Back to Journals » Nature and Science of Sleep » Volume 14
The Elevated Central Chemosensitivity in Obstructive Sleep Apnea Patients with Hypertension
Authors Wang X, Luo J , Huang R, Xiao Y
Received 15 February 2022
Accepted for publication 20 April 2022
Published 3 May 2022 Volume 2022:14 Pages 855—865
DOI https://doi.org/10.2147/NSS.S362319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Xiaona Wang, Jinmei Luo, Rong Huang, Yi Xiao
Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yi Xiao, Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuaifuyuan, Dongcheng District, Beijing, 100730, People’s Republic of China, Email [email protected]
Purpose: Hypertension is a common comorbidity in obstructive sleep apnea (OSA), in which dysfunction of the autonomic nervous system plays an integral part. Chemoreflex is essential for ventilatory control and cardiovascular activity. This study aimed to determine whether central chemosensitivity was increased in OSA patients with hypertension and the potential role of the autonomic nerve activity in this relationship.
Patients and Methods: A total of 77 men with OSA were included in this cross-sectional study. We measured hypercapnic ventilatory response (HCVR) by the rebreathing method under isoxic hyperoxia to test the central ventilatory chemosensitivity since hyperoxia silences the peripheral chemoreceptors’ response to CO2. To elevate the autonomic nerve activity, time-domain, frequency-domain, and non-linear variables of heart rate variability were calculated over 5-min records. Univariate and multivariate linear regression analyses were used to find the determinants of HCVR.
Results: The median HCVR was 2.3 (1.8, 3.3), 2.1 (1.6, 3.0), and 3 (2.2, 3.7) L/min/mmHg in all participants, OSA patients, and OSA patients with hypertension, respectively. Hypertension was significantly associated with elevated HCVR after adjusting for age, central obesity, OSA severity, daytime sleepiness, and diabetes mellitus. Compared with OSA patients, OSA patients with hypertension had higher body mass index, worse nocturnal hypoxia, and lower time-domain variables and frequency-domain variables. After adjusting for age, apnea-hypopnea index, central obesity, and beta-blocker usage, approximate entropy was independently negatively associated with HCVR in OSA patients with hypertension.
Conclusion: This study demonstrated elevated central chemosensitivity in OSA patients with hypertension. Compared with OSA patients, OSA patients with hypertension had attenuated parasympathetic nerve activity. This study preliminarily illustrated that elevated central chemosensitivity might be associated with weak adaptability of the cardiac autonomic nervous system in OSA patients with hypertension.
Keywords: obstructive sleep apnea, central chemosensitivity, hypercapnic ventilatory response, hypertension, autonomic nerve activity
Introduction
Obstructive sleep apnea (OSA) is a common sleep-disordered breathing that involves multiple mechanisms, including increased airway collapsibility, low respiratory arousal threshold, and unstable ventilatory control.1 Unstable ventilatory control, characterized as high loop gain, contributes to periodic apnea by amplifying the ventilation’s magnitude after recovery from airway collapse.2 Loop gain is an engineering theory utilized to evaluate the negative feedback control system’s stableness, which comprises controller gain, plant gain, and the delay between the two. Controller gain mirrors ventilatory chemoreflex in the respiratory system, and smaller lung volume might produce higher plant gain.3 Hyperactivity of chemoreceptors has been confirmed in patients with OSA, which contributes to unstable ventilatory control and repeated airway obstruction.4,5
Central and peripheral chemoreceptors modulate both ventilatory control and neural circulatory control.6 In patients with heart failure, activation of central chemoreceptors was shown to be correlated with the augmentation of ventilation and sympathetic drive.7 Familial dysautonomia, a rare hereditary autonomic neuropathy, shows the synergy between dysfunction of autonomic neurons and reduced ventilatory chemoreflex.8 In patients with Lewy body dementia, the reduced central chemoreflex was shown to be associated with impaired heart rate variability (HRV).9 The sympathovagal imbalance relating to apnea events is associated with arrhythmia and cardiovascular diseases for patients with OSA.10 Moreover, sustained increase of sympathetic nerve activity has been found in patients with OSA during sleep11 and resting wakefulness.12 However, in previous studies, conflicting findings were made regarding the alterations of parasympathetic nerve activity in patients with OSA during sleep and daytime.13 The mechanisms by which OSA leads to elevated sympathetic nerve activity include overactivity of peripheral chemoreceptors and inordinate central sympathetic outflow.14 The modification of parasympathetic activity in the central chemoreflex has been found in lamb models,15 while the relationship between central chemoreflex and autonomic nerve activity in patients with OSA has not been reported yet. Considering the complex interaction between central chemoreceptors and cardiovascular neural control,16,17 the correlation between central chemoreflex and autonomic nerve activity in patients with OSA is worth noting.
Hypertension is a common comorbidity or complication in patients with OSA. In spontaneously hypertensive rats, augmented central ventilatory chemosensitivity increases the ventilation, sympathetic nerve activity, and arterial blood pressure, which still existed when peripheral chemoreceptors were attenuated by hyperoxia.18 The melanocortin system19 and orexin neurons18 might play a remarkable role in the complex interaction between central sympathetic efferents and central chemoreceptor activity. However, the link between hypertension and central chemosensitivity in patients with OSA was ambiguous. Meanwhile, excessive sympathoexcitation20 and diminished parasympathetic activity21 play an integral part in the development and progression of hypertension. Thus, we assumed that there might be a significant relationship between autonomic nerve activity and central chemoreflex in OSA patients with hypertension.
Hypercapnic ventilatory response (HCVR) measured by the rebreathing method under isoxic hyperoxia has often been used to test the central ventilatory chemosensitivity since hyperoxia silences the peripheral chemoreceptors’ response to CO2.22,23 In this study, we intended to confirm the association between hypertension and HCVR in patients with OSA. We also explored the relationship between autonomic nerve activity and HCVR in OSA patients with hypertension.
Materials and Methods
Participants
This cross-sectional study was conducted in the sleep unit of Peking Union Medical College Hospital in Beijing, China. This study included adult men with OSA (age ≥ 18 years). The diagnosis for OSA was based on an apnea-hypopnea index (AHI) equal to or greater than 5/h combined with either associated symptoms (such as sleepiness, snoring, or observed apneas) or related diseases (such as hypertension, diabetes, and cardiovascular diseases). Besides, those with an AHI ≥ 15/h also met the diagnosis criteria, even when they were free of associated symptoms and diseases.24 Participants were excluded if one of the following criteria was met: (1) also suffering from cardiovascular diseases, such as arrhythmia, coronary disease, or heart failure; (2) impaired pulmonary function, such as percentage of predicted forced expiratory volume in 1 s (FEV1% predicted) < 80%, percentage of predicted forced vital capacity (FVC% predicted) < 80%, or FEV1/FVC ratio < 70%; (3) acute or chronic kidney disease, liver disease, or neuromuscular disease; (4) smoking in the past year; (5) receiving continuous positive airway pressure treatment in the past year; (6) refusal to undergo rebreathing tests; and (7) electrocardiography (ECG) data not appropriate for HRV analysis.
This study was conducted in accordance with the Declaration of Helsinki. The Clinical Research Ethics Committee of Peking Union Medical College Hospital approved the experimental protocol (JS-2627). All patients provided written informed consent.
Polysomnography and Data Collection
Whole-night polysomnography was performed using a standard device (Embla N7000; Natus Medical Incorporated, Broomfield, CO, USA). Sleep state and associated respiratory events were evaluated following the American Academy of Sleep Medicine manual (Version 2.3).25 Total sleep time (TST) and percentages of sleep stages [stages 1, 2, 3, and rapid eye movement sleep] were collected. The AHI was calculated as the number of apnea and hypopnea events per hour. An AHI ≥ 15/h was used as the threshold to divide participants into those with mild OSA and moderate-to-severe OSA. Obstructive apnea index (OAI) and central apnea index (CAI) were defined as the sum of obstructive or central apnea events per hour. Oxygen desaturation index (ODI), lowest values of peripheral blood oxygen saturation (LSpO2), and percent of time spent at peripheral blood oxygen saturation beneath 90% (T90) were used to describe the severity of nocturnal hypoxia.
The participants’ demographic data were collected, including age, body mass index (BMI), waist circumference (WC), and neck circumference. BMI was calculated as weight (in kg)/height2 (in m2). Obesity was defined as BMI ≥ 28 kg/m2.26 Central obesity was defined as WC ≥ 90 cm for men.27 Diabetes mellitus was diagnosed when the patients had a history of diabetes mellitus or had received treatment for this condition. Hypertension was diagnosed when the individuals currently used antihypertensive medications or met the 2020 definition of hypertension of the International Society of Hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg at two different times).28 In this study, the pharmacotherapy for hypertension included calcium channel blocker, beta-blocker, diuretic, and renin-angiotensin system inhibitor. All participants completed the Epworth Sleepiness Scale (ESS) questionnaire and Pittsburgh Sleep Quality Index (PSQI). ESS score ≥ 11 reflects daytime sleepiness,29 while PSQI > 5 is associated with poor sleep quality.30
HRV
Lead II ECG was used to record the R-R intervals with polysomnography equipment during the total time in bed. We aimed to analyze autonomic nerve activity during wakefulness, so we collected 5-min R-R intervals during wakefulness before sleep for each participant. The wakefulness was identified by an electroencephalogram. All subjects were asked to maintain a supine position and relaxed tidal breathing during the ECG acquisition. We used the software Kubios HRV (version 3.4; Kubios Co., Kuopio, Finland) for HRV analysis.31 We analyzed time-domain, frequency-domain, and non-linear variables in accordance with the guidelines.32,33 The time-domain variables included the mean heart rate, the standard deviation of R-R intervals (SDNN), square root mean squared differences between successive R-R intervals (RMSSD), and the percentage of adjacent R-R intervals with a difference greater than 50 ms (pNN50). With Welch’s periodogram method (300 s window width and 50% overlap window). Fast Fourier transformation was used for frequency-domain analysis. The very low-frequency range (VLF, 0–0.04 Hz), low-frequency range (LF, 0.04–0.15 Hz), high-frequency range (HF, 0.15–0.4 Hz), normalized LF power (LFnu), normalized HF power (HFnu), and LF/HF ratio were measured. In non-linear metrics, approximate entropy (ApEn) and sample entropy (SampEn) were used to quantify the regularity of the R-R series.
HCVR and Spirometry
In the morning, the participants undertook the rebreathing tests in accordance with Duffin’s method.23 They sat at a table, and breathed with a nose peg and a mouthpiece connected to a closed one-way circuit with a 6 L plastic rebreathing bag containing the test gas. The bag initially had a gas mixture of 13% O2/6% CO2 /balanced nitrogen, and 100% O2 was fed into the rebreathing bag to maintain end-expiratory oxygen partial pressure at 150 mmHg during the test. The test lasted 3–5 min or was terminated when end-tidal carbon dioxide partial pressure was higher than 60 mmHg, or minute ventilation exceeded 100 L/min. The volume transducer and gas sampling port of the cardiopulmonary exercise cart (MasterScreen CPX; Jaeger, Hoechberg, Germany) were connected with the mouthpiece and rebreathing bag to collect the data (end-tidal carbon dioxide partial pressure and minute ventilation). Breath-by-breath measurements monitored expired gas concentrations and minute ventilation. HCVR was evaluated by relating end-tidal carbon dioxide partial pressure with minute ventilation during the rebreathing test using linear regression, representing the central chemosensitivity.
After 30 min of rest, the participants underwent spirometry. The cardiopulmonary exercise cart was used to assess the pulmonary function of participants. The data of FEV1% predicted, FVC% predicted, and FEV1/FVC ratio were acquired.
Statistical Analysis
We calculated mean ± standard deviation or median (interquartile range) for continuous variables depending on the data distribution. Categorical variables were presented as the number and percentage. Univariate linear regression analyses were used to find the determinants of HCVR. The relationship between HCVR and hypertension was assessed using multivariate linear regression in three adjusted models [model 1 adjusted for age; model 2 adjusted for age, OSA severity (AHI ≥ 15 /h), and daytime sleepiness (ESS ≥ 11); model 3 adjusted for central obesity (WC ≥ 90 cm), diabetes mellitus, and all variables in model 2]. The comparison between OSA patients and OSA patients with hypertension was conducted by independent Student’s t-test, Mann–Whitney U-test, or Pearson’s χ2 test depending on the data distribution. For statistical analysis, the natural logarithmic transformation of VLF, LF, and HF was used. The factors correlating with HCVR in OSA patients with hypertension were selected by univariate linear regression analyses. Furthermore, we used multiple forward stepwise linear regression analyses (likelihood ratio) to find the determinants of HCVR in OSA patients with hypertension. SPSS version 21.0 (Chicago, IL, USA) was used for data analysis. A two-sided P < 0.05 was considered to reflect statistical significance.
Results
Characteristics of Recruited Participants
As shown in Supplementary Figure 1, 135 men took part in this study and 58 men were excluded. Overall, 5 participants showed abnormal pulmonary function (FEV1/FVC < 70%), 40 participants were current smokers, 5 participants did not complete the rebreathing test, and 8 participants had ECG data that were not appropriate for HRV analysis. Thus, 77 men were enrolled in this study. The clinical characteristics of the 77 enrolled men are shown in Table 1. The median age of all participants was 41 years. The median BMI was 27.4 kg/m2 and 40.3% of patients were obese. The median WC was 97.0 cm and 83.1% of patients were diagnosed with central obesity. The median values of FEV1% predicted, FVC% predicted, and FEV1/FVC ratio were 97.2 (90.0, 104.0) %, 100.5 (91.5, 105.0) %, and 79.6 (77.0, 83.8) %, respectively. The median HCVR was 2.3 (1.8, 3.3) L/min/mmHg. The median AHI was 38.8/h and 72.7% of participants were classified as having moderate-to-severe OSA. The incidences of daytime sleepiness, poor sleep quality, hypertension, and diabetes mellitus were 57.1%, 70.1%, 50.7%, and 13.0%, respectively.
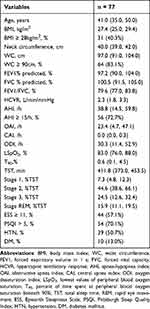 |
Table 1 Clinical Features of All Participants |
Elevated HCVR in OSA Patients with Hypertension
As shown in Figure 1, age, hypertension, and central obesity were positively associated with HCVR (all P values < 0.05). Patients with moderate-to-severe OSA tended to have augmented HCVR compared with patients with mild OSA. The relationships of obesity, poor sleep quality, daytime sleepiness, and diabetes mellitus with HCVR were not significant. In adjusted models, we found that hypertension was significantly associated with elevated HCVR after adjusting for age, central obesity, OSA severity, daytime sleepiness, and diabetes mellitus (Table 2).
 |
Table 2 The Relationship Between Hypertension and HCVR |
Comparison Between OSA Patients and OSA Patients with Hypertension
As shown in Table 3, the mean age did not differ between OSA patients and OSA patients with hypertension. The OSA patients with hypertension had higher BMI, percentage of obesity, and neck circumference. However, the WC and percentage of central obesity did not differ between the two groups. The OSA patients with hypertension had lower values of FEV1% predicted and FVC% predicted. The FEV1/FVC was similar between the two groups. The median HCVR was 3.0 (2.2, 3.7) L/min/mmHg in OSA patients with hypertension and 2.1 (1.6, 3.0) L/min/mmHg in OSA patients. The AHI, OAI, and CAI did not differ significantly between the two groups. The OSA patients with hypertension had worse nocturnal hypoxia (higher ODI and T90, and lower LSpO2). The TST and the percentages of sleep stages did not differ between the two groups. The percentages of daytime sleepiness, poor sleep quality, and diabetes mellitus also did not differ between the two groups.
 |
Table 3 The Comparison of Clinical Features Between OSA Patients and OSA Patients with Hypertension |
The mean heart rate was 71.9 ± 9.5 bpm in OSA patients and 74.6 ± 10.0 bpm in OSA patients with hypertension, which were not significantly different (Table 4). Compared with the OSA patients, the OSA patients with hypertension had lower time-domain (RMSSD and SDNN) and frequency-domain variables (ln VLF, ln HF, and HFnu). In addition, the OSA patients with hypertension had a higher LF/HF ratio than OSA patients. In non-linear metrics of HRV, SampEn and ApEn did not differ between the two groups.
 |
Table 4 The Comparison of HRV Variables Between OSA Patients and OSA Patients with Hypertension |
Factors Correlating with HCVR in OSA Patients with Hypertension
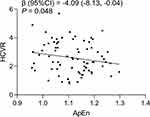
The relationships between clinical features, HRV variables, and HCVR in OSA patients with hypertension are shown in Supplementary Table 1. We only found a significantly negative relationship between ApEn and HCVR. Moreover, we found that ApEn was independently associated with HCVR after adjusting for age, AHI, central obesity, and beta-blocker usage (Figure 2).
Discussion
This study first demonstrated that OSA patients with hypertension had elevated central chemosensitivity compared with OSA patients. Moreover, using HRV analysis, we found that augmented central chemosensitivity was associated with reduced adaptability of the autonomic nervous system in OSA patients with hypertension.
The retrotrapezoid nucleus is the key nodal point of central ventilatory control.34 Hypercapnia activates the central chemoreceptors by increasing the central hydrogen ion concentration.35 Thus, HCVR might be a suitable method to measure the central chemosensitivity and the maintenance of isoxic hyperoxia disrupted the effect of peripheral chemoreceptors at the same time. There is controversy in the literature about the determinants and modified factors of HCVR in patients with OSA. The mechanism of central chemoreflex might differ between males and females. Sin et al36 found that age and daytime PaCO2 were primary determinants of HCVR in men, and BMI was a significant factor for HCVR in women. Wang et al37 also found that obstructive sleep apnea/hypopnea index was positively associated with HCVR in asymptomatic patients with OSA. In this study, we explored the determinants of HCVR only in male patients and found a positive relationship between age and HCVR. However, we did not find a significant relationship between AHI and HCVR. The relationships of HCVR with OAI, CAI, severity of nocturnal hypoxia, daytime sleepiness, and poor sleep quality were also not significant in this study. Ge et al38 found that ventilatory chemosensitivity was increased in obese men. Meanwhile, Earing et al39 found that BMI mediated the relationship between AHI and HCVR. We also found the increased HCVR in OSA patients with central obesity. Narkiewicz et al40 found that obesity selectively potentiated central chemosensitivity. Central obesity was also found to be linked to increased morbidity of metabolic diseases and cardiovascular diseases; thus, the relationship between BMI or central obesity and altered central chemoreflex requires further study. Furthermore, patients with OSA combined with diabetes41 and metabolic syndrome42 also had increased HCVR. In this study, we also found the elevated HCVR in OSA patients with hypertension after adjusting for age, central obesity, OSA severity, diabetes mellitus, and daytime sleepiness.
HRV analysis is an applicable approach to measure autonomic nerve activity. In HRV analysis, time-domain variables (RMSSD and pNN50) and frequency-domain variable (HF) are associated with parasympathetic preponderance. In this study, OSA patients with hypertension had lower RMSSD, ln HF, and HFnu values than OSA patients, which indicated decreased parasympathetic activity in the former. LF and LF/HF ratio are often used to reflect the sympathetic activity. LF reflected a mixture of sympathetic activity, parasympathetic activity, and other factors.43 In this study, there was no significant difference in lnLF or LFnu between the two groups. Moreover, we found a higher LF/HF ratio in OSA patients with hypertension. However, Billman43 demonstrated that it was inappropriate to interpret increased LF/HF ratio as reflective of sympathetic overactivity. Thus, there was no adequate evidence to support increased sympathetic nerve activity in OSA patients with hypertension in this study. On the other hand, SDNN directly reflects the autonomic nerve activity, and diminished values of SDNN revealed a poor prognosis in patients with cardiovascular disease.44 In this study, OSA patients with hypertension also showed unadaptable autonomic nerve activity and an intensive risk of cardiovascular events, according to the diminished values of SDNN. Moreover, in this study, OSA patients with hypertension had lower lnVLF. VLF is not a direct marker of sympathetic or parasympathetic activity. Previous studies found that VLF is related to the activity of the renin-angiotensin system.45 This system plays a vital role in regulating the progression of hypertension.46
Coupling between ventilation and sympathetic activity was reported in chronic intermittent hypoxia rats47 and spontaneously hypertensive rats.48 This study preliminarily explored the association between central chemosensitivity and autonomic nerve activity in OSA patients with hypertension. In this study, we found a negative relationship between ApEn and HCVR in OSA patients with hypertension. In terms of HRV, entropy is used to interpret the complexity of fluctuations in R-R intervals. Raised entropy indicates greater adaptability of the autonomic nervous system.49 Moreover, ApEn and SampEn were decreased when the sympathetic nerves were activated,50 or parasympathetic nerves were depressed.51 However, we did not find a significant relationship between SampEn and HCVR, which might have been due to the different theoretical ideas behind ApEn and SampEn.52 Thus, overactivity of the central chemoreceptors might be related to weak adaptability of the cardiac autonomic nervous system.
Strong evidence has demonstrated that elevated peripheral chemosensitivity contributes to excessive activation of the sympathetic nervous system.53 We described for the first time the relationship among hypertension, autonomic nerve activity, and central chemosensitivity in patients with OSA. Owing to the heterogeneity of OSA and the limited available therapies for OSA, the pharmacological remedies targeted at ventilatory chemoreflex might reduce the severity of OSA, the morbidity of cardiovascular disease, and mortality. However, several limitations of our study should be noted. First, this was a cross-sectional study, so conclusions on the causality between central chemosensitivity and hypertension could not be drawn. There are also many etiologies and types of hypertension, so more studies are needed to analyze the correlation between HCVR and different causes of hypertension. More studies are also needed to verify the relationship between central chemosensitivity and cardiovascular prognosis in patients with OSA. Second, the mechanism of ventilatory control is convoluted, which might be influenced by ethnicity, sex, and age. However, this study included only Chinese men. Thus, large real-world studies are needed and a simpler method to measure ventilatory chemosensitivity is also required for future research.
Conclusion
Compared with patients with OSA, OSA patients with hypertension had higher central chemosensitivity. OSA patients with hypertension also had attenuated parasympathetic nerve activity compared with OSA patients. Moreover, this study preliminarily illustrated that elevated central chemosensitivity might be associated with weak adaptability of the cardiac autonomic nervous system in OSA patients with hypertension.
Acknowledgments
The authors would like to thank Yang Zhao, Hairong Zhang, and Shuning Li for their help in collecting data.
Funding
The National Key Research and Development Project of China (No. 2018YFC1315103) funded this study.
Disclosure
Dr Xiaona Wang reports a patent for a device for measuring ventilatory control based on hypercapnic ventilatory response issued to ZL 2021 2 1127108.9. The authors declare that no financial or other relationships might lead to a conflict of interest in the present article.
References
1. Su L, Xiao Y. Application of personalized medicine to obstructive sleep apnea in China. Sleep Med. 2021;87:22–29. doi:10.1016/j.sleep.2021.08.014
2. Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114(7):911–922. doi:10.1152/japplphysiol.00747.2012
3. Deacon-Diaz N, Malhotra A. Inherent vs. Induced loop gain abnormalities in obstructive sleep apnea. Front Neurol. 2018;9:896. doi:10.3389/fneur.2018.00896
4. Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103(6):1929–1941. doi:10.1152/japplphysiol.00561.2007
5. Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99(9):1183–1189. doi:10.1161/01.cir.99.9.1183
6. Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand. 2003;177(3):377–384. doi:10.1046/j.1365-201X.2003.01083.x
7. Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100(3):262–267. doi:10.1161/01.cir.100.3.262
8. Palma JA, Gileles-Hillel A, Norcliffe-Kaufmann L, Kaufmann H. Chemoreflex failure and sleep-disordered breathing in familial dysautonomia: implications for sudden death during sleep. Auton Neurosci. 2019;218:10–15. doi:10.1016/j.autneu.2019.02.003
9. Mizukami K, Homma T, Aonuma K, Kinoshita T, Kosaka K, Asada T. Decreased ventilatory response to hypercapnia in dementia with Lewy bodies. Ann Neurol. 2009;65(5):614–617. doi:10.1002/ana.21613
10. May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. 2017;151(1):225–241. doi:10.1016/j.chest.2016.09.014
11. Bradicich M, Sievi NA, Grewe FA, Gasperetti A, Kohler M, Schwarz EI. Nocturnal heart rate variability in obstructive sleep apnoea: a cross-sectional analysis of the Sleep Heart Health Study. J Thorac Dis. 2020;12(Suppl 2):S129–s138. doi:10.21037/jtd-cus-2020-005
12. Qin H, Keenan BT, Mazzotti DR, et al. Heart rate variability during wakefulness as a marker of obstructive sleep apnea severity. Sleep. 2021;44(5). doi:10.1093/sleep/zsab018
13. Dissanayake HU, Bin YS, Ucak S, de Chazal P, Sutherland K, Cistulli PA. Association between autonomic function and obstructive sleep apnea: a systematic review. Sleep Med Rev. 2021;57:101470. doi:10.1016/j.smrv.2021.101470
14. Mansukhani MP, Kara T, Caples SM, Somers VK. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep. 2014;16(9):476. doi:10.1007/s11906-014-0476-2
15. Marsland DW, Callahan BJ, Shannon DC. The afferent vagus and regulation of breathing in response to inhaled CO2 in awake newborn lambs. Biol Neonate. 1975;27(1–2):102–107. doi:10.1159/000240765
16. Xu F, Owen J, Frazier DT. Cerebellar modulation of ventilatory response to progressive hypercapnia. J Appl Physiol. 1994;77(3):1073–1080. doi:10.1152/jappl.1994.77.3.1073
17. Tian Y, Geng D, Wang Y, et al. Contribution of retrotrapezoid nucleus neurons to CO(2) -amplified cardiorespiratory activity in spontaneously hypertensive rats. J Physiol. 2021;599(4):1115–1130. doi:10.1113/jp280246
18. Li A, Roy SH, Nattie EE. An augmented CO2 chemoreflex and overactive orexin system are linked with hypertension in young and adult spontaneously hypertensive rats. J Physiol. 2016;594(17):4967–4980. doi:10.1113/jp272199
19. Do Carmo JM, da Silva AA, Moak SP, da Silva FS, Spradley FT, Hall JE. Role of melanocortin 4 receptor in hypertension induced by chronic intermittent hypoxia. Acta Physiol. 2019;225(4):e13222. doi:10.1111/apha.13222
20. Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10(5):457–466. doi:10.1016/j.jash.2016.02.015
21. Walther LM, von Känel R, Heimgartner N, Zuccarella-Hackl C, Ehlert U, Wirtz PH. Altered cardiovascular reactivity to and recovery from cold face test-induced parasympathetic stimulation in essential hypertension. J Clin Med. 2021;10(12):2714. doi:10.3390/jcm10122714
22. Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med. 1967;16(1):20–32. doi:10.1111/imj.1967.16.1.20
23. Duffin J. Measuring the respiratory chemoreflexes in humans. Respir Physiol Neurobiol. 2011;177(2):71–79. doi:10.1016/j.resp.2011.04.009
24. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
25. Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV. The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications. 2.3 Version. Darien, IL: American Academy of Sleep Medicine; 2016.
26. Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17(3):370–374.
27. So ES, Yoo KS. Waist circumference cutoff points for central obesity in the Korean elderly population. J Appl Gerontol. 2015;34(1):102–117. doi:10.1177/0733464812464428
28. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi:10.1097/hjh.0000000000002453
29. Walker NA, Sunderram J, Zhang P, Lu SE, Scharf MT. Clinical utility of the Epworth sleepiness scale. Sleep Breathing. 2020;24(4):1759–1765. doi:10.1007/s11325-020-02015-2
30. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
31. Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–220. doi:10.1016/j.cmpb.2013.07.024
32. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065.
33. Sassi R, Cerutti S, Lombardi F, et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace. 2015;17(9):1341–1353. doi:10.1093/europace/euv015
34. Guyenet PG, Stornetta RL, Souza G, Abbott SBG, Shi Y, Bayliss DA. The retrotrapezoid nucleus: central chemoreceptor and regulator of breathing automaticity. Trends Neurosci. 2019;42(11):807–824. doi:10.1016/j.tins.2019.09.002
35. Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron. 2015;87(5):946–961. doi:10.1016/j.neuron.2015.08.001
36. Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO(2) matter? Chest. 2000;117(2):454–459. doi:10.1378/chest.117.2.454
37. Wang D, Grunstein RR, Teichtahl H. Association between ventilatory response to hypercapnia and obstructive sleep apnea-hypopnea index in asymptomatic subjects. Sleep Breath. 2007;11(2):103–108. doi:10.1007/s11325-006-0090-x
38. Ge RL, Stone JA, Levine BD, Babb TG. Exaggerated respiratory chemosensitivity and association with SaO2 level at 3568 m in obesity. Respir Physiol Neurobiol. 2005;146(1):47–54. doi:10.1016/j.resp.2004.11.009
39. Earing C, Owen J, Griffith-Mcgeever C, et al. An act of balance: interaction of central and peripheral chemosensitivity with inflammatory and anti-inflammatory factors in obstructive sleep apnoea. Respir Physiol Neurobiol. 2019;266:73–81. doi:10.1016/j.resp.2019.05.002
40. Narkiewicz K, Kato M, Pesek CA, Somers VK. Human obesity is characterized by a selective potentiation of central chemoreflex sensitivity. Hypertension. 1999;33(5):1153–1158. doi:10.1161/01.hyp.33.5.1153
41. Nishimura M, Miyamoto K, Suzuki A, et al. Ventilatory and heart rate responses to hypoxia and hypercapnia in patients with diabetes mellitus. Thorax. 1989;44(4):251–257. doi:10.1136/thx.44.4.251
42. Trombetta IC, Maki-Nunes C, Toschi-Dias E, et al. Obstructive sleep apnea is associated with increased chemoreflex sensitivity in patients with metabolic syndrome. Sleep. 2013;36(1):41–49. doi:10.5665/sleep.2298
43. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. doi:10.3389/fphys.2013.00026
44. Fang SC, Wu YL, Tsai PS. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs. 2020;22(1):45–56. doi:10.1177/1099800419877442
45. Taralov ZZ, Terziyski KV, Kostianev SS. Heart rate variability as a method for assessment of the autonomic nervous system and the adaptations to different physiological and pathological conditions. Folia Med. 2015;57(3–4):173–180. doi:10.1515/folmed-2015-0036
46. Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin auton res. 2019;29(2):231–243. doi:10.1007/s10286-018-0572-5
47. Zoccal DB, Simms AE, Bonagamba LG, et al. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586(13):3253–3265. doi:10.1113/jphysiol.2008.154187
48. Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587(3):597–610. doi:10.1113/jphysiol.2008.165902
49. Sequeira VCC, Bandeira PM, Azevedo JCM. Heart rate variability in adults with obstructive sleep apnea: a systematic review. Sleep Sci. 2019;12(3):214–221. doi:10.5935/1984-0063.20190082
50. Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R, Montano N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J Appl Physiol. 2007;103(4):1143–1149. doi:10.1152/japplphysiol.00293.2007
51. Bolea J, Pueyo E, Laguna P, Bailón R. Non-linear HRV indices under autonomic nervous system blockade. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:3252–3255. doi:10.1109/embc.2014.6944316
52. Bajić D, Japundžić-žigon N. On quantization errors in approximate and sample entropy. Entropy. 2021;24(1):73. doi:10.3390/e24010073
53. Iturriaga R, Alcayaga J, Chapleau MW, Somers VK. Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiol Rev. 2021;101(3):1177–1235. doi:10.1152/physrev.00039.2019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.