Back to Journals » Clinical Epidemiology » Volume 15
The Metabolic Score for Insulin Resistance (METS-IR) Predicts Cardiovascular Disease and Its Subtypes in Patients with Hypertension and Obstructive Sleep Apnea
Authors Yang W, Cai X , Hu J, Wen W , Mulalibieke H, Yao X, Yao L, Zhu Q, Hong J, Luo Q, Liu S, Li N
Received 17 November 2022
Accepted for publication 28 January 2023
Published 15 February 2023 Volume 2023:15 Pages 177—189
DOI https://doi.org/10.2147/CLEP.S395938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eyal Cohen
Wenbo Yang,1,* Xintian Cai,1,2,* Junli Hu,1 Wen Wen,1 Heizhati Mulalibieke,1 Xiaoguang Yao,1 Ling Yao,1 Qing Zhu,1 Jing Hong,1 Qin Luo,1 Shasha Liu,1 Nanfang Li1
1Hypertension Center, Xinjiang Hypertension Institute, NHC Key Laboratory of Hypertension Clinical Research, Key Laboratory of Xinjiang Uygur Autonomous Region, Xinjiang Clinical Medical Research Center for Hypertension Diseases, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, People’s Republic of China; 2Graduate School, Xinjiang Medical University, Urumqi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nanfang Li, Hypertension Center, Xinjiang Hypertension Institute, NHC Key Laboratory of Hypertension Clinical Research, Key Laboratory of Xinjiang Uygur Autonomous Region, Xinjiang Clinical Medical Research Center for Hypertension Diseases, People’s Hospital of Xinjiang Uygur Autonomous Region, NO. 91 TianChi Road, Urumqi, 830001, People’s Republic of China, Tel +86 8564818, Email [email protected]
Objective: We aimed to evaluate the METS-IR (metabolic score for insulin resistance) index for the prediction of incident cardiovascular disease (CVD) and its subtypes (coronary artery disease and stroke) in patients with hypertension and obstructive sleep apnea (OSA).
Methods: A retrospective cohort study was conducted with 2031 adults with hypertension and OSA, participants from the Urumqi Research on Sleep Apnea and Hypertension study (UROSAH). The hazard ratios and 95% CIs (credibility interval) for CVD and its subtypes were estimated using multivariate Cox proportional hazards regression models.
Results: After a median follow-up of 6.80 years (interquartile range: 5.90– 8.00 years), a total of 317 (15.61%) participants developed new-onset CVD, including 198 (9.75%) incident coronary heart disease (CHD) and 119 (5.86%) incident stroke. After adjusting for as many relevant confounding factors as possible, each SD increase in METS-IR was associated with a 30% increased risk of new onset overall CVD events, a 32% increased risk of new onset CHD, and a 27% increased risk of new onset stroke. When METS-IR was assessed as tertiles, after adjustment for fully confounding factors, the highest tertiles versus the lowest tertiles were associated with a greater hazard of CVD (HR 2.05; 95% CI 1.52,-2.77), CHD (HR 1.96; 95% CI 1.35– 2.84), and stroke (HR 2.24; 95% CI 1.35– 3.72). The results of various subgroups and sensitivity analyses were similar. When METS-IR was added, CVD predictions were reclassified and identified more accurately than baseline models for the C-index, continuous net reclassification improvement, and integrated discrimination index. CHD and stroke showed similar results.
Conclusion: METS-IR is a powerful predictor of CVD and its subtypes in patients with hypertension and OSA, which can facilitate the identification of high-risk individuals and provide individualized CVD prevention.
Keywords: cohort study, METS-IR, cardiovascular disease, hypertension, obstructive sleep apnoea
Introduction
CVD (cardiovascular disease) is the leading cause of death and disability in the global population,1,2 as well as a major contributor to reduced patient quality of life and increased social medical cost burden.3 Obstructive sleep apnea (OSA) and hypertension are both independent risk factors for CVD morbidity and mortality.4–7 However, the coexistence of OSA and hypertension will have a more adverse and extensive effect on the function of the cardiovascular system in individuals8–10 and may synergistically aggravate or lead to a series of metabolic abnormalities.11
Insulin resistance (IR) has been recognized as a major common feature in the pathophysiology of OSA and hypertension, which plays a crucial role in the occurrence and development of OSA and hypertension.12,13 Therefore, detecting IR early and accurately is crucial for optimizing the management and implementing preventive strategies for hypertension with OSA and preventing the progression of CVD.
The METS-IR (metabolic score for insulin resistance) index is a new and easily accessible index for assessing insulin resistance that was developed in recent years.14 METS-IR has better diagnostic efficacy than non-insulin-based IR indices such as TG (triglyceride) / HDL (high density lipoprotein) and TyG (triglyceride glucose index).14 Several studies have reported an association between METS-IR and CVD in patients with hypertension or OSA, however, to our knowledge, the studies related to the METS-IR index and CVD were mainly cross-sectional studies.15–19 Few longitudinal studies have been conducted on this topic, and they are limited to the general population, reporting the association between METS-IR and CHD risk.17,18,20 There are few studies that further investigate the relationship between METS-IR and the risk of CVD and its subtypes (CHD and stroke), especially in high-risk CVD individuals with hypertension and OSA. Hence, we aimed to investigate the association between METS-IR and CVD in hypertensive patients with OSA using relevant databases.
Materials and Methods
Study Population
We analyzed all the data from the UROSAH dataset, which was designed to evaluate the relationship between OSA and cardiovascular long-term outcomes in hypertensive patients.21 We included 3605 participants with hypertension and suspected OSA who visited the Hypertension Center of the People’s Hospital of Xinjiang Uygur Autonomous Region from January 2011 to December 2013. The final follow-up ended in January 2021, with 3329 participants completing the final follow-up and 276 participants lost to follow-up. We excluded 744 non-OSA participants who were diagnosed by polysomnography (PSG) with an AHI < 5, and participants who met either of the following criteria were also excluded: CVD at baseline and FBG, HDL-C, TG, or BMI data were missing (n=554). Ultimately, the main analysis included a total of 2031 participants (Figure 1). The Xinjiang Uygur Autonomous Region People’s Hospital’s Ethics Committee approved this study (reference: 2019030662), and written informed consent was provided by all study participants. Our study was conducted in accordance with the principles of the Declaration of Helsinki.
 |
Figure 1 Flow chart of selected participants. |
Data Collection
Baseline data collection was performed by professional researchers. Medical professionals collect lifestyle information, demographic characteristics, use of medicines, and medical history. Hip circumference, waist circumference, blood pressure, weight, and height were measured by trained nurses according to standardized methods described in previous studies.21 Overnight PSG (Compumedics E Series, Australia) examinations were performed on all participants in the laboratory (details of PSG are provided in the Supplementary Material).
Biochemical Parameters
A series of biochemical indicators of fasting blood in the morning were collected and analyzed using an automated biochemical analyzer (Hitachi 7600; Japan). Biochemical indices were detected and analyzed, including serum creatinine, blood urea nitrogen, FBG, TG, TC, HDL-C, and LDL-C. A Chronic Kidney Disease Epidemiology Collaborative Group equations was used to calculate the estimated glomerular filtration rate (eGFR).22
Definitions
The formula for calculating METS-IR was: Ln[(2 × FBG (mg/dL)) + TG (mg/dL)] × BMI (kg/m2))/(Ln[HDL-C (mg/dL)]).14 The diagnosis of hypertension was based on “China guidelines on prevention and treatment of hypertension 2010”, defined as resting blood pressure (BP) above 140/90 mmHg or using anti-hypertensive drugs currently. OSA was defined as an apnea hypopnea index (AHI) ≥ 5 events per hour, further, the severity of OSA was defined as follows: mild OSA (5 to 15), moderate OSA (15 to 30), and severe OSA (greater than 30). It was considered regular continuous positive airway pressure (CPAP) treatment if the patient used CPAP therapy more than 70% of the time per night and for at least four hours each night throughout the follow-up period.23,24 Regular oral appliance treatment was defined as at least 70% of consecutive nights and 4 hours or more of use per night.25 The definition of diabetes was OGTT 2-hour blood glucose above 11.1 mmol/L or fasting blood glucose above 7.0 mmol/L or a self-reported diabetes history.
Determination of Outcome Events
The primary outcome of this study was onset of CVD, including CHD and stroke. CVD was diagnosed by cardiologists from the tertiary hospital’s clinical events committee after reviewing all medical records. CHD was defined as a fatal or nonfatal myocardial infarction, unstable angina, and coronary revascularization (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty). Stroke (ischemic and hemorrhagic strokes) was defined as a rapid or sudden onset fixed neurologic deficit of at least 24h or until death (a detailed definition of CVD is provided in Supplementary Material). Inpatient medical records, outpatient interviews, and telephone interviews were used to obtain follow-up results. Deaths due to CVD are confirmed by population management consulting and hospital death certificates, and appraised by the clinical events committee at our tertiary hospital. Following the first visit, participants were followed until either a new cardiovascular disease or death occurred, whichever occurred first, or until the end of the follow-up period in January 2021.
Statistical Analysis
An appropriate descriptive statistic was used to summarize all data (median and range for continuous variables, frequency and count for categorical variables). For comparing the characteristics of the participants by METS-IR tertiles or quartiles, the Kruskal–Wallis test, univariate or multivariate analysis using the ANOVA test, Fischer’s exact test, or chi-square test were used where appropriate. The unadjusted cumulative hazards were visualized using Kaplan-Meier analysis, and Log rank tests were used to determine significance. Additionally, the variance inflation factor (VIF) was used to assess potential multicollinearity among explanatory variables, and variables with a VIF > 5 were discarded (Supplemental Table 1). A Cox proportional hazards regression model was used to determine the HRs and the 95% CIs of new-onset CVD or its subtypes (CHD and stroke) involving METS-IR (continuous, tertiles, and categories). There are three models: Model 1 is adjusted for sex and age; Model 2 further adjusts additional variables, including diabetes history, drinking status, smoking status, BMI, systolic blood pressure, and diastolic blood pressure; and Model 3 further fits all noncollinear variables. We tested for trends by assigning each participant to the median of their tertiles and entering these values as continuous terms in the logistic regression model.
Several sensitivity analyses were conducted to test the robustness of our results. First, to assess potential bias for residual confounding by smoking and drinking status, we repeated the analyses separately, excluding current smokers and current drinkers. Second, to eliminate potential bias, we performed a 2-year lagged analysis, excluding patients who had a new onset of CVD during the 2-year follow-up. Third, we performed the primary analysis again after excluding participants who received treatment for OSA regularly in order to assess residual confounding. Finally, based on Fine and Gray competing risk models, we estimated CVD risk by accounting for deaths from other causes. Moreover, through a generalized additive model and smoothed curve fitting, we explored the possibility of a nonlinear relationship between METS-IR and new-onset CVD, CHD, and stroke. We conducted stratified analyses and interactions based on age (<50 or ≥ 50 years), sex (female or male), drinking (never, past, or current), smoking (never, past, or current), BMI (<28 or ≥ 28 kg/m2), SBP (<140 or ≥ 140 mmHg), AHI (≥ 5 to < 15, ≥ 15 to < 30, or ≥ 30 events/h), DBP (<90 or ≥ 90 mmHg) and diabetes (no or yes).
To assess the improvement in the predictive power of models with and without METS-IR, the C-statistic, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated.
A two-sided probability test was used for all statistical tests with significance levels of P <0.005 and R, version 4.1.1.
Results
Baseline Characteristics of the Participants
Among 2031 participants involved in the analysis, the mean (standard deviation) age was 49.58 ± 10.77 years, and the proportion of men was 68.76%. In accordance with the levels of METS-IR, participants were categorized into three groups (Table 1). Participants with higher METS-IR had higher blood lipids, fasting blood glucose, diastolic blood pressure, and an AHI; a higher proportion of smoking, alcohol consumption, obesity, and diabetes; and were more likely to be male and have regular CPAP and oral therapy (Table 1).
 |
Table 1 Baseline Characteristics of Participants by METS-IR Tertiles |
Association Between METS-IR and Risk of CVD
During a median follow-up duration of 6.80 years (29,218.4 person-years), a total of 317 (15.61%) participants developed new-onset CVD, including 119 (5.86%) incident strokes and 198 (9.75%) incident CHDs. Furthermore, we show cumulative hazard curves for CVD events and its subtypes (CHD and stroke) across METS-IR tertiles in Figure 2. A clear increase in cardiovascular events was observed in the groups with higher METS-IR indices.
 |
Figure 2 Cumulative hazard curves for new-onset CVD events (A), CHD events (B), and stroke events (C) across METS-IR tertiles. |
Overall, there was a significant positive correlation between METS-IR and the risk of new-onset CVD, CHD, and stroke (Figures 3A–C). Consistently, the fully adjusted HRs of CVD, CHD, and stroke were 1.30 (1.16–1.47), 1.32 (1.14–1.53), and 1.27 (1.05–1.54), respectively, for each SD increase in METS-IR (Table 2).
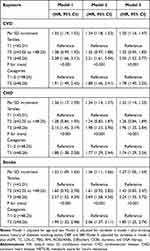 |
Table 2 Hazard Ratios (95% CI) of CVD, CHD, and Stroke, Stratified by METS-IR |
 |
Figure 3 Smoothed curves of the relationship between METS-IR and (A) CVD, (B) CHD, and (C) stroke. (Adjustments for all noncollinear variables). |
When METS-IR was assessed as tertiles, the highest tertiles versus the lowest tertiles were associated with a greater hazard of CVD (adjusted HR in tertiles 3 vs tertiles 1 2.05; 95% CI 1.52,-2.77), CHD (adjusted HR in tertiles 3 vs tertiles 1 1.96; 95% CI 1.35–2.84), and stroke (adjusted HR in tertiles 3 vs tertiles 1 2.24; 95% CI 1.35–3.72), after adjustment for fully confounders (Model 3). The risk of CVD, CHD, and stroke was increased in the second tertiles compared with the lowest tertiles; further analysis of the trend test indicated a trend of increased risk among the groups. Similar results were obtained for the relationship between METS-IR and cardiovascular disease when METS-IR was divided based on quartile (Tables S2).
Sensitivity Analysis
Several sensitivity analyses were conducted in order to determine whether our results are robust. First, similar results were obtained when we excluded current smokers and drinkers (Tables S3 and S4). Excluding undergoing OSA treatment, or excluding developed CVD in the first 2 years of follow-up, also did not change the strength of the association between METS-IR and the risk of new-onset CVD, CHD, and stroke appreciably (Tables S5 and S6). In addition, competing analyses produced similar results as Cox proportional hazards models (Table S7).
Subgroup Analysis
The association between METS-IR (per SD increment) and the risk of CVD, CHD, and stroke was assessed through stratified analysis according to age, gender, BMI, SBP, DBP, AHI, diabetes, current smoking, and current alcohol consumption (Figure 4A–C). No interaction was observed between the selected covariates and new-onset CVD.
 |
Figure 4 Associations of METS-IR with the risk of (A) new onset CVD, (B) new onset CHD, and (C) new onset stroke in different subgroups. |
Additive Effect of METS-IR on Established Risk Factors
We attempted to fit METS-IR to a Cox proportional hazards model (established risk factors) to determine whether the METS-IR had additive predictive value over established clinical risk factors based on C-statistic, continuous NRI, and IDI (Table 3). The model containing METS-IR was significantly better in predictive ability than the baseline risk model (c-statistic: 0.651 vs 0.618). Again, we observed highly consistent results from the risk prediction of CHD and stroke. Compared with the baseline risk model, the addition of METS-IR improved the reclassification and identification of CVD predictions with a continuous NRI of 0.140 (0.047, 0.203) and IDI of 0.009 (0.003, 0.022). Likewise, the addition of METS-IR greatly improved the ability to reclassify and discriminate predictions of coronary artery disease and stroke.
 |
Table 3 Discrimination of Each Predictive Model for Outcomes Using Continuous NRI and IDI |
Discussion
In this retrospective cohort study, we evaluated the association between METS-IR and the risk of CVD events in participants with hypertension and OSA. Our results found that METS-IR was a strong predictor of CVD and its subtypes (coronary artery disease and stroke) in hypertensive patients with OSA. In addition, compared with some traditional risk factors, the addition of METS-IR greatly improves the ability to predict and discriminate CVD risk events in participants with hypertension and OSA.
Insulin resistance has been described as a decreased sensitivity of peripheral tissues such as skeletal muscle, liver, and adipose tissue to the action of insulin, manifested by defects in insulin-stimulated glucose oxidation and uptake, decreased glycogen synthesis, and a decreased capacity for inhibiting lipid oxidation.26,27 Numerous epidemiological studies have shown a strong association between insulin resistance and CVD, and insulin resistance can cause and predict adverse cardiovascular events.28,29 The Insulin Resistance Atherosclerosis Study (IRAS) reported the first significant link between insulin resistance and atherosclerosis in 1996,30 and subsequent multiple prospective cohort studies further revealed that insulin resistance is an important and independent risk factor for CVD.28,31–34 Pathophysiological studies have found that insulin resistance can change the lipid metabolism of the whole body, increase the lipotoxicity of the heart, and cause oxidative stress and endothelial dysfunction,35,36 which together lead to dyslipidemia, hypertension, and cardiovascular disease.37
Therefore, to better evaluate IR and identify it early in the high-risk population of CVD, some evaluation indicators based on insulin measurement have been developed and have become predictors and markers of insulin resistance.27,38 The most commonly used evaluation index in epidemiological studies is the homeostasis model. However, its practical applicability is limited due to the impact of the cost of insulin testing and the bias of the calibration settings and conversion between units in the kit, which can easily lead to biased results.39
In recent years, emerging indicators for the evaluation of insulin resistance based on non-insulin measurements have been gradually discovered.40–45 The METS-IR index is a reliable indicator for evaluating IR and screening for cardiometabolic-related risk disorders, developed by Bello et al14,46,47 Several studies have reported a link between METS-IR and hypertension, arteriosclerosis, and CVD, consistently supporting METS-IR as a convenient and cost-effective monitoring indicator for assessing cardiovascular metabolic risk in healthy and high-risk populations.15–17
However, there have been very few longitudinal studies that have examined the relationship between METS-IR and CVD risk. One study from a Korean health checkup population without diabetes found that METS-IR had a good predictive value in ischemic heart disease,20 and another study found that elevated METS-IR levels were associated with the risk of developing HTN in a non-overweight Chinese population.48 There was also a strong association between METS-IR and CVD in the cardiometabolic high-risk population. A study of 18,609 hypertensive patients found a nearly J-shaped association between METS-IR and the risk of stroke.49 In our cohort study, our results are consistent with previous studies: METS-IR has a good predictive value for CVD, but we extend the previous research results and provide new evidence. First of all, our results show that METS-IR has a good predictive value not only for the risk of ischemic heart disease or stroke but also for CVD, including ischemic heart disease and stroke. In addition, our study involved a special population with hypertension and OSA, and these patients are at higher risk for CVD. Our findings emphasize the importance of reducing the METS-IR index and improving insulin sensitivity for controlling the progression of CVD in hypertensive patients with OSA, and more intensive efforts should be devoted to the combined treatment of improving insulin resistance based on reducing blood pressure and treating OSA. METS-IR was composed of FBG, TG, HDL-C, and BMI, suggesting that patients with hypertension and OSA should pay more attention to improving blood glucose, blood lipids, and BMI in their daily lives in order to better prevent the progression of CVD. Home mobile smart devices seem to be a good choice for long-term home applications.50–53
Underlying pathological mechanisms may partly explain our observations. At present, the mechanisms underlying IR-induced cardiovascular disease mainly include chronic hyperglycemia, dyslipidemia, endothelial dysfunction, and inflammation.37,54,55 As a result of decreased glycogen synthesis and increased gluconeogenesis in hepatic IR, there is elevated fasting blood glucose, dyslipidemia, and an increased risk of thrombosis.56,57 Insulin resistance that occurs in adipose tissue will result in elevated levels of free fatty acids, resulting in visceral fat accumulation associated with elevated levels of BP and plasminogen activator inhibitor 1, and resulting in ectopic and toxic lipid accumulation in blood vessels (which alters cell signaling and cardiac structure), thereby contributing to the increase in the prevalence of cardiovascular disease.57,58 IR-induced endothelial dysfunction, which is mainly caused by the decrease of nitric oxide production by endothelial cells through the PI3K/Akt pathway and the increase of reactive oxygen species, thrombogenic factors, and pro-inflammatory markers mediated by MAPK/ERK, all of which contribute to increasing the risk of CVD.57,59
In our results, there is a significant positive correlation between METS-IR and the risk of new-onset CVD. When the second tertile of METS-IR was compared with the first tertile, the risk of CVD increased. Unfortunately, the HR was wide with a 95% confidence interval, but there was a significant statistical difference in the trend test of METS-IR tertiles, whether in the unadjusted or adjusted model. It is possible that the small sample size limits the clinical significance of these findings. In addition, we speculate that smoking as a confounding factor may be another reason for the lack of statistical significance in HR between the second tertile group of METS-IR and the first group, because the results of the above COX regression analysis are based on the analysis after adjusting for smoking. Sensitivity analyses were performed when current smokers were excluded directly. As shown in Supplemental Table S2, the METS-IR second tertile group had 1.92-fold and 1.64-fold statistical significance in the risk of stroke and total cardiovascular events, respectively.
We found that when METS-IR was grouped into four categories, the relationship between METS-IR and the risk of incident stroke was statistically significant only in the highest quartile group. Previous studies have confirmed that smoking is a risk factor for stroke,60 but this relationship is more likely to be an ischemic stroke. As shown in a 14-year follow-up cohort study in Japan, compared with non-smokers, the greater the number of daily cigarettes smoked, the greater the effect on cerebral infarction, but the effect on cerebral hemorrhage was not significant.61 Since the defined end event is new-onset CVD, the potential impact of hemorrhagic stroke on the outcome cannot be ruled out.
Moreover, in this study, we enhanced the power of risk stratification significantly through the addition of METS-IR to the baseline risk model, which already included traditional risk factors. Our results are helpful for further screening of high-risk individuals in patients with hypertension and OSA, as well as further verifying the correlation between METS-IR and CVD.
To the best of our knowledge, the present study is the first to examine the association between METS-IR and CVD risk in a high-risk group of hypertensive patients with OSA. The strengths of this study include its cohort study design, long-term and complete follow-up data, adjustment of multiple CVD risk factors, reliable measurement of clinical parameters, and accurate diagnosis of OSA patients. However, our research also has some limitations. First of all, we carried out a series of sensitivity analyses in order to control the confounding factors, but because we did not consider the time and dependence of antiplatelet and lipid-lowering therapy, this may affect our results. However, there was no statistical difference in the proportion of antiplatelet and lipid-lowering therapy among the three groups, and we also adjusted these factors, the final results are also highly consistent in different model analyses, which makes up for our shortcomings to a certain extent. Of course, in future research, we need to strengthen the comprehensive collection of information to make the research results more universal and persuasive. Second, our study was did not analyze the dynamic changes of METS-IR during 6.8 years of follow-up, because we were a retrospective cohort study and failed to obtain multiple follow-up data during 6.8 years of follow-up. Therefore, the relationship between the different trend tracks of METS-IR and new-onset CVD remains unclear. In future studies, we will obtain more follow-up data to observe the relationship between METS-IR dynamic trajectories and CVD. In addition, since our results are based on a single center, their universality is limited.
Conclusion
In conclusion, METS-IR is an independent predictor of CVD and its subtypes (coronary artery disease and stroke) in hypertensive patients with OSA. As a simple and reliable marker based on non-insulin measurements, METS-IR has great potential for predicting and preventing CVD. Our results provide new insights into individualized CVD prevention strategies for hypertensive patients with OSA.
Data Sharing Statement
If requested, the corresponding author can provide the data supporting the findings.
Ethics Approval and Consent to Participate
Xinjiang Uygur Autonomous Region People’s Hospital’s Ethics Committee approved this study (reference: 2019030662), and written informed consent was provided by all study participants.
Funding
Funding for this study was provided by Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01C173).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788.
2. Roth Gregory A, Mensah George A, Johnson Catherine O, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
3. Timmis A, Vardas P, Townsend N, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43(8):716–799. doi:10.1093/eurheartj/ehab892
4. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144(3):e56–e67. doi:10.1161/CIR.0000000000000988
5. Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124(19):2049–2051. doi:10.1161/CIRCULATIONAHA.111.062190
6. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/NEJM200005113421901
7. Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi:10.1016/S0140-6736(19)32008-2
8. Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379–1386. doi:10.1378/chest.06-2703
9. Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension. 2009;53(1):64–69. doi:10.1161/HYPERTENSIONAHA.108.119420
10. Floras JS. Hypertension, sleep apnea, and atherosclerosis. Hypertension. 2009;53(1):1–3. doi:10.1161/HYPERTENSIONAHA.108.123711
11. Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi:10.1001/jama.290.14.1906
12. Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi:10.1164/ajrccm.165.5.2103001
13. Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317(6):350–357. doi:10.1056/NEJM198708063170605
14. Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–544. doi:10.1530/EJE-17-0883
15. Han KY, Gu J, Wang Z, et al. Association between METS-IR and prehypertension or hypertension among normoglycemia subjects in Japan: a retrospective study. Front Endocrinol. 2022;13:851338. doi:10.3389/fendo.2022.851338
16. Liu XZ, Fan J, Pan SJ. METS-IR, a novel simple insulin resistance indexes, is associated with hypertension in normal-weight Chinese adults. J Clin Hypertens. 2019;21(8):1075–1081. doi:10.1111/jch.13591
17. Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, et al. Prediction of incident hypertension and arterial stiffness using the non-insulin-based metabolic score for insulin resistance (METS-IR) index. J Clin Hypertens. 2019;21(8):1063–1070. doi:10.1111/jch.13614
18. Wang Z, Li W, Li J, Liu N. The nonlinear correlation between a novel metabolic score for insulin resistance and subclinical myocardial injury in the general population. Front Endocrinol. 2022;13:889379. doi:10.3389/fendo.2022.889379
19. Kim T, Kang J. Relationship between obstructive sleep apnea, insulin resistance, and metabolic syndrome: a nationwide population-based survey. Endocr J. 2022;70:107–119. doi:10.1507/endocrj.EJ22-0280
20. Yoon J, Jung D, Lee Y, Park B. The metabolic score for insulin resistance (METS-IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Pers Med. 2021;11(8):742. doi:10.3390/jpm11080742
21. Cai X, Li N, Hu J, et al. Nonlinear relationship between Chinese visceral adiposity index and new-onset myocardial infarction in patients with hypertension and obstructive sleep apnoea: insights from a cohort study. J Inflamm Res. 2022;15:687–700. doi:10.2147/JIR.S351238
22. Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63(5):820–834. doi:10.1053/j.ajkd.2013.12.006
23. Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest. 2009;135(3):704–709. doi:10.1378/chest.08-2182
24. Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. doi:10.1056/NEJMoa1306187
25. Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi:10.1136/thoraxjnl-2012-201900
26. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37.
27. Park SY, Gautier JF, Chon S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab J. 2021;45(5):641–654.
28. Jeppesen J, Hansen TW, Rasmussen S, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49(21):2112–2119.
29. Eddy D, Schlessinger L, Kahn R, et al. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–366.
30. Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) investigators. Circulation. 1996;93(10):1809–1817.
31. Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153(4):559–565.
32. Gast KB, Tjeerdema N, Stijnen T, et al. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7(12):e52036.
33. Yun JE, Won S, Sung J, et al. Impact of metabolic syndrome independent of insulin resistance on the development of cardiovascular disease. Circ J. 2012;76(10):2443–2448.
34. Rutter MK, Meigs JB, Sullivan LM, et al. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham offspring study. Diabetes. 2005;54(11):3252–3257.
35. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520.
36. Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97(4):1784–1789.
37. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122.
38. Sarafidis PA, Lasaridis AN, Nilsson PM, et al. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21(9):709–716.
39. Manley SE, Stratton IM, Clark PM, et al. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53(5):922–932.
40. Tao LC, Xu JN, Wang TT, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
41. Sato F, Nakamura Y, Kayaba K, Ishikawa S. TG/HDL-C ratio as a predictor of stroke in the population with healthy BMI: the Jichi medical school cohort study. Nutr Metab Cardiovasc Dis. 2022;32(8):1872–1879.
42. Wu Z, Zhou D, Liu Y, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134.
43. Yan Y, Wang D, Sun Y, et al. Triglyceride-glucose index trajectory and arterial stiffness: results from Hanzhong adolescent hypertension cohort study. Cardiovasc Diabetol. 2022;21(1):33.
44. Hayıroğlu Mİ, Çınar T, Çiçek V, Palice A, Ayhan G, Tekkeşin Aİ. The triglyceride-glucose index can predict long-term major adverse cardiovascular events in Turkish patients with high cardiovascular risk. J Lipid Atheroscler. 2022;11(3):280–287.
45. Hu J, Cai X, Li N, et al. Association between triglyceride glucose index-waist circumference and risk of first myocardial infarction in Chinese hypertensive patients with obstructive sleep apnoea: an observational cohort study. Nat Sci Sleep. 2022;14:969–980.
46. Cai XT, Zhu Q, Liu SS, et al. Associations between the metabolic score for insulin resistance index and the risk of type 2 diabetes mellitus among non-obese adults: insights from a population-based cohort study. Int J Gen Med. 2021;14:7729–7740.
47. Cai X, Gao J, Hu J, et al. Dose-response associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese Chinese population: retrospective evidence from a population-based cohort study. Dis Markers. 2022;2022:4930355.
48. Xu C, Song G, Hu D, Li G, Liu Q, Tang X. Association of METS-IR with incident hypertension in non-overweight adults based on a cohort study in Northeastern China. Eur J Public Health. 2022;32(6):884–890.
49. Cai X, Hu J, Zhu Q, et al. Relationship of the metabolic score for insulin resistance and the risk of stroke in patients with hypertension: a cohort study. Front Endocrinol. 2022;13:1049211.
50. Tekkeşin Aİ, Hayıroğlu Mİ, Çinier G, et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: a pragmatic randomised clinical trial. Atherosclerosis. 2021;319:21–27.
51. Hayıroğlu Mİ, Çınar T, Çinier G, et al. The effect of 1-year mean step count on the change in the atherosclerotic cardiovascular disease risk calculation in patients with high cardiovascular risk: a sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 2021;79(10):1140–1142.
52. Hayıroğlu Mİ, Çinier G, Yüksel G, et al. Effect of a mobile application and smart devices on heart rate variability in diabetic patients with high cardiovascular risk: a sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 2021;79(11):1239–1244.
53. Hayiroğlu Mİ, Çinier G, Pay L, et al. The relation between average 1-year home blood pressure and the change in pro-BNP and left ventricle mass index. Blood Press Monit. 2022;27(5):327–333.
54. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):2237–2238.
55. Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. 2016;2016:3634948.
56. Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97.
57. Li M, Chi X, Wang Y, et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7(1):216.
58. Montani JP, Carroll JF, Dwyer TM, et al. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S58–S65.
59. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia. 2010;53(7):1270–1287.
60. OOhsawa M, Ogasawara K, Omama S, et al. Abstract P259: smoking increases risks of death and stroke in both men and women. absolute risk difference of stroke is likely to be larger in women. Circulation. 2017;135(suppl_1):1.
61. Ueshima H, Choudhury SR, Okayama A, et al. Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke. 2004;35(8):1836–1841.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
