specific than ultrasound.16–18 AMH has been reported as an independent predictor of PCOS.19–22 Previous studies have shown that serum AMH level in PCOS patients is 2–3 times higher than that in ordinary women of reproductive age, and women with higher AMH levels (≥4.45 ng/mL) are more likely to develop PCOS than those with lower AMH level.23 Besides, high levels of AMH in women with PCOS are associated with reduced ovulation induction response and the risk of early abortion after in vitro fertilization and embryo transfer (IVF-ET) treatment.24,25 AMH appears to predict the response of letrozole-induced ovulation and gonadotropin-stimulated ovaries to IVF in women with PCOS,26,27 suggesting the importance of AMH in the diagnosis and treatment of PCOS.
however, serum AMH levels are affected by multiple factors, such as the environment and heredity.15,28 Previous studies have suggested that AMH levels in PCOS are related to HA levels and are affected by obesity and IR.23,29–31 Obesity and IR, as two underlying pathophysiological processes of PCOS, although not included in the diagnostic criteria, are closely associated with PCOS.32 Many clinical and epidemiological data show that about 70% of women with PCOS have IR, and up to 80% are overweight or obese, especially with abdominal obesity.33 Obesity and IR may exacerbate PCOS-related ovulation disorders by enhancing follicular excess through AMH dysregulation or the HA pathway.23 Appropriate weight management and improvement of IR can improve ovulation function in women with PCOS.32 However, the exact relationship between AMH levels and obesity and IR remains unclear. Furthermore, those relationships in different phenotypes and races are still controversial.34–38 Understanding the influencing factors of AMH in women with PCOS is advantageous for a better understanding of the clinical significance of AMH level fluctuations. Furthermore, it may help clinicians detect PCOS predisposition and intervene early to improve metabolic and reproductive outcomes. Therefore, we designed a retrospective, single-center cohort study to explore the relationship between serum AMH levels and IR and obesity in Chinese women with PCOS, which aimed to add to the current literature and provide insight into optimal clinical treatment.
Methods
Participants
We enrolled 220 Chinese women with PCOS aged 20–39 who visited the endocrinology clinic at Shengjing Hospital of China Medical University between January 2018 and January 2022. The inclusion criteria were (1) subjects meeting the diagnostic criteria of the 2018 Chinese PCOS Guidelines for women of reproductive age,9 which include the following: irregular menstrual cycle, amenorrhea, irregular uterine bleeding, or irregular menstrual volume. The diagnosis was also based on at least one of the following criteria: hyperandrogenic performance, hirsuteness, or HA; manifestations of HA, including acne and hirsutism; and biochemical indexes of HA (ie, testosterone [T] > 0.75 ng/mL) or a polycystic ovary on ultrasonography; (2) subjects aged between 18 and 40 years; and (3) subjects who had not received any prescription or non-prescription drugs that affect insulin sensitivity or ovarian function, including hormonal contraceptives, within three months before the trial.
The exclusion criteria were as follows:
- Subjects with Cushing’s syndrome and adrenal cortex hyperplasia or tumors
- Subjects with abnormal uterine bleeding, primary amenorrhea, hypothalamic amenorrhea, pituitary amenorrhea, and uterine amenorrhea
- Subjects with hyperprolactinemia
- Subjects with thyroid dysfunction
- Subjects with premature ovarian aging, functional ovarian tumors, and theca cell proliferation
- Subjects who were pregnant or breastfeeding
- Subjects with a 17 hydroxyprogesterone level > two ng/mL.
- Subjects with autoimmune disease, malignant tumors, diseases of the central nervous system, or other conditions caused by HA and ovulation disorders
A complete medical history was required for all subjects. The Institutional Review Board approved this study at China Medical University (approval number: 2022PS674K), and informed consent was obtained from each patient before the study.
Assessments
This was a retrospective, single-center cohort study. After obtaining informed consent, the following data were obtained: (1) height, weight, and age; (2) menstrual cycle; and (3) medical history. The height and weight of each subject wearing light clothes were measured to the nearest 0.1 cm and 0.1 kg, respectively. The BMI was calculated as weight (kg) divided by height (m) square. Menstrual cycle disorders included oligomenorrhea and amenorrhea. Oligomenorrhea refers to patients with less than six menstrual periods within 12 months, and amenorrhea refers to patients who have stopped menstruating for more than six months. Each bleeding incidence counts as one menstrual cycle.
Venous blood levels were measured on days 2–5 of the menstrual cycle or when no dominant follicles were found on gynecological ultrasound, and the subjects fasted for 8–12 h overnight. All samples were measured in a vital laboratory in the hospital using standard laboratory techniques. Serum AMH levels were determined by enzyme-linked immunosorbent assay. Levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and T were measured using an electrochemical luminescence analyzer on Beckman Coulter Unicel Dlx 800. We calculated LH/FSH = LH (mIU/mL)/FSH (mIU/mL); fasting plasma glucose (FPG) and fasting insulin (FINS) levels were measured using an automatic biochemical analyzer. Glycated hemoglobin (HbA1c) levels were determined using an automatic HbA1c detector (high-pressure liquid chromatography). On the day of blood collection, the ovarian volume and number and size of ovarian follicles on each side were determined by ultrasound examination. Those who had never engaged in sexual activity underwent a transabdominal ultrasound examination, and those who had engaged in sexual activity underwent a transvaginal ultrasound examination.
The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) level was calculated using the following formula to evaluate IR:39
HOMA-IR is currently the most commonly used clinical indicator to evaluate the degree of IR. China’s diabetes cooperative group defines HOMA-IR ≥ 2.69 as IR, and obesity is defined as a BMI ≥ 25 kg/m2 according to the World Health Organization (WHO)’s standard in Asia.40,41
Based on age, participants were divided into the 20–29-year-old group (131 women) and the 30–39-year-old group (89 women). The participants were also divided into two groups according to the level of HOMA-IR: the IR group (HOMA-IR ≥ 2.69, 126 patients) and the non-IR (NIR) group (HOMA-IR < 2.69, 94 patients). Furthermore, according to BMI, 93 women were categorized into the non-obese group (BMI < 25 kg/m²), and 127 women were categorized into the obese group (BMI ≥ 25 kg/m²). Finally, combined with the current standard classification of PCOS,42 we divided PCOS into three phenotypes according to our inclusion criteria, that is, the polycystic ovary (PCO) and oligomenorrhea (OA) (102 patients), OA and hyperandrogenism (HA) (34 patients), and PCO+OA+HA (84 patients) groups. Among them, the group of OA+HA and PCO+OA+HA belonged to the classic phenotype of the Rotterdam criteria, while PCO+OA was the non-hyperandrogenemia phenotype.42
Statistical Analyses
SPSS (version 25.0; IBM, Armonk, NY, USA) and GraphPad Prism 8.0.1 (GraphPad Software, Chicago, IL, USA) were used to perform all statistical analyses. All data were tested for normality and homogeneity of variance. Normally distributed data are expressed as means ± standard deviations. Independent sample t-tests were performed to compare two groups, and R software (version 3.5.0, MathSoftCorp, AT&T Bell Laboratories) was used to correct P-values. Effects in three groups were analyzed by analysis of variance, and the Bonferroni test was performed as a posthoc test to determine where the statistical differences existed. Pearson correlation analysis was performed to study the correlation between AMH and age, obesity, IR, and other indicators of PCOS, and multiple linear regression analysis was performed to determine the factors influencing AMH. Bilateral tests were performed for all statistical tests. P < 0.05 was considered statistically significant.
Results
Demographics
The mean age, AMH level, BMI, HOMA-IR level, and T level of our participants were 28.13 ± 4.29 years, 7.97 ± 5.10 ng/mL, 27.21 ± 4.85 kg/m2, 4.31 ± 3.10, and 0.79 ± 0.34 ng/mL, respectively. IR was present in 126 (57%) participants; 127 (57%) were obese, 110 (50%) had abnormal glucose tolerance, 167 (80%) had an LH/FSH >1, and 119 (54.1%) had HA. Combined with the current standard classification of PCOS, 102 (46.4%) had PCO+OA, 34 (15.5%) had OA+HA, and 84 (38.2%) had PCO+OA+HA (Figure 1).
 |
Figure 1 General characteristics and classification proportion of PCOS population. |
Differences Between Age Groups
By comparing the two age groups, we found that women in the 20–29-year-old group showed significantly higher AMH, LH, T, FINS, HOMA-IR, and LH/FSH levels than women in the 30–39-year-old group. There was no significant difference in BMI and HbA1c, FPG, and FSH levels between the two age groups. Notably, AMH [(8.90±5.22) ng/mL vs (6.61±4.61) ng/mL, P < 0.05]; LH/FSH (1.83±0.912 vs 1.39±0.85, P < 0.05); LH [(11.84±6.24) mIU/mL vs (8.70±5.75) mIU/mL, P < 0.01]; and T [(0.99±0.29) ng/mL vs (0.50±0.14) ng/mL, P < 0.01] levels remained significantly different after adjusting for P values (Table 2), which suggests that these hormone levels decrease with an increase in the age of patients with PCOS.
 |
Table 2 PCOS General Information and Comparison of PCOS General Information Among Different Age Groups |
Correlation Between AMH and Other Indicators in the HOMA-IR Groups
In comparing the IR group and the NIR group at the cut-off point of HOMA-IR 2.69, we found that the ages of the participants in the NIR group were significantly higher than those in the IR group. AMH, LH, LH/FSH, and T levels were significantly higher in the IR group than in the NIR group; a larger HOMA-IR suggests higher AMH, LH, LH/FSH, and T levels and lower age. However, only AMH [(6.03±3.74) ng/mL vs (9.39±5.48) ng/mL, P < 0.00] showed a significant difference after adjusting P values. There were no differences in other indicators between the two groups (Table 3).
 |
Table 3 Comparison of AMH Levels and Other Indicators Under Different HOMA-IR Conditions |
Differences in BMI Groups
According to BMI, 93 women were categorized into the non-obese group (BMI < 25 kg/m²), and 127 women were categorized into the obese group (BMI ≥ 25 kg/m²). We found that HbA1c was significantly higher in the obese group than in the non-obese group. AMH, LH/FSH, and LH levels in the obese group were significantly lower than in the non-obese group. There were no differences in T, FPG, FSH, FINS, and HOMA-IR levels, as well as age between the two groups. However, we found that the previous correlation was not significant after the correction of P values (P>0.05) (Table 4).
 |
Table 4 Comparison of AMH Level and Other Indexes Under Different BMI |
Differences in Different PCOS Phenotypes
We divided PCOS into three phenotypes according to the diagnostic criteria and compared them between multiple groups. AMH, T, LH, LH/FSH, and age differed among different phenotypes. Compared with PCO+OA type, AMH [(6.60±4.53) ng/mL vs (9.81±5.30) ng/mL vs (8.89±5.27) ng/mL, P < 0.01], T [(0.53±0.15) ng/mL vs (1.02±0.28) ng/mL vs (1.02±0.29) ng/mL, P < 0.01], LH [(8.93±5.78) mIU/mL vs (13.16±6.91) mIU/mL vs (11.51±5.98) mIU/mL, P < 0.01], and LH/FSH (1.41±0.86 vs 1.94±0.94 vs 1.84±0.91, P < 0.01) were increased in the OA+HA group and PCO+OA+HA group, while age [(31.85±2.47) years vs (24.91±2.54) years vs (24.90±2.58) years, P < 0.01] decreased. There were no significant differences in other indicators. In addition, there was no significant difference between the OA+HA group and the PCO+OA+HA group (Table 5 and Figure 2).
 |
Table 5 Comparison of AMH Level and Other Indexes in Different PCOS Phenotypes |
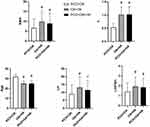 |
Figure 2 Indicators of significant differences in different PCOS phenotypes. *P <0.05, #P <0.01 vs. PCO+OA. |
Correlation Between AMH and Other Indicators and Multiple Linear Regression Analysis Affecting AMH Levels
In Pearson correlation analysis, a significant moderate positive correlation between AMH and the following factors was noted: T (r = 0.356, P < 0.01); LH/FSH (r = 0.311, P < 0.01); FINS (r = 0.265, P < 0.01); HOMA-IR (r = 0.223, P < 0.01); and LH (r = 0.223, P < 0.01). Conversely, AMH was negatively correlated with age (r = −0.315, P < 0.01) and BMI (r = −0.208, P < 0.01). In order of size of correlation, T showed the highest correlation, whereas BMI was the lowest (T > Age > LH/FSH > FINS > HOMA-IR = LH > BMI). There was no correlation between AMH and HbA1c, FPG, and FSH levels (Table 6). Multiple linear regression analysis using AMH level as the dependent variable and LH, LH/FSH, T, FINS, HOMA-IR levels, age, and BMI as independent variables revealed statistically significant results (R2= 0.259, F = 10.574, P < 0.01). We found that AMH levels could be explained in terms of T, LH/FSH, FINS, and BMI. Meanwhile, T, LH/FSH, and FINS positively affected AMH, and BMI negatively affected AMH. T has the most significant influence on the level of AMH among them. HOMA-IR was one of the independent determinants of AMH level in women with PCOS. The regression coefficients of the other variables are shown in Table 7.
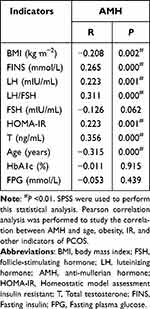 |
Table 6 Correlation Analysis of AMH and Various Indexes |
 |
Table 7 Multiple Linear Regression Analysis of AMH Influencing Factors |
Correlation and Multiple Linear Regression Analysis in Different PCOS Phenotypes
Due to the different expression of AMH between the OA+HA group and PCO+OA+HA group (classical group for short) and PCO+OA group, we further analyzed the correlation factors and independent determinants of AMH in these two groups. As shown in Table 8 and Table 9, T (r = 0.347, P < 0.01) had the highest correlation with AMH in the classical group, while LH/FSH (r = 0.410, P < 0.01) had the highest correlation in the PCO+OA group. In addition, a weak correlation between FPG (r = 0.148, P < 0.05) and AMH was observed in the classical group. Other related factors were not significantly different from the correlation analysis of AMH in general. These related factors were further incorporated into multiple linear regression analysis. Moreover, we found that T, LH/FSH, and BMI levels in the classical group were independent determinants of AMH (R2= 0.262, F=9.330, P< 0.01), whereas only LH/FSH was the influencing factor of AMH in the PCO+OA group (R2= 0.295, F=5.628, P< 0.01).
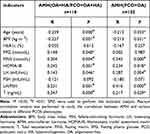 |
Table 8 Correlation Analysis of AMH and Various Indexes in Different PCOS Phenotypes |
 |
Table 9 Multiple Linear Regression Analysis of AMH Influencing Factors in Different PCOS Phenotypes |
Discussion
Our study aimed to lay a foundation for the future exploration of ovulation and metabolic abnormalities in PCOS patients in China. We found that AMH levels in the IR group were significantly higher than in the NIR group. Although differences are insignificant after adjusting for P values, HbA1c levels were higher, and AMH, LH/FSH, and LH levels were lower in obese individuals than in non-obese individuals. The classical phenotype (PCO+OA+HA and OA+HA) had higher AMH, T, LH/FSH, and LH levels and lower ages than the phenotype of PCO+OA. Furthermore, AMH levels were positively correlated with LH, LH/FSH, T, HOMA-IR, and FINS levels and negatively correlated with age and BMI. Besides, there was also a weak correlation in FPG in the classical PCOS phenotype. Through multiple linear regression, we found that AMH levels could be explained by T, LH/FSH, FINS, and BMI. In addition, among different phenotypes, AMH has the highest correlation with T in the classical phenotype, and T, LH/FSH, and BMI are independent determinants of AMH. However, the correlation between AMH and LH/FSH was strongest in non-hyperandrogenemia PCOS, and AMH was only affected by LH/FSH levels.
Normal ovarian development is affected by the factors inside and outside the ovary. The former include growth factors, cytokines, and inhibin in the follicular fluid, and the latter includes FSH deficiency, LH hypersecretion, high androgen levels in the ovaries and adrenal gland, IR, and hyperinsulinemia.43,44 AMH is recognized as the primary hormonal regulator of ovarian follicular development by concurrently stimulating preantral follicle growth and inhibiting antral follicle maturation.43 The above endogenous and exogenous factors could also influence serum AMH levels, which limits the accurate interpretation of AMH values in a clinical setting.45 Previous studies have determined that AMH levels are positively correlated with HA and LH and significantly negatively correlated with age in patients with PCOS.46 As we know, age is the most critical factor affecting the quality of the ovarian reserve.47 AMH levels increase steadily till nine years of age, decline slightly during the pubertal ages, and peak at around 25. A gradual decline follows this by reducing the primordial follicle pool with age until it reaches undetectable levels at an average of 50–51 years of age, corresponding to menopause.48 Serum AMH levels have decreased with age in healthy women and women with PCOS.48 This is consistent with our results, and age was significantly increased in the PCO+OA phenotype compared with the classical phenotype and was positively correlated with AMH. Fertility begins to decline in women in their 30s, and Tehrani et al suggest ovarian reserve screening should be considered in women older than 30.48 Our study shows that, compared with PCOS women aged 20–29, AMH, LH, LH/FSH, and T levels in women aged 30–39 showed a downward trend, suggesting a decline in ovarian reserve function. In addition, much effort is spent to identify a correct algorithm that considers women’s age and ovarian reserve markers as a tool to optimize the recombinant follicle-stimulating hormone (rFSH) starting dose in IVF procedure.49,50 Nevertheless, current evidence regarding PCOS women, particularly the ones with high AMH, seems inadequate. Further studies are necessary to prove this clinical utility.
LH is one of the biological features typically found in PCOS women.51 Researchers believed LH could cause a four-fold elevation in AMH production in ovarian granulosa cells of women with PCOS and elevate AMH expression with or without ovulation.52 The correlation between AMH and LH is independent of serum androgen and FSH levels.53 A high level of LH can stimulate the secretion and expression of AMH. AMH can also increase GnRH neurons’ activity and up-regulate LH’s pulsating secretion.53 AMH is secreted before the FSH-dependent selection of the dominant follicle,29 which is consistent with our current results. In our study, LH and LH/FSH were positively correlated with AMH levels in each phenotype and were independent determinants of AMH, while FSH did not correlate with AMH. Androgens promote the early stages of folliculogenesis, and androgen excess has been recognized as an essential factor in the development of most of the reproductive and metabolic alterations characterizing this syndrome.17,52 Excess androgen increases the ratio of estradiol receptor α to estradiol receptor β, resulting in increased AMH expression.53 Bongrani et al found that plasma AMH concentration was independently associated with PCO and intraovarian hyperandrogenism.54 In our study, we observed a positive correlation between serum T levels and AMH, especially in the classical PCOS phenotype, which corroborates data in the literature supporting the pathogenic role of androgen excess in the development of PCO.
The relationship between AMH and metabolic abnormalities such as IR has been controversial in the literature. IR is one of the essential mechanisms of PCOS pathogenesis,55 and insulin sensitizers, such as inositol, have shown significant advantages in improving ovulation and fertilization in PCOS women in the past two decades.55,56 IR and compensatory hyperinsulinemia can lead to androgen-dependent anovulation through different mechanisms, and a direct correlation has been found between sinus follicle count, ovarian volume, and hyperinsulinemia.57 Furthermore, LH and androgens appear to be related to the correlation between IR and AMH levels in PCOS.58 Although the internal mechanism of the relationship between AMH levels and IR in PCOS is unclear, some authors have reported a positive correlation between AMH and IR. IR-induced hyperinsulinemia appears to increase the premature differentiation of granulosa cells, suggesting that IR plays a role in AMH secretion in these cells. Excessive insulin levels have also been shown to alter granulosa cell receptivity and AMH production.59–62 Furthermore, relevant analysis of AMH genotypes in PCOS found significant differences in the distribution of AMH (rs10407022) gene polymorphisms between women with PCOS with IR and healthy women. However, there were no differences in the distribution of AMH genotypes between women with PCOS without IR and healthy women.63 When metformin, an insulin sensitizer, was used to treat PCOS for two months, the serum AMH level decreased, and ovulation increased, suggesting an etiological relationship between AMH levels and IR-PCOS.64 In this study, when IR was defined by HOMA-IR≥2.69, AMH levels in women with IR-PCOS were significantly higher than in women with NIR-PCOS.
In previous studies on the correlation between AMH and IR, the results are contradictory due to the different classifications of PCOS and the difference in the HOMA-IR boundary point. Wiweko et al’s study also revealed that serum AMH was significantly correlated with the HOMA-IR level, and there were differences between different PCOS phenotypes.65 Forenseca et al defined IR as HOMA-IR >3 and found higher serum AMH concentrations in IR-PCOS compared to NIR-PCOS.66 Sezai et al used the HOMA-IR cut-off as 2.5 in women with PCOS, and no significant difference was found in serum AMH levels between the IR and non-IR groups.35 The cut-off point of 2.69 was obtained according to the IR survey data of the China Diabetes Association, which was more consistent with the value of the Chinese IR population.40 In addition, all patients that met our inclusion criteria also met Rotterdam standards, providing comparability. Previous studies have shown that AMH is associated with the PCOS phenotype, as defined by the Rotterdam criteria (phenotype A: OA + HA + PCO; Phenotype B: OA + HA; Phenotype C: HA + PCO; Phenotype D: OA+ PCO). Among them, the highest serum AMH levels were found in phenotype A, whereas phenotype C was found to have the lowest mean serum level of AMH.61,67,68 The inclusion criteria of this study covered the population with phenotypes A, B, and D, which were more in line with AMH levels. Besides, our study with a large sample size was more suitable for studying the influencing factors of AMH in the Chinese context. We also divided the PCOS population into three phenotypes, A, B, and D, mentioned above. We found that the levels of AMH, LH, LH/FSH, and T were significantly higher in phenotypes A and B, and there was no significant difference between A and B. Although HOMA-IR showed no significant difference among different phenotypes, FINS and HOMA-IR were significantly positively correlated with AMH levels in classical phenotypes (phenotypes A and B) with high expression of AMH.
Li et al demonstrated that high levels of AMH may increase the risk of IR in obese PCOS patients,69 suggesting AMH’s effect on IR. A possible mechanism is that the release of AMH by the granulosa cells of PCOS patients through the uptake of androgens leads to hyperinsulinemia.23 In vitro studies have shown that elevated levels of AMH in beta cells of the pancreas may trigger insulin secretion. High peripheral AMH may affect islet function and increase the risk of IR and diabetes later in life.69 In our study, AMH was slightly positively associated with fasting glucose levels under the classical phenotype. A recent epidemiological survey of 3293 female participants found that lower age-specific AMH levels were associated with a higher risk of type 2 diabetes in women, suggesting a negative effect of AMH on islet function.
In China, 34.1–43.3% of women with PCOS are obese.70 The adverse effects of obesity on reproductive health and fertility, such as ovulation dysfunction, infertility, abortion, and related pregnancy complications, are well documented.71 Studies have also found that obese women with PCOS have higher infertility rates, poor response to ovulation induction drugs, poor embryo quality, a low success rate of in vitro fertilization, and significantly increased adverse pregnancy outcomes.72 In obese women, changes in the ovarian follicular microenvironment, including steroidogenesis, metabolism, and inflammation, indirectly affect AMH levels.73 A decrease in AMH levels has been suggested to be the result of metabolism, storage, and clearance in obese individuals.73 Increased leptin production associated with obesity directly inhibits AMH production.74 It has also been suggested that reduced AMH levels in obese women may be due to the blood-thinning effect of increased body size.62 Piouka et al demonstrated that the serum AMH levels of overweight and obese women with PCOS were significantly lower than those of lean women with PCOS.75 A meta-analysis based on 26 studies demonstrated that BMI was negatively correlated with AMH.67 Moreover, Buyukkaba et al found that the significantly increased AMH levels by losing weight with bariatric surgery in patients with morbid obesity with and without PCOS may indicate the improvement of fertilization potential, which could be considered when evaluating fertility in patients with morbid obesity.73 In this study, although differences were not significant with adjustment for potential confounding factors, AMH, LH/FSH, and LH levels were lower in obese individuals than in non-obese individuals. Furthermore, correlation and regression analysis of AMH showed that BMI was independently and negatively associated with AMH levels, supporting the concept that follicular development may be impaired in women with PCOS with increased BMI.
However, the relationship between obesity biomarkers and AMH levels in women with PCOS has also revealed conflicting reports, which can vary depending on the definition of obesity and grouping based on BMI. Zeng et al found that AMH was independently associated with central obesity but not general obesity in women with PCOS.36 Although obese women with PCOS primarily show abdominal obesity, large waist and hip circumferences, and a high waist-to-hip ratio, these were not included in our present study. Therefore, there may be some bias in the committed relationship between obesity and AMH levels, as one of the limitations of our study.
Other limitations of our retrospective cross-sectional study population consisted of only subfertile PCOS women; hence, it is impossible to generalize the findings to adolescent PCOS. Moreover, the antral follicle count was not included because of the lack of specific values once their number exceeded 12 on ultrasound and the large number of reports on the correlation between AMH and AFC. The exact mechanism of the relationship between AMH levels and IR and obesity in PCOS needs to be further elucidated. There is no consistent serum AMH diagnostic threshold for PCOS.71,76–78 Moreover, because of the lack of a control group, we did not further elaborate on the diagnostic significance of AMH levels in PCOS. Finally, due to the selection of Chinese PCOS diagnosis and treatment standards, patients with HA+PCO phenotype were not included, which may be biased in the correlation analysis of AMH. However, the sample size of this study is large, and the diagnosis of PCOS follows Chinese diagnosis and treatment standards, which is more consistent with the characteristics of PCOS in the Chinese population and provides a new perspective for AMH-related research in Chinese PCOS women. Therefore, we hope that more preclinical and clinical studies are conducted to verify the role of AMH levels in the prediction, prevention, and treatment of PCOS and to provide more of a theoretical basis for exploring the etiology of PCOS.
Conclusion
We found that serum AMH levels were associated with metabolic abnormalities in Chinese women with PCOS. Chinese women with PCOS-IR showed an association with greater AMH levels. AMH was significantly correlated with BMI under PCOS classical phenotype, while AMH seems to be affected only by LH/FSH in the non-hyperandrogenic PCOS. Future studies are needed to explore the potential mechanism linking BMI, IR, and AMH that might lead to important insights into ovarian physiology in patients with PCOS.
Abbreviations
PCOS, Polycystic ovary syndrome; AMH, Anti-Mullerian Hormone; T, Testosterone; BMI, Body mass index; FPG, Fasting plasma glucose; FINS, Fasting insulin; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LH, Luteinizing hormone; FSH, Follicle-stimulating hormone; IR, Insulin resistant; NIR, Non-insulin resistant; HA, Hyperandrogenism; PCO, Polycystic ovary; OA, Oligomenorrhea.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
Study complies with the Declaration of Helsinki. The Institutional Review Board approved our study at China Medical University (approval number: 2022PS674K), and informed consent was obtained from each patient before the study.
Author Contributions
H.Z. conceived the study. C.L. and L.Z. designed the study. H.Z. and Z.D. wrote the study design and registered the study protocol. H.Z. and Z.D. developed the statistical methods and analyzed the data. H.Z. wrote the first draft of the manuscript. Z.D., C.L., and L.Z. critically checked its content and approved its final version. All authors agreed with the results and conclusions of this article and agreed on the journal to which the article will be submitted. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version was accepted for publication, and any significant changes were introduced at the proofing stage. All authors agreed to take responsibility and be accountable for the article’s contents.
Funding
This work was supported by a grant from the 345 talent project plan of Shengjing Hospital of China Medical University.
Disclosure
The authors declare that they have no competing interests.
References
1. Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351. doi:10.18632/oncotarget.19180
2. Chen X, Yang D, Mo Y, et al. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Bio. 2008;139(1):59–64. doi:10.1016/j.ejogrb.2007.12.018
3. Deswal R, Narwal V, Dang A, et al. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. 2020;13(4):261. doi:10.4103/jhrs.JHRS_95_18
4. Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi:10.1016/j.fertnstert.2016.05.003
5. Podfigurna-Stopa A, Luisi S, Regini C, et al. Mood disorders and quality of life in polycystic ovary syndrome. Gynecol Endocrinol. 2015;31(6):431–434. doi:10.3109/09513590.2015.1009437
6. Amiri M, Bidhendi YR, Nahidi F, et al. The relationship between clinical and biochemical characteristics and quality of life in patients with polycystic ovary syndrome. Clin Endocrinol. 2019;90(1):129–137. doi:10.1111/cen.13858
7. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston. 1992;1992:77–84.
8. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop groxup. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Rep. 2004;19:41–47. doi:10.1093/humrep/deh098
9. Azziz R, Carmina E, Dewailly D, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi:10.1210/jc.2006-0178
10. Chinese Endocrinologist Association, Chinese Medical Doctor Association. Endocrinologist consensus on the management of polycystic ovary syndrome. Chin J Endocrinol Metab. 2018;34(1):1–7.
11. Wolf WM, Wattick RA, Kinkade ON, et al. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health. 2018;15(11):2589. doi:10.3390/ijerph15112589
12. De LV, Musacchio MC, Cappelli V, et al. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(1):38. doi:10.1186/s12958-016-0173-x
13. Yumiko T, Yoshikazu K, Yuko H, et al. Anti-Müllerian hormone levels in the diagnosis of adolescent polycystic ovarian syndrome: a systematic review and meta-analysis. Endocr J. 2022;69:897–906. doi:10.1507/endocrj.EJ22-0081
14. Salman BM, Javeria S, Sobia A, et al. Serum anti-Müllerian hormone as a predictor of polycystic ovarian syndrome among women of reproductive age. BMC Womens Health. 2022;22:199. doi:10.1186/s12905-022-01782-2
15. Iris H, Preston PJ, Klara B, et al. Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo stimulation with letrozole: a prospective cohort study. J Clin Med. 2022;11:2597.
16. Moolhuijsen LME, Louwers YV, Anke M, et al. Association between an AMH promoter polymorphism and serum AMH levels in PCOS patients. Hum Reprod. 2022;37:1544–1556. doi:10.1093/humrep/deac082
17. Ewa R, Michał K, Anna C-K, et al. Anti-müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci. 2021;23:22. doi:10.3390/ijms23010022
18. Bell RJ, Islam RM, Skiba MA, et al. Substituting serum anti-Müllerian hormone for polycystic ovary morphology increases the number of women diagnosed with polycystic ovary syndrome: a community-based cross-sectional study. Hum Reprod. 2021;37:109–118. doi:10.1093/humrep/deab232
19. de Loos Alexandra D, Martin H, Katharina B, et al. Antimüllerian hormone to determine polycystic ovarian morphology. Fertil Steril. 2021;116:1149–1157. doi:10.1016/j.fertnstert.2021.05.094
20. Evliyaoglu O, Imöhl M, Weiskirchen R, et al. Age-specific reference values improve the diagnostic performance of AMH in polycystic ovary syndrome. Clin Chem Lab Med. 2020;58(8):1291–1301. doi:10.1515/cclm-2019-1059
21. Calzada M, López N, Noguera JA, et al. AMH in combination with SHBG for the diagnosis of polycystic ovary syndrome. J Obstet Gynaecol. 2019;39(8):1130–1136. doi:10.1080/01443615.2019.1587604
22. Abbara A, Eng PC, Phylactou M, et al. Anti-müllerian hormone (AMH) in the diagnosis of menstrual disturbance due to polycystic ovarian syndrome. Front Endocrinol. 2019;10:656. doi:10.3389/fendo.2019.00656
23. Deshmukh H, Papageorgiou M, Kilpatrick EC, et al. Development of a novel risk prediction and risk stratification score for polycystic ovary syndrome. Clin Endocrinol. 2019;90(1):162–169. doi:10.1111/cen.13879
24. Wiweko B, Maidarti M, Priangga MD, et al. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet. 2014;31(10):1311–1316. doi:10.1007/s10815-014-0300-6
25. Mumford SL, Legro RS, Diamond MP, et al. Baseline AMH level associated with ovulation following ovulation induction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(9):3288–3296. doi:10.1210/jc.2016-1340
26. Knez J, Kovačič B, Medved M, et al. What is the value of anti-Müllerian hormone in predicting the response to ovarian stimulation with GnRH agonist and antagonist protocols. Reprod Biol Endocrinol. 2015;13:58. doi:10.1186/s12958-015-0049-5
27. Xin L, Ying H, Xinyan W, et al. Serum anti-Müllerian hormone levels are associated with early miscarriage in the IVF/ICSI fresh cycle. BMC Pregnancy Childbirth. 2022;22:279. doi:10.1186/s12884-022-04591-5
28. Di Clemente N, Chrystèle R, Alice P, et al. Anti-müllerian hormone in female reproduction. Endocr Rev. 2021;42:753–782. doi:10.1210/endrev/bnab012
29. Didier D, Anne-Laure B, Agathe D, et al. Role of anti-müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol. 2020;11:641. doi:10.3389/fendo.2020.00641
30. Skałba P, Cygal A, Madej P, et al. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):254–259. doi:10.1016/j.ejogrb.2011.06.006
31. Alviggi C, Conforti A, De RP, et al. The distribution of stroma and antral follicles differs between insulin-resistance and hyperandrogenism-related polycystic ovarian syndrome. Front Endocrinol. 2017;8:117. doi:10.3389/fendo.2017.00117
32. Er L, Jinxiao Z, Jiahui S, et al. Serum anti-müllerian hormone levels were negatively associated with body fat percentage in PCOS patients. Front Endocrinol. 2021;12:659717. doi:10.3389/fendo.2021.659717
33. Sadeghi HM, Adeli I, Calina D, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. 2022;23(2):583. doi:10.3390/ijms23020583
34. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol. 2021;95(4):531–541. doi:10.1111/cen.14421
35. Monica G, Ritu Y, Reeta M, et al. Correlation of body mass index (BMI), anti-mullerian hormone (AMH), and insulin resistance among different polycystic ovary syndrome (PCOS) phenotypes - a cross-sectional study. Gynecol Endocrinol. 2019;35:970–973. doi:10.1080/09513590.2019.1613640
36. Sezai S, Begum AM, Nigar S, et al. Serum AMH levels and insulin resistance in women with PCOS. Eur J Obstet Gynecol Reprod Biol. 2018;224:159–164. doi:10.1016/j.ejogrb.2018.03.007
37. Xiying Z, Yinxiang H, Mulin Z, et al. Anti-Müllerian hormone was independently associated with central obesity but not with general obesity in women with PCOS. Endocr Connect. 2022;11. doi:10.1530/EC-21-0243
38. Miaoxian O, Pei X, Han L, et al. AMH is a good predictor of metabolic risk in women with PCOS: a cross-sectional study. Int J Endocrinol. 2021;2021:9511772. doi:10.1155/2021/9511772
39. Yaqi W, Li W, Zhengyu Y, et al. Association of body mass index with serum anti-Müllerian hormone and inhibin B levels among 8323 women attending a reproductive medical center: a cross-sectional study. Endocrine. 2022;75:284–292. doi:10.1007/s12020-021-02839-2
40. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
41. Xiao-yan X, Wwn-ying Y, Zhao-jun Y. The diagnostic significance of homeostasis model assessment of insulin resistance in Metabolic Syndrome among subjects with different glucose tolerance. Chin J Diabetes. 2004;12(3):182–186.
42. Haixia S, Zhenqing Y, Ping L, et al. HOMA-IR for predicting clinical pregnancy rate during IVF. Gynecol Endocrinol. 2021;2021:1–6.
43. Paolo M, Flavia T, Cecilia B, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E628–37. doi:10.1210/jc.2012-3908
44. Bellver J, Rodríguez-Tabernero L, Robles A, et al. Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet. 2018;35(1):25–39. doi:10.1007/s10815-017-1047-7
45. Qiao J, Feng HL. Extra-and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17–33. doi:10.1093/humupd/dmq032
46. Cui Y, Shi Y, Cui L, et al. Age-specific serum antimullerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril. 2014;102:230–236 e232. doi:10.1016/j.fertnstert.2014.03.032
47. Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28(9):2562–2569. doi:10.1093/humrep/det262
48. Liu S, Hong L, Mo M, et al. Association of antimüllerian hormone with polycystic ovarian syndrome phenotypes and pregnancy outcomes of in vitro fertilization cycles with fresh embryo transfer. BMC Pregnancy Childbirth. 2022;22(1):171. doi:10.1186/s12884-022-04518-0
49. Rossana DP, Simone G, Sara G, et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch Gynecol Obstet. 2018;298:1029–1035. doi:10.1007/s00404-018-4906-2
50. Tanja BP, Eda VB, Uršula PZ, et al. PGR and PTX3 gene expression in cumulus cells from obese and normal weighting women after administration of long-acting recombinant follicle-stimulating hormone for controlled ovarian stimulation. Arch Gynecol Obstet. 2019;299:863–871. doi:10.1007/s00404-018-5031-y
51. Tehrani FR, Firouzi F, Behboudi-Gandevani S. Investigating the clinical utility of the anti-mullerian hormone testing for the prediction of age at menopause and assessment of functional ovarian reserve: a practical approach and recent updates. Aging Dis. 2022;13(2):458–467. doi:10.14336/AD.2021.0825
52. Silva MSB, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic–pituitary–gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78(1):1–16. doi:10.1007/s00018-020-03576-x
53. Franks S, Hardy K. Androgen Action in the Ovary. Front Endocrinol. 2018;9:452. doi:10.3389/fendo.2018.00452
54. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian Hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. doi:10.1210/er.2015-1104
55. Simone LA, Paola R, Massimo B, et al. Metabolism and ovarian function in PCOS women: a therapeutic approach with inositols. Int J Endocrinol. 2016;2016:6306410. doi:10.1155/2016/6306410
56. Cristiana P, Simone LA, Paolo M, et al. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-The-art and future perspectives. Gynecol Endocrinol. 2016;32:431–438. doi:10.3109/09513590.2016.1144741
57. Bongrani A, Plotton I, Mellouk N, et al. High androgen concentrations in follicular fluid of polycystic ovary syndrome women. Reprod Biol Endocrinol. 2022;20:88. doi:10.1186/s12958-022-00959-6
58. Xu Y, Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. 2022;2022:9240569. doi:10.1155/2022/9240569
59. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi:10.1210/er.2011-1034
60. Carmina E, Orio F, Palomba S, et al. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril. 2005;84:413–419. doi:10.1016/j.fertnstert.2004.12.061
61. Fonseca HP, Brondi RS, Piovesan FX, et al. Anti-Mullerian hormone and insulin resistance in polycystic ovary syndrome. Gynecol Endocrinol. 2014;30:667–670. doi:10.3109/09513590.2014.920004
62. Tokmak A, Kokanali D, Timur H, et al. Association between anti-Mullerian hormone and insulin resistance in non-obese adolescent females with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32:926–930. doi:10.1080/09513590.2016.1193140
63. Oldfield AL, Kazemi M, Lujan ME. Impact of obesity on anti-mullerian hormone (AMH) Levels in women of reproductive age. J Clin Med. 2021;10(14):3192. doi:10.3390/jcm10143192
64. Zheng MX, Li Y, Hu R, et al. Anti-Müllerian hormone gene polymorphism is associated with androgen levels in Chinese polycystic ovary syndrome patients with insulin resistance. J Assist Reprod Genet. 2016;33(2):199–205. doi:10.1007/s10815-015-0641-9
65. Foroozanfard F, Samimi M, Almadani AH, et al. Effect of metformin on the anti-Müllerian hormone level in infertile women with polycystic ovarian syndrome. Electron Physician. 2017;9(19):5969–5973. doi:10.19082/5969
66. Wiweko B, Indra I, Susanto C, et al. The correlation between serum AMH and HOMA-IR among PCOS phenotypes. BMC Res Notes. 2018;11(1):114. doi:10.1186/s13104-018-3207-y
67. Piouka A, Farmakiotis D, Katsikis I, et al. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metabol. 2009;296:E238–43. doi:10.1152/ajpendo.90684.2008
68. Catteau-Jonard S, Bancquart J, Poncelet E, et al. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40:223–229. doi:10.1002/uog.11202
69. Sahmay S, Atakul N, Oncul M, et al. Serum anti-Mullerian hormone levels in the main phenotypes of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;170:157–161. doi:10.1016/j.ejogrb.2013.05.019
70. Li X-J, Wang H, Lu D-Y, et al. Anti-müllerian hormone accelerates pathological process of insulin resistance in polycystic ovary syndrome patients. Horm Metab Res. 2021;53:504–511. doi:10.1055/a-1499-7718
71. Kim JY, Tfayli H, Michaliszyn SF, et al. Anti-Müllerian hormone in obese adolescent girls with polycystic ovary syndrome. J Adolesc Health. 2017;60(3):333–339. doi:10.1016/j.jadohealth.2016.10.015
72. Jungheim ES, Travieso JL, Carson KR, et al. Obesity and reproductive function. Clin North Am. 2012;39(4):479–493.
73. Hirschberg AL. Polycystic ovary syndrome, obesity and reproductive implications. Women’sHealth. 2009;5(5):529–542.
74. Buyukkaba M, Turgut S, Ilhan Mahmut M, et al. Anti-mullerian hormone levels increase after bariatric surgery in obese female patients with and without polycystic ovary syndrome. Horm Metab Res. 2022;54:194–198. doi:10.1055/a-1756-4798
75. Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–1669. doi:10.1093/humrep/det072
76. Moslehi N, Shab-Bidar S, Tehrani FR, et al. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A Meta-Analysis Menopause. 2018;25(9):1046–1055. doi:10.1097/GME.0000000000001116
77. Yue CY, Lu LKY, Li M, et al. Threshold value of anti-Mullerian hormone for the diagnosis of polycystic ovary syndrome in Chinese women. PLoS One. 2018;13(8):e0203129. doi:10.1371/journal.pone.0203129
78. Sathyapalan T, Al-Qaissi A, Kilpatrick ES, et al. Salivary and serum androgens with anti-Müllerian hormone measurement for the diagnosis of polycystic ovary syndrome. Sci Rep. 2018;8(1):3795. doi:10.1038/s41598-018-22176-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.