Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Baseline Serum BCAAs are Related to the Improvement in Insulin Resistance in Obese People After a Weight Loss Intervention
Authors Zhang C, Wang S , Wu Y , Guo Y, Wang X
Received 30 August 2022
Accepted for publication 6 January 2023
Published 19 January 2023 Volume 2023:16 Pages 179—186
DOI https://doi.org/10.2147/DMSO.S388117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gian Paolo Fadini
Chenghui Zhang,* Suyuan Wang,* Yunhong Wu, Yanhong Guo, Xi Wang
Endocrinology Department, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Wang, Email [email protected]
Background: Branched chain amino acids (BCAAs) have been revealed to be closely related to insulin resistance and obesity. This study aimed to investigate if BCAA levels at baseline are related to an improvement in insulin resistance after implementing a weight loss program intervention.
Methods: Stored blood samples from participants in previous trials were used for BCAA evaluation. Linear regression was used to analyze the relationship between baseline amino acid levels and changes in the insulin resistance index (HOMA-IR) and blood glucose.
Results: A total of 48 participants were enrolled. After the intervention, the body weight (78.29± 12.68 vs 72.06 ± 13.30 kg, p=0.020), fasting glucose (4.76 ± 0.43 vs 4.48 ± 0.39 mmol/L, p=0.001), fasting insulin (18.41± 13.58 vs 12.87± 10.88, p=0.028), and HOMA-IR (4.01± 3.39 vs 2.62± 2.18, p=0.018) were improved significantly. BCAA levels were related to the improvement in HOMA-IR (β=− 0.006, p=0.039), and valine was found to be the most closely related to the improvement in HOMA-IR (β=− 0.013, p=0.017).
Conclusion: The baseline BCAA is related to the improvement in insulin resistance among participants after a weight loss intervention.
Keywords: obesity, branched chain amino acid, weight loss, insulin resistance
Introduction
In the past decades, the incidence of overweight and obesity has been increasing rapidly and has become one of the major public health issues in China. The prevalence of obesity in China has been estimated to be around 15% according to epidemic studies.1
Branched chain amino acids (BCAAs) were revealed to be strongly related to obesity and insulin resistance in both population and animal studies.2,3 An epidemiology study showed that a higher intake of BCAAs in daily life may lead to a higher risk of obesity and insulin resistance.4 The circulating BCAA level has been reported to decrease with a decrease in the body weight after diet intervention or weight loss surgery.5,6
To achieve weight-loss, several diet interventions have been developed and used. Among them, an intervention system integrating a mobile app, wireless body composition scale, and diet replacement product, called the Metawell program, was developed in China and has been proven to be effective in previous studies.7,8 However, whether this intervention can improve insulin resistance in obese patients, and whether the circulating BCAAs are related to the improvement in glucose metabolism remains unknown.
Therefore, this study analyzed the association between the Metawell weight loss intervention program and improvement in insulin resistance in obese patients, as well as the association between BCAAs and improvement in insulin resistance in patients.
This study complies with the Declaration of Helsinki and have been approved by the ethics committee of Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region.
Methods
Population and Weight Loss Interventions
The samples and related data were acquired from the DIOP study; the inclusion criteria for participants and diet intervention programs have been described in detail in the previously published protocol of this study.9
The participants’ age was between 18 and 70 years and body mass index 25–40 kg/m2, and they had at least one of the following condition listed: history of hypertension or either systolic blood pressure >120 mmHg or diastolic blood pressure >80 mmHg; abdominal circumference >96 cm (90 cm for women); fasting triglycerides >1.69 mmol/L; history of type 2 diabetes mellitus managed with lifestyle interventions (not on insulin or oral medications) or fasting blood glucose >5.6 mmol/L; HDL cholesterol <1.04 mmol/L (1.3 mmol/L for women).
In this study, we chose 48 participants in the intervention group and sent their blood samples, from before and after the intervention, stored in a −80℃ freezer, for the evaluation of amino acid levels.
During the intervention period, all these Participants in intervention group had been guided to use meal replacement product to replace their daily meal, monitor and upload their body composition data using a wireless scale. Their daily energy intake had been limited to 800–1200 kcal and daily protein intake would be about 10g/day.
Clinical Measurements
Age, weight, waist circumstance, hip circumstance, fasting glucose, and fasting insulin level of the participants were extracted from the database of DIOP study. The detection methods have been described in our previous study9 As a brief, body weight and height were measured using an ultrasonic height-weight scale (DHM-200, Dinghengkeji, Hennan China) and alanine aminotransferase (ALT) blood glucose and insulin levels were tested by automatic biochemical analyzer (7180, HITACHI, Japan). The insulin resistance index was calculated using Homeostatic Model Assessment for Insulin resistance index (HOMA-IR) model with the fasting glucose and fasting insulin levels.10
Serum Amino Acid Test
The serum samples were dried with nitrogen and re-dissolved. Then, 20 μL of the samples was added to an HPLC-MS/MS system (SHIMADZU LC20, API 3200MD) with a mobile phase of methanol and 0.2% formic acid (80:20, V/V) at a flow rate of 30 μL/min for separation. The mass spectrometry detection was performed using an electrospray ionization source, and multiple reaction monitoring (MRM) scanning.
Statistical Methods
Data were presented as mean ± standard deviation (SD) for continuous data and n (n%) for categorical data. The differences in the clinical measurements before and after the intervention were analyzed using the paired t-test. According to Yin’s study (Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers), the participants were divided into two groups, participants with a HOMA-IR >3 would be defined as insulin resistant, and those with HOMA-IR≤3 defined as non-insulin resistant. Differences in the amino acids levels between the two groups were analyzed using the independent t-test. Linear regression was used to analyze the relationship between baseline amino acid levels and the change in HOMA-IR and glucose levels, the age and gender of participants, baseline BMI will be adjusted. P<0.05 was considered as statistically significant.
Results
Changes in Anthropometrical and Clinical Measurements After the Intervention
A total of 48 participants were enrolled in this study, out of whom, 30 (62.50%) participants were women. The average age was 34.55 years. After the intervention, the bodyweight of the participants decreased along with an improvement in the fasting glucose and insulin resistance. The changes in the participants’ weight, BMI, waist circumstance, hip circumstance, fasting glucose, and HOMA-IR were statistically significant. The changes in the clinical measurements are summarized in Table 1.
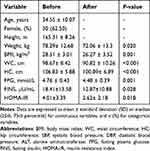 |
Table 1 Changes in the Anthropometrical and Clinical Measurements (n=48) |
Relationship Between Aromatic Amino Acids and Improvement in Insulin Resistance, and Other Metabolic Indices
Patients were divided into two groups based on their baseline HOMA-IR, and the differences in amino acids and other clinical characteristics were compared between the groups (Table 2). The levels of leucine, valine, phenylalanine, tryptophan, aromatic amino acids (AAAs), and BCAAs were higher in the insulin resistance group.
 |
Table 2 Differences in the Baseline Amino Acid Levels and Clinical Characteristics Between the Non-Insulin Resistance and Insulin Resistance Groups |
The results of correlation analysis showed that the decrease in HOMA-IR after weight loss correlated with the baseline levels of BCAAs (Figure 1). Also, changes in baseline phenylalanine and valine were statistically significant in relation to the improvement in insulin resistance. After adjusting for the age, gender and baseline BMI of participants, the mean HOMA-IR levels decreased by 0.6 (95% confidence interval [CI]: 0.03–0.13, p=0.042) for every 100 increase in the BCAA levels (Table 3). The decrease in the fasting glucose level was associated with baseline levels of AAA (Figure 2). After adjustment, Isoleucine, tryptophan, and tyrosine levels were statistically significant in relation to the improvement in the fasting glucose while the relationship between baseline AAA and decrease in glucose level was not statistically significant after adjustment (Table 4).
 |
Table 3 Correlation Between the Baseline Amino Acid Levels and Change in HOMA-IR |
 |
Table 4 Correlation Between the Baseline Amino Acid Levels and Change in Fasting Glucose |
In addition to insulin resistance, there was a statistically significant correlation between baseline aromatic amino acids/branched-chain amino acids and aminotransferase level (Supplementary Table 1).
Discussion
Our main finding was that the baseline BCAA level was associated with an improvement in insulin resistance in participants after a diet intervention program for weight loss with a lowering of the fasting glucose level.
After a three-month intervention using a diet replacement product, our participants showed a significant improvement in insulin resistance along with a decrease in the body weight. The results of DiRECT study showed that a primary care-led weight loss intervention program can lead to a remission of type 2 diabetes, with diabetes remission found in about 36% of the participants in the intervention group.11 The results of our study confirmed that diet replacement interventions can also improve insulin resistance in non-diabetic participants.
On comparing the baseline BCAA and AAA levels between the non-insulin resistance and insulin resistance group, we found that the BCAA and AAA levels were significantly higher in the insulin resistance group, and baseline BCAA level is related to the improvement of insulin resistance after weight loss intervention. BCAAs were usually considered closely related to metabolic unhealthy obesity and a consequence of insulin resistance, they indicated the abnormal protein breakdown. However, we can still find robust evidence that BCAAs also play an important role in the development of insulin resistance.12 Many epidemic studies have revealed elevated BCAA levels in participants with insulin-resistance.2 Many studies also revealed that a higher baseline BCAA level was correlated with a higher baseline HOMA-IR as well as a greater decrease in HOMA-IR after weight loss,5 and after weight loss interventions, such as restricting protein intake and surgery, the blood BCAAs will decrease along with a decreasing in body weight.13–15 Zhou’s team had performed a study using ob/ob mice to further confirm the causal relationship between BCAAs and insulin resistance. They find that in ob/ob mice, restoring BCAA catabolic flux or limiting the intake of BCAA can reduce the abundance of BCAA and attenuate insulin resistance of these mice.16 Our findings were consistent with the findings of the above study.
Valine was found to be the most closely associated with the improvement in HOMA-IR, and tyrosine was most closely related to the improvement in blood glucose after weight loss. This finding was similar of Newgard’s research, which indicated that a reduction in valine or isoleucine alone is sufficient to recapitulate the beneficial effects of reducing all BCAAs,2 also, Yu’s team found that a restriction in valine intake will promote the metabolic health in mice.17 The effect of valine was mediated by 3-HIB, an intermediate of valine, and first reported by Jang. An accumulation of valine will lead to the elevation of 3-hydroxyisobutyrate (3-HIB), and the elevated 3-HIB level will activate endothelial fatty acid transport and promote muscle lipid accumulation.18 Meanwhile, 3-HIB will mediate the increase in basal muscle glucose uptake, then leading to glucotoxicity, both effects will lead to an result of insulin resistance.19 Lee et al also found elevated 3-HIB levels in individuals with dysglycaemia.20 Elevated oxidative stress is another possible way that valine causing insulin resistance. Hu’s team found that L-Val levels were associated with a higher fasting plasma glucose level and oxidative stress. However, further studies should be performed to confirm this mechanism.21 Tyrosine, one of the aromatic amino acids, was considered as a promising biomarker to distinguish metabolic unhealthy obesity from metabolic healthy obesity,12 Li’s study suggests that the risk of people with tyrosine level higher than 46μmol/L to be diabetic was higher.22
Furthermore, we find that the baseline BCAA level is correlated with the improvement in liver function through the lowering of ALT. A study on the relationship between BCAAs and Non-alcoholic Fatty Liver Disease (NAFLD) reported that the during follow-up, participants with a higher baseline valine level showed a greater increment in the hepatic fat, and the increase in the area under the curve (AUC) of plasma valine for predicting hepatic fat fraction (HFF) was 0.803.23 Therefore, we assume that the correlation between plasma BCAAs and improvement in liver function was caused by the improvement in NAFLD in our participants; however, further studies are required to confirm this.
The main limitation of our study is that because of the short follow-up period, it was difficult to observe the effect of the dietary intervention and BCCAs on insulin resistance in the medium- and long-term. Comparing baseline BCAA levels, the change in BCAAs was more of interest and a study on the relationship of change in BCAAs and the intermediate products of BCAAs and improve of insulin resistance will provide more clues on the underlying mechanism, limited by funding, we did not perform the evaluation of BCAAs and any of the intermediates of BCAA levels after intervention, this is another limitation of our study.
Data Sharing Statement
These data won’t uploaded online, please contact corresponding author to acquire data.
Ethical Approval
This study was approved by the ethics committee of the Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region. All participants had been signed the informed consent before enrollment.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by Bethune Charitable Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
2. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi:10.1016/j.cmet.2009.02.002
3. Lee J, Vijayakumar A, White PJ, et al. BCAA supplementation in mice with diet-induced obesity alters the metabolome without impairing glucose homeostasis. Endocrinology. 2021;162(7):bqab062. doi:10.1210/endocr/bqab062
4. Lu J, Gu Y, Liu H, et al. Daily branched-chain amino acid intake and risks of obesity and insulin resistance in children: a cross-sectional study. Obesity. 2020;28(7):1310–1316. doi:10.1002/oby.22834
5. Zheng Y, Ceglarek U, Huang T, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103(2):505–511. doi:10.3945/ajcn.115.117689
6. Golzarand M, Toolabi K, Hedayati M, Azam K, Douraghi M, Djafarian K. Comparative study of resting metabolic rate and plasma amino acid profile in patients who underwent laparoscopic roux-en-y gastric bypass and laparoscopic sleeve gastrectomy: 6-month follow-up study. Obes Surg. 2019;29(10):3125–3132. doi:10.1007/s11695-019-03969-3
7. Senecal C, Widmer RJ, Larrabee BR, et al. A digital health weight loss program in 250,000 individuals. J Obes. 2020;2020:9497164. doi:10.1155/2020/9497164
8. Senecal C, Collazo-Clavell M, Larrabee BR, et al. A digital health weight-loss intervention in severe obesity. Digit Health. 2020;6:2055207620910279. doi:10.1177/2055207620910279
9. Wang X, Wang S, Zhang C, et al. Internet-based platform for a low-calorie dietary intervention involving prepackaged food for weight loss in overweight and obese individuals in China: protocol for a randomised controlled trial. BMJ Open. 2022;12(1):e048106. doi:10.1136/bmjopen-2020-048106
10. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
11. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–355. doi:10.1016/S2213-8587(19)30068-3
12. Cheng D, Zhao X, Yang S, Cui H, Wang G. Metabolomic signature between metabolically healthy overweight/obese and metabolically unhealthy overweight/obese: a systematic review. Diabetes Metab Syndr Obes. 2021;14:991–1010. doi:10.2147/DMSO.S294894
13. Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–530. doi:10.1016/j.celrep.2016.05.092
14. Tan HC, Khoo CM, Tan MZ, et al. The effects of sleeve gastrectomy and gastric bypass on branched-chain amino acid metabolism 1 year after bariatric surgery. Obes Surg. 2016;26(8):1830–1835. doi:10.1007/s11695-015-2023-x
15. Lips MA, Van Klinken JB, van Harmelen V, et al. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care. 2014;37(12):3150–3156. doi:10.2337/dc14-0195
16. Zhou M, Shao J, Wu CY, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730–1746. doi:10.2337/db18-0927
17. Yu D, Richardson NE, Green CL, et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021;33(5):905–922.e6. doi:10.1016/j.cmet.2021.03.025
18. Jang C, Oh SF, Wada S, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22(4):421–426. doi:10.1038/nm.4057
19. Bishop CA, Machate T, Henning T, et al. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle. Nutr Diabetes. 2022;12(1):20. doi:10.1038/s41387-022-00200-8
20. Lee S, Gulseth HL, Langleite TM, et al. Branched-chain amino acid metabolism, insulin sensitivity and liver fat response to exercise training in sedentary dysglycaemic and normoglycaemic men. Diabetologia. 2021;64(2):410–423. doi:10.1007/s00125-020-05296-0
21. Hu W, Yang P, Fu Z, et al. High L-Valine Concentrations associate with increased oxidative stress and newly-diagnosed type 2 diabetes mellitus: a cross-sectional study. Diabetes Metab Syndr Obes. 2022;15:499–509. doi:10.2147/DMSO.S336736
22. Li J, Cao YF, Sun XY, et al. Plasma tyrosine and its interaction with low high-density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Investig. 2019;10(2):491–498. doi:10.1111/jdi.12898
23. Goffredo M, Santoro N, Tricò D, et al. A branched-chain amino acid-related metabolic signature characterizes obese adolescents with non-alcoholic fatty liver disease. Nutrients. 2017;9(7):642. doi:10.3390/nu9070642
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


