Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway
Authors Xiang J , Qin L , Zhong J, Xia N , Liang Y
Received 12 June 2023
Accepted for publication 5 August 2023
Published 15 August 2023 Volume 2023:16 Pages 2433—2446
DOI https://doi.org/10.2147/DMSO.S425642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Jie Xiang,1 Liyan Qin,2 Jinling Zhong,3 Ning Xia,2 Yuzhen Liang3
1Department of Clinical Nutrition, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Geriatric Endocrinology, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 3Department of Endocrinology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
Correspondence: Yuzhen Liang, Department of Endocrinology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China, Email [email protected] Ning Xia, Department of Geriatric Endocrinology, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China, Email [email protected]
Background: Obesity is related to the loss of skeletal muscle mass and function (sarcopenia). The co-existence of obesity and sarcopenia is called sarcopenic obesity (SO). Glucagon like peptide-1 receptor agonists (GLP-1RA) are widely used in the treatment of diabetes and obesity. However, the protective effects of GLP-1RA on skeletal muscle in obesity and SO are not clear. This study investigated the effects of GLP-1RA liraglutide and semaglutide on obesity-induced muscle atrophy and explored the underlying mechanisms.
Methods: Thirty-six male C57BL/6J mice were randomly divided into two groups and fed a regular diet and a high-fat diet for 18 weeks, respectively. After establishing an obesity model, mice were further divided into six groups: control group, liraglutide (LIRA) group, semaglutide (SEMA) group, high-fat diet (HFD) group, HFD + LIRA group, HFD + SEMA group, and subcutaneous injection for 4 weeks. The body weight, muscle mass, muscle strength, glycolipid metabolism, muscle atrophy markers, myogenic differentiation markers, GLUT4 and SIRT1 were analyzed. C2C12 myotube cells treated with palmitic acid (PA) were divided into four groups: control group, PA group, PA + LIRA group, PA + SEMA group. The changes in glucose uptake, myotube diameter, lipid droplet infiltration, markers of muscle atrophy, myogenic differentiation markers, GLUT4 and SIRT1 were analyzed, and the changes in related indicators were observed after the addition of SIRT1 inhibitor EX527.
Results: Liraglutide and semaglutide reduced HFD-induced body weight gain, excessive lipid accumulation and improved muscle atrophy. Liraglutide and semaglutide eliminated the increase of muscle atrophy markers in skeletal muscle and C2C12 myotubes. Liraglutide and semaglutide restored impaired glucose tolerance and insulin resistance. However, these beneficial effects were attenuated by inhibiting SIRT1 expression.
Conclusion: Liraglutide and semaglutide protects skeletal muscle against obesity-induced muscle atrophy via the SIRT1 pathway.
Keywords: liraglutide, semaglutide, muscle atrophy, obesity, insulin resistance, SIRT1
Introduction
Obesity (defined as BMI ≥ 30 kg/m2) is a chronic disease characterized by abnormal body fat or over-accumulation, which affects more than 1 billion people worldwide.1 Due to a sedentary lifestyle, adipose tissue disorders, comorbidities (acute and chronic diseases) and metabolic changes during aging, loss of skeletal muscle mass and function (sarcopenia) is common in obese patients.2 The decrease in muscle mass/function coexists with an increase in fat mass, is referred to as sarcopenic obesity (SO).3 Compared to obesity alone, SO is associated with an increased risk of adverse health consequences, such as disability, cardiovascular metabolic diseases, other comorbidities and mortality.4 The deposition of ectopic fat caused by obesity can lead to biological dysfunction of skeletal muscles, including inflammation, insulin resistance (IR) and oxidative stress.5 These changes further exacerbate the loss of skeletal muscles and physical dysfunction.6 Therefore, a deep understanding of the potential mechanisms of SO is crucial for its accurate diagnosis, effective prevention and treatment. Currently, the main treatment for SO is lifestyle intervention, including calorie restriction and physical activity.7 However, there are still some limitations in the treatment of SO.8 Therefore, determining the therapeutic targets of SO has attracted widespread attention.
Glucagon like peptide-1 (GLP-1) is a hormone secreted by intestinal L cells, which reacts to nutrients and plays a glucose dependent insulin promoting role in pancreatic islet cells.9 In addition, GLP-1 has multiple effects on various organ systems. Especially the decrease in appetite and food intake, leading to weight loss with long term use. These mechanisms support the clinical development of glucagon like peptide-1 receptor agonist (GLP-1RA) in the treatment of obesity. At present, the Food and Drug Administration (FDA) has approved some anti-obesity drugs, including GLP-1RA liraglutide and semaglutide.1 However, when obese individuals successfully lose weight, their muscle mass usually decreases as their body fat decreases.10 Since the skeletal muscle is the main site of glucose treatment, the decrease in skeletal muscle mass may be related to the deterioration of glucose metabolism in patients. Moreover, the decrease in skeletal muscle mass may be associated with an increased risk of sarcopenia and weakness in elderly patients. Therefore, it is better to mainly reduce fat without significant reducing muscle mass when weight loss is achieved. Some recent reports suggest that GLP-1RA can reduce weight without significantly reducing muscle mass, but the specific mechanism is not yet clear. In the Liraglutide Effect and Action in Diabetes trial (LEAD-3), GLP-1RA liraglutide treatment significantly reduced body fat, but there was no significant change in lean body mass.11 Perna et al reported that in obese elderly patients receiving 24 weeks of treatment with liraglutide, there was a significant reduction in fat mass, but no reduction in appendicular lean mass.12 Blundell et al investigated the impact of a new GLP-1RA semaglutide on obese patients. After 12 weeks of treatment, the average body fat and lean mass decreased by 3.5 kg and 1.1 kg, respectively.13 Ozeki et al reported that semaglutide effectively reduced body fat while maintaining the muscle mass in obese type 2 diabetic patients.14 These results indicated that GLP-1RA can reduce body fat with little impact on muscle, but the mechanism of action is still unclear.
Sirtuin1 (SIRT1) is a Sirtuin family protein with nicotinamide adenine dinucleotide (NAD) protein deacetylase activity.15 SIRT1 is a key regulator of skeletal muscle biological function, such as muscle atrophy, glycolipid metabolism, muscle cytokine secretion, and mitochondrial function.16 Overexpression of SIRT1 reduces muscle dysfunction by inhibiting key atrophy genes in skeletal muscles.17 Li et al reported that paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via SIRT1 pathway.18 Therefore, SIRT1 might be a key therapeutic target for muscle atrophy. Xu et al reported that SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis.19 GLP-1RA Exendin-4, alleviated hepatic steatohepatitis in HFD induced obese C57BL/6J mice in a SIRT1 dependent manner.20 Jeon et al reported that GLP‑1 improves PA induced insulin resistance in human skeletal muscle via SIRT1 activity.21 There may be a correlation between the activity of GLP-1RA and SIRT1. However, the relationship between GLP-1RA and SIRT1, as well as the effect of GLP-1RA on skeletal muscles is still poorly understood. Thus, the present study investigated whether GLP-1RA liraglutide and semaglutide has a protective effect in the skeletal muscle of HFD-fed mice and in PA-treated myotubes. In addition, this study explored whether the effect of GLP-1RA occurs through the activation of SIRT1.
Materials and Methods
Animals and Groups
A total of 36 Male C57BL/6 mice (8 weeks old) weighing 20.1±1.1 g, purchased from Guangzhou Yancheng Biotechnology Co., Ltd. were included in this study. The Mice were placed in a pathogen free environment at a temperature of 22 ± 2°C, with 12-hour light/12-hour dark cycles, and provided with adequate food and water. After one week of adaptive feeding, the animals were randomly fed a normal diet (Control, 12 kcal% fat, 24 kcal% protein, 64 kcal% carbohydrate; Sibeifu Biotechnology Co., Ltd, n=18) or a high-fat diet (HFD,60 kcal% fat, 20 kcal% protein, 20 kcal% carbohydrate; Research Diets, Inc, n=18) for more than 4 months. Weighed the feed to calculate the food intake of mice. In addition, mice were weighed weekly to monitor their weight gain. Mouse models of SO often exhibit weight gain accompanied by decreased muscle mass and strength. After 18 weeks feeding, the weight of the HFD group exceeded the average weight of the control group by 20%, which was the standard for successful modeling of obese mice. After modeling obesity, mice were further divided into six groups: normal diet group (Control), liraglutide (LIRA, Novo Nordisk) group, semaglutide (SEMA, Novo Nordisk) group, high-fat diet (HFD) group, HFD + LIRA group, HFD + SEMA group. Liraglutide (400ug/kg/d) and semaglutide (60ug/kg/d) was daily subcutaneous injection for 4 weeks. All animal experiments meet the requirements of National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No.8023) and has been were approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University (approval number 2022-KY0772).
Assessment of Body Weight and Lower Limb Muscles Weight
Measured the weight of mice every week. After the mice were anesthetized and sacrificed, the lower limb muscles including gastrocnemius muscle, tibialis anterior muscle, soleus muscle and quadriceps femoris muscle were quickly separated and weighed.
Intraperitoneal Glucose Tolerance Test (IPGTT) and Insulin Tolerance Test (ITT)
After 16 hours of fasting, the mice were intraperitoneally injected with 20% glucose at a dose of 2.0 g/kg body weight. Used the glucose meter to measure the glucose concentration in tail blood at 0, 15, 30, 60 and 120 minutes (Accu-Chek Active; Roche, Germany). After resting for a week, the mice fasted for 4 hours, then they were intraperitoneally injected with insulin at a dose of 0.5 U/kg body weight. Used a Glucose meter to measure the glucose concentration in tail blood at 0, 15, 30, 45, 60 and 90 minutes.
Biochemical Index Analysis
After anesthesia, blood samples were collected from the orbit of the mice. Then centrifuged the sample to separate the serum (3000g, 10 minutes). Fasting blood glucose was measured by a blood glucometer. Fasting insulin, triglycerides (TG), and total cholesterol (TC) levels were measured by ELISA assay kits (Quanzhou jiubang, China). Homeostasis model assessment of insulin resistance (HOMA-IR) was also assessed: 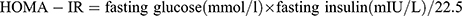 .
.
Grip Strength Test
Measured the grip strength of mice by using an electronic grip meter (DS2-500N, Shanghai Puxin, China). The mouse grasped the metal grid with its limbs, then gently pulled its tail backwards to make it parallel to the surface of the table until it released the grid. The peak force will be displayed on the sensor to measure the grip strength of the mouse. Repeated three times and record the maximum value.
Four-Limb Hanging Test
Each mouse was placed on a grid and grabbed with four claws. Inverted the grid and measured the suspension time. Repeated three times and recorded the maximum suspension time.
Micro Computed Tomography (Micro CT) Test
After anesthetized with tribromoethanol (0.03mL/g), laid the mouse prone on the scanner. After scanning, the lean body mass was measured using Micro CT (Aloka Latheta LCT 200, Hitachi, Japan).
Tissue Preparation and Histological Analysis
Fixed the gastrocnemius muscle (GAS) in muscle stationary liquid (Servicebio, China). Embedded the sample in paraffin and cut it into 5μm thick sections. The sections were stained with Haematoxylin and eosin (H&E) or Oil Red O solution. Image Pro Plus software was used to analyze the average cross-sectional area (CSA) and Oil Red O positive area of GAS muscle fibers.
Cell Culture
C2C12 cells (Procell, Wuhan, China) were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% foetal bovine serum (Gibco) and 1% penicillin-streptomycin solution (Biosharp). C2C12 cells were seeded at 2×105 cells per 6-well plate. When Cell fusion was 90% −100%, switched to DMEM containing 2% horse serum for incubation (Solarbio, Beijing, China) for 5 days to differentiate into myotubes. Then the myotubes were treated with 1.0 mM PA (Kunchuang, Xian, China) for 24 hours to simulate in vitro obesity. They were divided into four groups: control group, PA group, PA + LIRA (400 nM) group, PA + SEMA (60 nM) group.
Inhibitor Intervention
The SIRT1 inhibitor EX-527 (Med Chem Express) was used to inhibit the expression of SIRT1. C2C12 myotube cells were treated with PA and Liraglutide or semaglutide for 24 hours with or without EX-527.
2-NBDG Glucose Uptake Test
C2C12 myotubes were treated with DMEM containing fluorescent deoxyglucose analogues (2-NBDG, AAT Bioquest) for 30 minutes. Then washed the cells with buffer solution, analyzed cells using flow cytometer with 530/30 nm filter (FITC channel).
Immunofluorescence
C2C12 myotubes were incubated at room temperature with 50–100ul of membrane breaking working solution for 20 minutes, followed by PBS washing three times for 5 minutes each time. Then blocked cell serum with 10% normal goat for 30 minutes and incubated with anti-myosin heavy chain 2 (MYH2) antibody (1:1000, Signalway Antibody) overnight at 4 °C. The nuclei were stained with DAPI. Imaging was analyzed using Image-Pro Plus software, measuring the diameters of 5 myotubes in each slice and calculating the average value.
Oil Red O Staining
C2C12 myotubes were washed twice with PBS, and then incubated with oil red O working solution (Servicebio, China) at 37°C for 10 minutes. Used a photography microscope (Nikon, Japan) to select the target area for imaging, and then used Image Pro Plus software for analysis.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Extracted total RNA using RNAiso Plus (TaKaRa) and measured RNA concentration. Synthesized cDNA using Prime Script RT kit (TaKaRa). Performed QRT-PCR using the StepOnePlus real-time PCR system (ThermoFisher). The 2−ΔΔCt threshold cycling method was used to analyze the results. GAPDH was used as a reference gene. The relative mRNA expression of the control group was standardized to 1. The following PCR primers were used: Atrogin 1 (forward, F):5′- CATTGATGCGTGGGGTAGTC-3′, (reverse, R):5′-CAGGAGATGGTGCTGATAAAGAA-3′;
MURF1 (F) 5′-GGAAGGAAGCCAAGACAATAGA-3′, (R) 5′-GAGTGCTGGGATCAAAGGGT-3′.
MyoD (F) 5′-ACAGAACAGGGAACCCAGACC-3′, (R) 5′-TGAAGAACCAGGGACACCATC-3′.
MyoG (F) 5′-CGGTGGAGGATATGTCTGTTGC-3′, (R) 5′-GGTGTTAGCCTTATGTGAATGGG-3′.
SIRT1 (F): 5′-TCGGCTACCGAGGTCCATAT-3′, (R): 5′-GGAATCCCACAGGAGACAGAA-3′.
GLUT4 (F): 5′- TTCCTTCTATTTGCCGTCCTC-3′, (R): 5′-CTGTTTTGCCCCTCAGTCATT-3′.
GAPDH (F): 5′- GGTTGTCTCCTGCGACTTCA-3′, (R): 5′-TGGTCCAGGGTTTCTTACTCC-3′.
Western Blotting
Isolation and transfer of total protein onto PVDF membrane through SDS-PAGE. Incubated the membrane with the corresponding primary antibody, namely anti-SIRT1 (1:1000, Abcam), anti-GLUT4 (1:1000, Abcam), anti-Atrogin1 (1:1000, Abcam), anti- Myogenin (1:1000, Abcam), and anti-GAPDH (1:5000, Servicebio) overnight at 4°C. Then incubated the membrane with the second antibody at 37°C for 1 hour. Treated with a chemiluminescence substrate kit (Shanghai Yamei) and then use ImageJ software to detect protein bands.
Statistical Analysis
The experimental results were analyzed using SPSS 23.0 software. The data was expressed as mean ± standard deviation (SD). A Student’s t-test was used for comparison of two dependent groups and ANOVA was used for comparison of multiple groups. P<0.05 was considered as statistically significant.
Results
Beneficial Effects of Liraglutide and Semaglutide on Body Weight, Muscle Strength, and Muscle Mass in HFD Mice
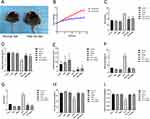
There was no significant difference in body weight between the normal diet group and the HFD group at the initial stage. However, after 18 weeks of feeding, the average body weight of the HFD group was 40% higher than that of the normal diet group, indicating the successful construction of an obese mouse model. Treatment with liraglutide and semaglutide significantly reduced the weight of obese mice (Figure 1A–C). Then we measured grip strength and limb suspension time to evaluate muscle strength and found that the relative grip strength and limb suspension time significantly decreased in HFD fed mice, while liraglutide and semaglutide treatment dramatically reduced HFD induced muscle atrophy (Figure 1D and E). Moreover, we used Micro CT to measure the body fat rate, visceral fat, and lean body weight (mainly composed of skeletal muscle) of mice to evaluate their muscle mass. Compared with the control group, the HFD group mice showed an increase in body fat rate and visceral fat, and a decrease percentage of lean body weight, which was significantly improved after treatment with liraglutide and semaglutide (Figure 1F and H). We also analyzed the weight of lower limb muscles (including gastrocnemius muscle, tibialis anterior muscle, soleus muscle and quadriceps femoris muscle), and found that high-fat diet significantly reduced the relative weight of lower limb muscles, while liraglutide and semaglutide evidently reversed the effect of high-fat diet on lower limb muscle weight (Figure 1I).
Liraglutide and Semaglutide Significantly Improved Glucose Tolerance, Insulin Resistance, Dyslipidemia, and Muscle Atrophy in HFD Mice
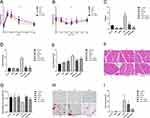
We also investigated the role of liraglutide and semaglutide in regulating glucose and lipid metabolism. IPGTT showed that liraglutide and semaglutide treatment improved HFD induced impaired glucose tolerance (Figure 2A). ITT and HOMA-IR suggested that HFD group mice exhibited insulin resistance compared to the control group, while treatment with liraglutide and semaglutide showed significant mitigation (Figure 2B and C). Liraglutide and semaglutide also alleviated HFD induced hyperlipidemia by reducing TG and TC levels (Figure 2D and E). These data showed that liraglutide and semaglutide treatment improved muscle atrophy, insulin resistance, and metabolic disorders in HFD mice. In addition, we further demonstrated the protective effect of liraglutide and semaglutide in improving skeletal muscle atrophy induced by HFD in obese mice. H&E staining showed that administration of liraglutide and semaglutide improved the decrease of CSA in HFD mice (Figure 2F and G). Moreover, oil red O staining indicated that lipid droplet infiltration in GAS muscles in HFD mice can also be alleviated by liraglutide and semaglutide treatment (Figure 2H and I).
Liraglutide and Semaglutide Improved Muscle Atrophy and Insulin Resistance in HFD Mice Through the SIRT1 Pathway
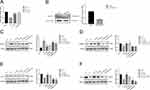
Next, we further determined whether liraglutide and semaglutide alleviated HFD induced skeletal muscle atrophy and insulin resistance by activating SIRT1 pathway. The results indicated that HFD up-regulated the expression of muscle atrophy factor Atrogin-1 (Figure 3A and B) but suppressed the expression of myogenic factor Myogenin (Figure 3C and D). However, liraglutide and semaglutide treatment could reduce the expression of Atrogin-1 and increased the expression of Myogenin. In addition, liraglutide and semaglutide treatment effectively alleviated the decrease in GLUT4 expression induced by HFD, which is related to the insulin resistance (Figure 3E and F). SIRT-1 is a key factor in regulating the biological function of skeletal muscles. We also found that liraglutide and semaglutide treatment eliminated the reduction of SIRT1 signaling pathway induced by HFD (Figure 3G and H). Therefore, the activation of SIRT-1 may be important for liraglutide and semaglutide to alleviate HFD induced muscle atrophy and insulin resistance.
Liraglutide and Semaglutide Alleviated PA Induced Muscle Atrophy, Lipid Accumulation, and Insulin Resistance in C2C12 Myotubes
To further explored the molecular mechanism of liraglutide and semaglutide in preventing HFD induced muscle atrophy, C2C12 myotubes were incubated with PA. PA was used to simulate obesity that can induce skeletal muscle atrophy, lipid droplet accumulation, and insulin resistance. We found that compared with the control group, PA significantly increased the expression of muscle atrophy markers Atrogin-1 and MuRF-1 (Figure 4A and B), while the expression of myogenic differentiation markers MyoD and Myogenin was significantly reduced (Figure 4C and D). In addition, the immunofluorescence of MYH2 showed that PA resulted in atrophy of myotubes, which was reversed by treatment with liraglutide and semaglutide. (Figure 4E and F). The above data indicated that liraglutide and semaglutide effectively alleviated PA induced muscle atrophy in C2C12 myotubes. Moreover, Oil Red O staining showed that liraglutide and semaglutide could improve lipid accumulation caused by PA (Figure 4G and H). We also found that liraglutide and semaglutide treatment attenuated the PA induced decrease in GLUT-4 expression (Figure 4I). In addition, the 2-NBDG test revealed that liraglutide and semaglutide mitigated PA induced insulin resistance (Figure 4J). It indicated that liraglutide and semaglutide ameliorated PA induced lipid accumulation and insulin resistance in C2C12 myotubes.
Liraglutide and Semaglutide Mitigated PA Induced Myotube Atrophy and Insulin Resistance via the SIRT1 Pathway
SIRT1 is a key regulatory factor in muscle metabolism. Therefore, we further verified whether liraglutide and semaglutide activated SIRT1 in the C2C12 myotubes to exert muscle protective effects. We found that compared with the control group, PA caused a decrease in SIRT1 expression, and liraglutide and semaglutide treatment significantly relieved the inhibition of SIRT1 in PA treated cells (Figure 5A). Then, the SIRT1 signal inhibitor EX-527 was added to further clarified the role of SIRT1 signal pathway in the influence of liraglutide and semaglutide. The concentration of EX-527 (100 µM) was determined (Figure 5B). Compared with the control group, PA resulted in increased expression of Atrogin1 protein and decreased expression of Myogenin, GLUT4 and SIRT1 protein, while liraglutide and semaglutide treatment showed significant recovery. However, after adding EX-527, the improvement effect of liraglutide and semaglutide was attenuated (Figure 5C–F). Therefore, liraglutide and semaglutide might alleviated myotube atrophy and insulin resistance caused by PA through the activation of SIRT1.
Discussion
Obesity and aging are common public health issues worldwide, both of which can cause loss of muscle mass and strength. When muscle mass reduction and fat mass accumulation coexist, it leads to sarcopenic obesity.3 Obesity can cause biological dysfunction of skeletal muscles, including lipid infiltration, insulin resistance, and inflammation.22,23 High-fat diet (HFD) has a higher energy density than a regular diet, which can lead to animal obesity and muscle atrophy.24,25 In animal studies, when the weight gain of the HFD group exceeds the control group by 10–25%, it belongs to mild to moderate obesity. If it is greater than 40%, it belongs to severe obesity.26,27 In our study, we used a high-fat diet containing 60% fat (mainly lard) to induce muscle atrophy in mice through lipid toxicity pathways.28 Our study found that mice fed HFD gradually gained weight, and after 18 weeks, the weight of the HFD group exceeded that of the control group by 49%, indicating severe obesity and can be used for subsequent experiments.
Glucagon-like peptide-1 receptor agonist (GLP-1RA) is a kind of hypoglycemic drug. In addition to reducing blood glucose, it also shows broad application prospects in reducing the weight of obese patients.29 There are six GLP-1RA approved for glucose reduction, but only liraglutide and semaglutide are approved for weight loss treatment in the United States at present.30 The mechanism of liraglutide and semaglutide on weight loss in obese patients is mainly to inhibit appetite and reduce energy intake.31 In animal studies, it has also been reported that liraglutide32 and semaglutide33 can reduce the weight of obese rats and mice by inhibiting appetite. For patients, liraglutide is injected once a day, and semaglutide is injected once a week. In this animal study, we referred to previous literature,34 and the use of liraglutide and semaglutide is subcutaneous injection once a day. We observed that after subcutaneous injection of liraglutide and semaglutide, the appetite of mice was suppressed. Four months later, mice fed with HFD lost 17.8% of their weight when using liraglutide, and 20.1% of their weight when using semaglutide. Interestingly, although the weight of mice in the normal diet group did not reach obesity, their weight also decreased (<10%) after liraglutide and semaglutide administration, indicating that liraglutide and semaglutide also had appetite inhibition and weight reduction effects on normal weight mice.
Skeletal muscle atrophy in obese mice is mainly evaluated through a decrease in muscle mass and muscle strength.35 We first evaluated the muscle strength of mice through grip strength testing and limb suspension testing. The mouse grip strength test36 can measure the muscle strength of its limbs, and the mouse limb suspension test37 can evaluate balance, coordination, and muscle condition. We found that the relative grip strength of HFD group mice decreased, and the longest suspension time was shortened, which was significantly improved after the treatment of liraglutide and semaglutide. These results suggested that the muscle strength of HFD group mice has decreased due to obesity. Liraglutide and semaglutide can not only reduce the weight of obese mice, but also increase the muscle strength.
Micro CT is a precise X-ray based imaging technique for small animal models.38 Pasetto et al used Micro CT for non-invasive assessment of muscle atrophy with good results.39 Therefore, we used Micro CT of small animals to measure the body fat rate, visceral fat, and lean body weight (mainly composed of skeletal muscle) of mice to evaluate their muscle mass. Our research found that the body fat rate and visceral fat of mice in HFD group increased significantly, and the percentage of lean body weight in total body weight decreased, which was significantly improved after treatment with liraglutide and semaglutide. It is suggested that the muscle mass of HFD group mice has decreased, and liraglutide and semaglutide can effectively reduce visceral fat of obese mice while maintaining muscle mass. After the mice were sacrificed, we weighed and recorded the lower limb muscles (Gastrocnemius muscle, soleus, tibialis anterior, Quadriceps) to further evaluate the effect of obesity on their muscle mass and the therapeutic effect of liraglutide and semaglutide. The results suggested that liraglutide and semaglutide treatment significantly reduced muscle mass loss caused by obesity.
Skeletal muscles regulates lipid and glucose metabolism.40 Obesity can lead to skeletal muscle fat deposition, leading to obesity related low-grade inflammation, releasing inflammatory factors, weakening the effect of insulin, and causing insulin resistance.41 Insulin resistance is one of the core pathophysiological mechanisms of sarcopenic obesity, leading to skeletal muscle atrophy.42 Therefore, we further verify whether HFD mice exhibit skeletal muscle lipid infiltration and insulin resistance, leading to muscle atrophy. The Gastrocnemius muscle muscle is the largest muscle in the hind limbs of mice and plays an important role in sports. Through the Oil Red O staining inspection of Gastrocnemius muscle, we found that there were a lot of lipid droplets in HFD group mice, which were significantly improved after treatment with liraglutide and semaglutide. It showed that obese mice had already had heterotopic fat deposition in skeletal muscle, and treatment with liraglutide and semaglutide could significantly reduce the lipid infiltration in skeletal muscle. The intraperitoneal injection of glucose tolerance test in mice can measure the clearance rate of the injected glucose load in vivo and evaluate whether there is any abnormality in glucose tolerance.43 The mouse insulin tolerance test can be used to evaluate insulin sensitivity.44 Measurement of fasting glucose, fasting insulin and calculation of HOMA-IR can further evaluate insulin resistance. Our results suggested that the HFD group mice had abnormal glucose tolerance, insulin resistance, and elevated triglycerides and total cholesterol, which were significantly improved after treatment with liraglutide and semaglutide. These results suggested that obese mice had insulin resistance due to lipid infiltration in skeletal muscle, and the intervention of liraglutide and semaglutide could significantly reduce insulin resistance, which may be one of the important mechanisms of improving muscle atrophy. We further performed HE staining of Gastrocnemius muscle and found that the cross sectional area of muscle fibers in HFD group mice decreased, which was significantly improved after treatment with liraglutide and semaglutide. It is proved that HFD can induce skeletal muscle atrophy in mice, and liraglutide and semaglutide can play a muscle protective role.
Maintaining a balance between skeletal muscle protein synthesis and degradation is crucial.45 Obese and sarcopenic obesity patients often experience muscle atrophy.46 Muscle protein degradation is mainly mediated by ubiquitin Proteasome and autophagy Lysosome systems.47 Atrogin-1 plays a key role in muscle atrophy as muscle specific ubiquitin ligase.48 Muscle derived regulatory factor Myogenin is crucial for promoting muscle recovery.49 GLUT-4 is a glucose transporter protein that maintains glucose homeostasis and insulin sensitivity.50 SIRT-1 is a key regulatory factor in muscle metabolism.51 We used qRT-PCR and Western blot methods to detect the expression levels of the aforementioned biomarkers. Our study found that the expression of Atrogin-1 in HFD group mice increased, and the expression of Myogenin, GLUT4 and SIRT1 decreased, which was significantly improved after treatment with liraglutide and semaglutide. It indicated that HFD induced muscle atrophy, insulin resistance, and decreased SIRT1 expression in obese mice. Liraglutide and semaglutide treatment can significantly reduce skeletal muscle atrophy and increase insulin sensitivity, which may play a role through the SIRT1 pathway.
In the cell experiment, we further explored the effects of liraglutide and semaglutide on the myotube atrophy of C2C12 cells induced by Palmitic acid. C2C12 cells are an ideal model for studying muscle diseases, which differentiate into myotubes after being treated with 2% horse serum.52 Palmitic acid (PA) is a saturated fat that can cause insulin resistance in skeletal muscle cell.53 PA treatment of C2C12 myotube cells can simulate in vitro obesity and cause myotube atrophy.54 Skeletal muscle cell absorb about 80% glucose through GLUT4, and the decrease of GLUT4 expression indicates that insulin signaling pathway is damaged.55 2-NBDG is a fluorescent labeled glucose tracer that can be used to evaluate insulin resistance of cells using immunofluorescence staining or flow cytometry.56 Our results showed that PA induced insulin resistance in C2C12 myotube cells, which could be improved after liraglutide and semaglutide intervention. Palmitic acid can also lead to lipid accumulation in myotube cells. We found that a large number of lipid droplets were observed in C2C12 myotube cells of PA group, which were significantly reduced after treatment with liraglutide and semaglutide. The immunofluorescence staining showed that the myotubes of C2C12 cells in PA group were significantly atrophied, and the diameter of myotubes increased after treatment with liraglutide and semaglutide.
Sirtuin family consists of seven subtypes, which are nicotinamide adenine dinucleotide (NAD) dependent proteins. Since the past two decades, sirtuin has evolved into a key epigenetics regulator of aging. Recent studies have shown that activation of SIRT1 plays a crucial role in preventing age-related muscle atrophy.57 SIRT1 belongs to the sirtuin family and has multiple functions in regulating metabolic disorders, cancer, and cardiac stress.58 Recent reports have found that activation of SIRT1 may help improve skeletal muscle disease.59 However, it is not yet clear whether GLP1-RA can improve skeletal muscle atrophy by activating SIRT1. Our results showed that in PA group, the expression of muscle atrophy markers (Atrogin-1, MuRF-1) of C2C12 myotube cells increased, and the expression of myogenic differentiation markers (MyoD, Myogenin) and SIRT1 decreased, which was significantly improved after treatment with liraglutide and semaglutide. These results suggested that liraglutide and semaglutide may improve skeletal muscle atrophy by activating SIRT1. EX-527 is an inhibitor of SIRT1. If the aforementioned improvement effect is eliminated after treating C2C12 myotube cells with EX-527, it can further confirm the regulatory effect of SIRT1. We found that the expression of Atrogin-1 in C2C12 myotube cells in the PA group increased, while the expression of Myogenin, GLUT4, and SIRT1 decreased. After treatment with liraglutide and semaglutide, the effect of PA was significantly improved, which was eliminated after adding EX-527. It suggested that SIRT1 may be involved in the regulation of liraglutide and semaglutide on PA induced myotube atrophy in C2C12 cells.
In summary, this study indicated that GLP1-RA liraglutide and semaglutide attenuated HFD-induced muscle atrophy and improved lipid and glucose metabolism via the SIRT1 pathway (Figure 6).
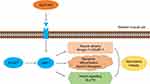 |
Figure 6 Schematic diagram of beneficial effects of liraglutide and semaglutide on obesity-induced skeletal muscle atrophy. |
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 8176030057) and Guangxi Natural Science Foundation (No.2017GXNSFDA198010).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Yang M, Liu S, Zhang C. The related metabolic diseases and treatments of obesity. Healthcare. 2022;10(9):1616. doi:10.3390/healthcare10091616
2. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15(3):321–335. doi:10.1159/000521241
3. Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12(6):887–888. doi:10.1038/oby.2004.107
4. Zhang X, Xie X, Dou Q, et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr. 2019;19(1):183. doi:10.1186/s12877-019-1195-y
5. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43–54. doi:10.1172/JCI88880
6. Lipina C, Hundal HS. Lipid modulation of skeletal muscle mass and function. J Cachexia Sarcopenia Muscle. 2017;8(2):190–201. doi:10.1002/jcsm.12144
7. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi:10.1038/s41574-018-0062-9
8. El Bizri I, Batsis JA. Linking epidemiology and molecular mechanisms in sarcopenic obesity in populations. Proc Nutr Soc. 2020;1–9. doi:10.1017/S0029665120000075
9. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. doi:10.1111/dom.13129
10. Kim B, Tsujimoto T, So R, Zhao X, Oh S, Tanaka K. Changes in muscle strength after diet-induced weight reduction in adult men with obesity: a prospective study. Diabetes Metab Syndr Obes. 2017;10:187–194. doi:10.2147/DMSO.S132707
11. Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11(12):1163–1172. doi:10.1111/j.1463-1326.2009.01158.x
12. Perna S, Guido D, Bologna C, et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016;28(6):1251–1257. doi:10.1007/s40520-015-0525-y
13. Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251. doi:10.1111/dom.12932
14. Ozeki Y, Masaki T, Kamata A, et al. The effectiveness of GLP-1 receptor agonist semaglutide on body composition in elderly obese diabetic patients: a Pilot Study. Medicines. 2022;9(9):47. doi:10.3390/medicines9090047
15. Milne JC, Denu JM. The sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12(1):11–17. doi:10.1016/j.cbpa.2008.01.019
16. Gurd BJ. Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):589–597. doi:10.1139/h11-070
17. Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem. 2013;288(42):30515–30526. doi:10.1074/jbc.M113.489716
18. Li Q, Wu J, Huang J, et al. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1α-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022;13:859723. doi:10.3389/fphar.2022.859723
19. Xu F, Li Z, Zheng X, et al. SIRT1 mediates the effect of GLP-1 receptor agonist exenatide on ameliorating hepatic steatosis. Diabetes. 2014;63(11):3637–3646. doi:10.2337/db14-0263
20. Lee J, Hong SW, Park SE, et al. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014;19(5):649–656. doi:10.1007/s12192-013-0490-3
21. Jeon JY, Choi SE, Ha ES, et al. GLP‑1 improves palmitate‑induced insulin resistance in human skeletal muscle via SIRT1 activity. Int J Mol Med. 2019;44(3):1161–1171. doi:10.3892/ijmm.2019.4272
22. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi:10.1016/j.arr.2016.09.008
23. Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambélé-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3):467–483. doi:10.1007/s10522-015-9626-4
24. Sishi B, Loos B, Ellis B, et al. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol. 2011;96(2):179–193. doi:10.1113/expphysiol.2010.054189
25. Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol. 1995;268(4 Pt 1):E546–E550. doi:10.1152/ajpendo.1995.268.4.E546
26. Rothwell NJ, Stock MJ. The development of obesity in animals: the role of dietary factors. Clin Endocrinol Metab. 1984;13(3):437–449. doi:10.1016/s0300-595x(84)80032-8
27. Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R46–R54. doi:10.1152/ajpregu.2002.282.1.R46
28. Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100(6):3077–3082. doi:10.1073/pnas.0630588100
29. Ladenheim EE. Liraglutide and obesity: a review of the data so far. Drug Des Devel Ther. 2015;9:1867–1875. doi:10.2147/DDDT.S58459
30. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi:10.1038/s41573-021-00337-8
31. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–793. doi:10.1038/ijo.2013.162
32. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. doi:10.1172/JCI75276
33. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi:10.1172/jci.insight.133429
34. Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3(6):844–857. doi:10.1016/j.jacbts.2018.09.004
35. You B, Dun Y, Fu S, et al. The treatment of rhodiola mimics exercise to resist high-fat diet-induced muscle dysfunction via sirtuin1-dependent mechanisms. Front Pharmacol. 2021;12:646489. doi:10.3389/fphar.2021.646489
36. Connolly AM, Keeling RM, Mehta S, Pestronk A, Sanes JR. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin alpha2-deficient mice. Neuromuscul Disord. 2001;11(8):703–712. doi:10.1016/s0960-8966(01)00232-2
37. Rafael JA, Nitta Y, Peters J, Davies KE. Testing of SHIRPA, a mouse phenotypic assessment protocol, on Dmdmdx and Dmdmdx3cv dystrophin-deficient mice. Mamm Genome. 2000;11(9):725–728. doi:10.1007/s003350010149
38. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13(8):724–728. doi:10.1007/s12603-009-0204-9
39. Pasetto L, Olivari D, Nardo G, et al. Micro-computed tomography for non-invasive evaluation of muscle atrophy in mouse models of disease. PLoS One. 2018;13(5):e0198089. doi:10.1371/journal.pone.0198089
40. Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–195. doi:10.1007/s00223-014-9915-y
41. Lauterbach MAR, Wunderlich FT. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch. 2017;469(3–4):385–396. doi:10.1007/s00424-017-1955-5
42. Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003;284(4):E741–E747. doi:10.1152/ajpendo.00514.2002
43. Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. doi:10.1152/ajpendo.90617.2008
44. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. doi:10.1242/dmm.006239
45. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–4314. doi:10.1111/febs.12253
46. Maliszewska K, Adamska-Patruno E, Krętowski A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol Arch Intern Med. 2019;129(11):809–816. doi:10.20452/pamw.15025
47. Milan G, Romanello V, Pescatore F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670. doi:10.1038/ncomms7670
48. Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi:10.1016/s0092-8674(04)00400-3
49. Knight JD, Kothary R. The myogenic kinome: protein kinases critical to mammalian skeletal myogenesis. Skelet Muscle. 2011;1:29. doi:10.1186/2044-5040-1-29
50. Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem. 2019;294(30):11369–11381. doi:10.1074/jbc.REV119.008351
51. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem. 2005;280(16):16456–16460. doi:10.1074/jbc.M501485200
52. Fujita H, Shimizu K, Nagamori E. Novel method for fabrication of skeletal muscle construct from the C2C12 myoblast cell line using serum-free medium AIM-V. Biotechnol Bioeng. 2009;103(5):1034–1041. doi:10.1002/bit.22318
53. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi:10.1172/JCI10842
54. Guo A, Li K, Tian HC, et al. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-α pathway. J Cell Mol Med. 2021;25(7):3585–3600. doi:10.1111/jcmm.16448
55. Cheema U, Yang SY, Mudera V, Goldspink GG, Brown RA. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskeleton. 2003;54(3):226–236. doi:10.1002/cm.10095
56. Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64(3):207–215. doi:10.1016/j.jbbm.2005.08.001
57. Anwar M, Pradhan R, Dey S, Kumar R. The role of sirtuins in sarcopenia and frailty. Aging Dis. 2023;14(1):25–32. doi:10.14336/AD.2022.0622
58. Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64(1):166–187. doi:10.1124/pr.110.003905
59. Tonkin J, Villarroya F, Puri PL, Vinciguerra M. SIRT1 signaling as potential modulator of skeletal muscle diseases. Curr Opin Pharmacol. 2012;12(3):372–376. doi:10.1016/j.coph.2012.02.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.