Back to Journals » Journal of Asthma and Allergy » Volume 15
Tezepelumab for Patients with Severe Uncontrolled Asthma: A Systematic Review and Meta-Analysis
Authors Zoumot Z, Al Busaidi N, Tashkandi W , Aljohaney AA, Isse S, Vidyasagar K , Ukwaja KN
Received 11 June 2022
Accepted for publication 26 October 2022
Published 18 November 2022 Volume 2022:15 Pages 1665—1679
DOI https://doi.org/10.2147/JAA.S378062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Zaid Zoumot,1 Nasser Al Busaidi,2 Wail Tashkandi,3 Ahmed A Aljohaney,4 Said Isse,1 Kota Vidyasagar,5 Kingsley Nnanna Ukwaja6
1Respiratory Institute Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates; 2Department of Pulmonology, Royal Hospital, Muscat, Sultanate of Oman; 3Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 4Department of Internal Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia; 5Department of Pharmacy, University College of Pharmaceutical Sciences, Kakatiya University, Warangal, Telangana, 506009, India; 6Department of Medicine, Alex Ekwueme Federal University Teaching Hospital, Abakaliki, Ebonyi State, Nigeria
Correspondence: Zaid Zoumot, Respiratory Institute Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates, Email [email protected]
Abstract: Tezepelumab is a human monoclonal antibody that blocks thymic stromal lymphopoietin, an epithelial-cell-derived cytokine implicated in the pathogenesis of asthma. It was approved by the United States Federal Drug Administration (US FDA) as an add-on maintenance treatment for patients with severe uncontrolled asthma in December 2021. We conducted a systematic review and meta-analysis to investigate the safety and efficacy of tezepelumab on forced expiratory volume (FEV1) (L), the rate of asthma exacerbations, health-related quality of life, fractional exhaled nitric oxide (FeNO) (ppb), and blood eosinophil count (cells/mL) in patients with severe, uncontrolled asthma. Mean changes for efficacy and proportions (safety) with their corresponding 95% confidence intervals (CIs) were used to provide pooled estimates. A total of six randomized controlled trials comprising 2667 patients were included, of whom 1610 were treated with tezepelumab and 1057 received placebo. The pooled analysis showed that tezepelumab treatment resulted in an improvement in FEV1 of 0.15 L (95% CI: 0.12 to 0.17), a reduction in the asthma exacerbation rate per year of 0.60 (95% CI: 0.51 to 0.70), and a reduction in FeNO of − 12.41 ppb (95% CI: − 14.28 to − 10.53) when compared to placebo. Improvements in FEV1 and FeNO levels were maintained at 24 and 52 weeks. As for safety, patients did not experience a higher incidence of adverse drug reactions with tezepelumab (0.79 (95% CI: 0.55 to 1.12)) as compared to placebo. As for quality of life, different doses of the tezepelumab intervention group depicted non-significant improvement in the QoL, from 0.15 (95% CI: − 0.09 to 0.38) for 70 mg, 0.18 (95% CI: − 0.10 to 0.46) for 210 mg, 0.08 (95% CI: − 0.16 to 0.32) for 280 mg as compared to the placebo. Tezepelumab significantly reduced exacerbation rates and improved FEV1 with an acceptable safety profile.
Keywords: tezepelumab, asthma, quality of life, systematic review, meta-analysis
Introduction
Asthma is a heterogeneous disease that affects an estimated 339 million people across the globe, of whom around 70% have moderate to severe asthma (Global Initiative for Asthma [GINA] step 3 to 5).1–3 In most patients, asthma can be controlled using conventional therapies including inhaled corticosteroids (ICS), long acting beta agonists (LABA), long acting anti-muscarinics (LAMA), and oral leukotriene receptor antagonists amongst other treatment options.4,5 For more severe cases which remain uncontrolled with standard treatment, there are biological therapies currently available.6 However, these biological therapies are only suitable for small subsets of patients with severe asthma, and are ineffective in those with non-allergic or non-eosinophilic asthma phenotypes. The heterogenous response to asthma treatment is likely directly related to differences in patterns of airway inflammation, immune-cell activation, and responsiveness to glucocorticoids.7 Existing biological approaches that inhibit only particular molecular targets, including IgE and type 2 (T2) helper cytokines, such as interleukin-4, interleukin-5, interleukin-13 and their respective receptors, therefore, only benefit that specific phenotype of patients. Hence, there exists an unmet need for new therapies that are effective in a wider population of asthmatics.8,9
Thymic stromal lymphopoietin (TSLP) is an epithelial-cell-procured cytokine mediating the immune response to inhaled environmental insults such as allergens and organic and non-organic pollutants, leading to a cascade of downstream inflammatory processes included in the pathophysiology of asthma.10 In asthma patients, TSLP titres were found to correlate with airway obstruction, disease severity, and glucocorticoid resistance. Moreover, TSLP was found to be a driver of type 2 inflammation in the airway, but also to mediate interactions between airway structural cells and immune cells, which are not completely driven by T2 inflammation.9,11,12
Tezepelumab is a first-in-class human IgG2 monoclonal antibody that binds to TSLP, preventing its interaction with the TSLP receptor complex. A proof-of-concept study in patients with mild, atopic asthma demonstrated that tezepelumab hindered both early and late asthmatic responses and suppressed biomarkers of T2 inflammation after inhaled allergen challenge.13,14 Early studies revealed that tezepelumab is associated with a significantly positive outcome in managing severe, uncontrolled asthma.13,15–17 These were followed by randomized clinical trials (RCTs) investigating the effect of tezepelumab on the annual rate of exacerbations amongst other outcomes.13,15–17 However there remains a significant need for more extensive data to guide future research and clinical use. Therefore, this study seeks to perform a systematic review and meta-analysis of the published literature to investigate the safety and efficacy of tezepelumab for patients with severe and uncontrolled asthma.
Materials and Methods
The current meta-analysis was performed according to Preferred Reporting Items for Systematic Review (PRISMA) guidelines18 (eTable S1).
Eligibility Criteria
The research question was framed in the PICOS format (Population, Intervention, Comparison, Outcome, Study Design) format to explore eligibility: We included published and unpublished randomized controlled trials (RCTs) evaluating patients who had uncontrolled, severe asthma despite other therapies, irrespective of age, sex, country, and ethnic group. We also included studies assessing tezepelumab compared placebo subcutaneously for the management of severe, uncontrolled asthma. We excluded non-human studies and in vitro research, Phase I clinical trials, case reports, editorials, conference proceedings, commentaries, expert opinions, reviews, studies without original data, non-RCTs, non-English publications, and duplicate publications; and studies that lacked a control-treated group for comparison with tezepelumab.
Search Strategy
A comprehensive literature search was performed in multiple electronic databases: PubMed, the Cochrane Central Register of Controlled Trials Library, and Epistemonikos for relevant studies from inception until January 19, 2022. The search terms included were “Asthma” OR “asthmatic” OR “Bronchial Hyperreactivity” OR “Respiratory Hypersensitivity” OR “bronchospas*” AND “tezepelumab” OR “amg 157”. Detailed search strategies are listed in Supplemental eTable S2. Additional reference lists from review articles, Google Scholar, and bibliographies were also searched manually to find published and unpublished trials. No date restrictions were applied, but the language restriction was used in electronic searches for studies.
Study Selection
Two authors (VK, ZZ) independently performed first-pass screening (FPS) by reviewing the titles and abstracts of all the records retrieved to identify articles that potentially meet the predefined eligibility criteria. The full texts of eligible titles were downloaded and reviewed independently by two authors (VK, AA) in the second-pass screening (SPS) to determine relevant inclusion in the final analysis. The discrepancies between the two reviewers (VK, AA) during the FPS and SPS were sorted by discussion with a third reviewer (KU).
Data Extraction and Management
Two authors (VK, KU) independently performed the data extraction, and all the relevant data were extracted from the included RCTs using data extraction templates. Discrepancies during the data extraction were resolved through discussion with a third reviewer (AA). The following details were extracted: study identification, authors’ details, study objectives, study design, the setting of intervention, study population, measures, and main findings (rates of asthma exacerbations, FEV1 before bronchodilation, and quality of life score, representing an approximately 52-week follow-up). This duration point was chosen because it was the most reported follow-up duration in the existing literature. Efficacy data reported until 52 weeks was collected, including extracting data from figures by using web application called WebPlotDigitizer19 if numerical data were not reported in the text.
Methodological Quality Assessment in Included Studies
Two reviewers independently (KU, VK) used Cochrane collaboration’s Risk of Bias (RoB) assessment tool20 which comprises six domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Further results were presented as low risk of bias, unclear risk of bias or high risk of bias.
Statistical Analysis
All the analyses were performed using STATA MP 16.1 software (StataCorp LLC, College Station, TX, USA). All the efficacy estimates were expressed as mean changes and 95% confidence interval (CI) from baseline. Standard deviations (SD) were calculated from the standard error or 95% CI and were imputed where it was not reported according to the Cochrane handbook for systematic review of interventions.21 Annual rate of Exacerbations and Safety estimates were presented as a pooled proportion with 95% CI.
Heterogeneity Testing
The Higgins I2 statistics and Cochran’s Q test were used to assess the potential statistical heterogeneity among trials. The meta-analysis was conducted using a fixed-effect model (using inverse-variance) or a random-effect model (Restricted maximum likelihood method) based on low heterogeneity (<50%) or high heterogeneity (>50%). Further, subgroup analyses were conducted as per total daily dose and various follow-up duration point wise. The publication bias was not assessed since the number of included trials was less than ten.
Results
Study Characteristics
A total of 452 records were identified through several sources. From these, 14 duplicate articles were removed. The remaining 438 records were considered for the primary screening. After screening titles and abstracts, 416 were excluded, and the remaining 22 articles were considered relevant for full-text evaluation. Sixteen articles were excluded as their outcome of interest was found irrelevant, insufficient, or ambiguous (Supplemental eTable S3). Finally, 6 unique RCTs met the inclusion criteria and were included in systematic review and meta-analysis13,15–17,22 (Figure 1).
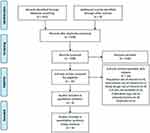 |
Figure 1 PRISMA flowchart of literature search and study selection. |
All the included trials were published before or in the year 2021 and included 2667 (range: 116–1059) participants with severe, uncontrolled asthma; all the studies were parallel design and multinational RCTs. All the studies included asthma patients uncontrolled despite maintenance inhaled corticosteroid therapy. Baseline mean age was 51.4 years and 64.2% of the participants were female, and follow-up duration ranged from 4 to 52 weeks. Other characteristics of the RCTs, such as study details, drug- and outcome-specific data, are shown in Table 1.
 |
Table 1 Study Characteristics of Included Studies |
Quality Assessment of Included Studies
Overall, the risk of bias in eligible RCTs is shown in Figure 2. All included studies exhibit a low risk of selection bias, performance bias, detection bias, and reporting bias. The risk of bias in randomization process was generally unclear apart from the Corren et al, 2021(a), Corren et al, 2021(b), and Emson et al, 2021 study, and all these studies have a low risk of bias.13,15,17,23
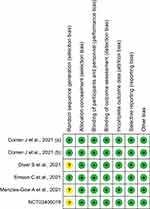 |
Figure 2 Assessment of the risk of bias in included studies with Cochrane domain-based quality assessment tool. |
Meta-Analysis
Efficacy of Tezepelumab on FEV1 Levels
Data on the effect of tezepelumab on FEV1 were available from three RCTs.13,22,23 Figure 3 shows the efficacy of tezepelumab therapy on FEV1 using a random-effects model. The included studies displayed varying degrees of improvement in FEV1. Overall, meta-analyses showed a significant improvement with tezepelumab versus placebo by 0.15 L (95% CI: 0.12 to 0.17, p = 0.00; I2 = 65%). Studies were sub-grouped based on the duration of intervention and showed consistent improvement in the FEV1 levels after 4 weeks [0.11 L (95% CI: 0.07 to 0.16)], after 12 weeks [0.13 L (95% CI: 0.08 to 0.18)], after 24 weeks [0.13 (95% CI: 0.08 to 0.18)] while at after 52 weeks [0.16 L (95% CI: 0.11 to 0.21)] showed decreased trend. More details can be found in Figure 3.
 |
Figure 3 Efficacy of tezepelumab versus placebo on FEV1 based on duration of intervention. |
Efficacy of Tezepelumab on Asthma Exacerbation Rate per Year
The pooled estimate of four trials13,15–17 revealed that tezepelumab significantly decreased the number of asthma exacerbations in a year by 0.60 (95% CI: 0.51 to 0.70) exacerbations when compared to the placebo. No statistically significant heterogeneity was observed (I2 = 0.00%; P = 0.91). Both doses of tezepelumab 210 and 280 mg groups depicted a significant decrease, 0.55 (95% CI: 0.42 to 0.71) and 0.59 (95% CI: 0.38 to 0.90) for 210 mg and 280 mg, respectively. Whereas tezepelumab 70 mg showed non-significant decrease in asthma exacerbations by 0.70 (95% CI: 0.47 to 1.04) (Figure 4).
 |
Figure 4 Efficacy of tezepelumab versus placebo on rate of asthma exacerbation based on different doses of intervention. |
Only one included study23 further reported the annualized rate of asthma exacerbations over a period of 52 weeks in the overall population and according to different baseline biomarker categories such as blood eosinophil count, FeNO levels, and allergic status. We performed subgroup analysis as per the patient’s ratio change in different categories of blood eosinophil count levels from baseline to end of the treatment. The pooled analysis of (<150 (cells/µL), (150 to <300 (cells/µL), (300 to <450 (cells/µL), and (≥450 (cells/µL) categories showed a significant decrease in annualized rate of asthma exacerbations in Tezepelumab group by 0.61 (95% CI: 0.42 to 0.88), 0.57 (95% CI: 0.41 to 0.79), 0.41 (95% CI: 0.27 to 0.64), and 0.23 (95% CI: 0.15 to 0.34), respectively, as compared to the placebo group. We also performed subgroup analysis as per the patient’s ratio change in different categories of FeNO levels from baseline to end of the treatment. The pooled analysis of (<25 (ppb) and (≥25 (ppb) categories showed a significant decrease in annualized rate of asthma exacerbations in Tezepelumab group by 0.68 (95% CI: 0.51 to 0.92) and 0.32 (95% CI: 0.25 to 0.42), respectively, as compared to the placebo group. Finally, we also performed a subgroup analysis based on allergic status from baseline to end of the treatment period. The pooled analysis of positive and negative status for any perennial allergen categories showed a significant decrease in annualized rate of asthma exacerbations in Tezepelumab group by 0.42 (95% CI: 0.33 to 0.53) and 0.49 (95% CI: 0.36 to 0.67), respectively, as compared to the placebo group.
Efficacy of Tezepelumab on Asthma-Related QoL
Data on the effect of tezepelumab on QoL were available from three RCTs.15,22,23 The meta-analysis of available data showed a significant improvement in QoL of 0.15 (95% CI: 0.00 to 0.29) versus placebo. However, assessing the four different doses of the tezepelumab intervention groups individually compared to placebo depicted non-significant improvements, 0.15 (95% CI: −0.09 to 0.39) for 70 mg, 0.20 (95% CI: −0.10 to 0.50) for 210 mg, 0.09 (95% CI: −0.17 to 0.35) for 280 mg, and 0.04 (95% CI: −0.15 to 0.23) for overall dose (Figure 5). There was statistically significant heterogeneity among these studies (I2 = 60.03%, p = 0.02).
Efficacy of Tezepelumab on Blood Eosinophil Count
Values of blood eosinophil count were available from five RCTs.13,16,17,22,23 The meta-analysis of available data showed a significant reduction in blood eosinophil count of −151.05 cells/μL (95% CI: −165.99 to −136.12) versus placebo at all different follow-up durations; after 4 weeks −186.59 cells/μL (95% CI: −238.71 to −134.47), after 12 weeks −160.15 cells/μL (95% CI: −198.08 to −122.23), after 24 weeks −189.16 cells/μL (95% CI: −23.135 to −146.97), and after 52 weeks −117.17 cells/μL (95% CI: −164.02 to −70.32) (Figure 6). Heterogeneity among these studies was not statistically significant (I2 = 12.11%, p = 0.32).
 |
Figure 6 Efficacy of tezepelumab versus placebo on blood eosinophil count based on duration of intervention. |
Efficacy of Tezepelumab on FeNO Levels
Data on FeNO levels were available from five RCTs.13,16,17,22,23 Fixed effects meta-analysis showed a significant reduction in FeNO levels in the tezepelumab group compared to placebo by −0.12.41 ppb (95% CI: −14.28 to −10.53), after 4 weeks −14.50 ppb (95% CI: −19.98 to −9.0.2), after 12 weeks −10.99 ppb (95% CI: −15.60 to −6.39), after 24 weeks −12.11 ppb (95% CI: −17.05 to −7.17), and after 52 weeks −12.46 ppb (95% CI: −14.93 to −9.99) was observed (Figure 7). -Heterogeneity among these studies was not statistically significant (I2 = 0.00%, p = 0.82).
 |
Figure 7 Efficacy of tezepelumab versus placebo on FeNO levels based on duration of intervention. |
Efficacy of Tezepelumab on Total Serum IgE Levels
Data on the effect of tezepelumab on total serum IgE levels were available from four RCTs.13,16,22,23 Figure 8 shows the efficacy of tezepelumab therapy on total serum IgE using a fixed effect model. The included studies displayed varying degrees of total serum IgE efficacy. Overall, meta-analyses showed a significant reduction in tezepelumab versus placebo/controls by −122.90 IU/mL (95% CI: −167.80 to −78.01, p = 0.00; I2 = 9.40%). Studies were sub-grouped based on the duration of intervention and showed more consistent reduction in total serum levels after 24 weeks [−136.99 IU/mL (95% CI: −251.01 to −22.98)] and 52 weeks [−157.78 IU/mL (95% CI: −217.13 to −98.44)], while duration after 4 weeks [−53.50 IU/mL (95% CI: −183.43 to 76.43)] and after 12 weeks [−32.45 IU/mL (95% CI: −147.15 to 82.24)] showed non-significant reduction in the tezepelumab group compared to the placebo group. More details can be found in Figure 8.
 |
Figure 8 Efficacy of tezepelumab versus placebo on total serum IgE levels based on duration of intervention. |
Safety of Tezepelumab on Patients with Adverse Drug Reactions (ADRs)
The pooled estimate of three trials15,22,23 revealed that, tezepelumab use was associated with a non-significant decrease in the incidence of adverse drug reactions (ADRs) 0.79; (95% CI: 0.55 to 1.12) when compared to the placebo. A statistically significant heterogeneity was observed among the studies (I2 = 96.9%; P = 0.00). All the doses of tezepelumab 70, 210, and 280 mg groups depicted non-significant decrease in the incidence of ADRs, by 0.92 (95% CI: 0.80 to 1.05), 1.00 (95% CI: 0.84 to 1.18) for 210 mg, 280 mg, and 1.02 (95% CI: 0.86 to 1.21) for 70 mg, respectively. Whereas overall a significant decrease in ADRs by 0.33 (95% CI: 0.26 to 0.41) was seen as compared to placebo (Figure 9).
Discussion
Several novel biological therapies have been proven to be effective in improving asthma control and reducing exacerbation rates for patients with the severe, uncontrolled asthma. These include omalizumab (anti-IgE), mepolizumab, benralizumab (anti-IL5R) and Reslizumab (all anti-IL5), and dupilumab (anti-IL4R [inhibits IL4/, IL13]).24 However, despite these advances, many patients fail to achieve optimal asthma control likely because these therapies only inhibit specific molecular targets.25 Inhibiting TSLP, CCR3, IL-5, PGD2, IL-4, IL-13, IL-9 and/or IgE may be effective in the management of allergic eosinophilic asthma.25 Tezepelumab is a novel human monoclonal antibody administered once in every 4 weeks that inhibits both T2 helper cytokines and its interaction with the TSLP receptor complex. In individual RCTs, tezepelumab was shown to improve FEV1 and FeNO levels compared with a placebo.13,16,17,22,23 However, to date, no extensive systematic compilation of the available evidence of tezepelumab has been performed.
We present the relative efficacy and safety of tezepelumab compared with placebo and provide a comprehensive analysis. Compared to placebo, the human monoclonal antibody tezepelumab improved FEV1 (0.17 to 0.40 (L)), asthma-related quality of life (0 to 0.22), reduced total serum IgE (−0.29 to −142 IU.mL), reduced FeNO (−0.48 to −0.35 ppb), and peripheral blood eosinophil count (−0.72 to −0.59 cells/μL). These effects were consistently maintained from week 4 to week 52.13,16,17,22,23 The improvements in FEV1 and exacerbation rates are clinically significant, whereas the magnitude of reduction in serum eosinophils and FeNO, although statistically significant, are quite modest and unlikely to have clinical significance. The most recent phase IIb trial (PATHWAY) has indeed demonstrated a similar reduction in FEV1, blood eosinophil count, and FeNO in patients with severe uncontrolled asthma treated with tezepelumab.15 We await the results of a more recently completed Phase 3 trial on tezepelumab, which may further support these findings.22
Our meta-analysis results demonstrated that tezepelumab showed significant reduction of the asthma exacerbation rates compared to the pooled placebo. This observation was in contrast to a previously published meta-analysis where tezepelumab did not show statistically significant improvement of the exacerbation rate compared to the pooled placebo, mainly due to the lower number of included trials (n = 1).26 In terms of safety, tezepelumab did not lead to a higher incidence of ADRs as compared to placebo, and individual studies indicated that most ADRs were mild or moderate in severity. We also found that none of the doses of tezepelumab (70 mg, 210 mg, and 280 mg) improved the QoL to a greater extent than placebo. These, findings are consistent with the PATHWAY study15 but contradict with the Menzies-Gow et al.23 Ongoing trials will provide longer term data on tezepelumab’s safety and quality of life.22
To the best of our knowledge, this is the first systematic review and meta-analysis that combined the data of all tezepelumab Phase 2 and phase 3 RCTs with a placebo comparison, providing a comprehensive picture of the effect of tezepelumab on clinical outcomes, biomarkers and safety. However, limitations of this study include the relatively small number of trials, and only including studies and data published in journal articles or available at ClinicalTrials.gov. Due to limitations in the published data, we were able to report pooled efficacy data for FEV1, blood eosinophil count, FeNO, total serum IgE, rate of exacerbations and asthma-related quality of life but we could not conduct a subgroup analysis to investigate the source of heterogeneity for some of the outcomes. Finally, the duration of follow-up of the studies was limited to 52 weeks with no longer term data available for analysis.
Conclusion
Clinical trials of TSLP inhibitor with tezepelumab completed to date have produced favourable results in patients with different kinds of asthma phenotypes, who experienced substantial reductions in exacerbation rates and improvements in lung function, symptom control, and QoL. Ongoing trials will provide further evidence of whether tezepelumab can be effective in a long-term treatment for a wider population of patients with severe asthma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi:10.3389/fped.2019.00246
2. Trivedi M, Denton E. Asthma in children and adults-what are the differences and what can they tell us about asthma? Front Pediatr. 2019;7:256. doi:10.3389/fped.2019.00256
3. Asthma GIf. Global; 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
4. Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
5. Ricciardolo FLM, Bertolini F, Carriero V. The role of dupilumab in severe asthma. Biomedicines. 2021;9(9):1096. doi:10.3390/biomedicines9091096
6. Eger K, Amelink M, Hashimoto S, Hekking PP, Longo C, Bel EH. Overuse of oral corticosteroids, underuse of inhaled corticosteroids, and implications for biologic therapy in asthma. Respiration. 2022;101(2):116–121. doi:10.1159/000518514
7. Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690(1–2):24–39. doi:10.1016/j.mrfmmm.2009.09.005
8. Esteban-Gorgojo I, Antolín-Amérigo D, Domínguez-Ortega J, Quirce S. Non-eosinophilic asthma: current perspectives. J Asthma Allergy. 2018;11:267–281. doi:10.2147/JAA.S153097
9. Menzies-Gow A, Wechsler ME, Brightling CE. Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir Res. 2020;21(1):268. doi:10.1186/s12931-020-01505-x
10. Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845–852. doi:10.1016/j.jaci.2012.07.010
11. Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75(1):68–78. doi:10.1016/j.cyto.2015.05.014
12. Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–2262. doi:10.4049/jimmunol.1701455
13. Corren J, Ambrose CS, Sałapa K, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma and perennial allergy. J Allergy Clin Immunol Pract. 2021;9(12):4334–42.e6. doi:10.1016/j.jaip.2021.07.045
14. Parnes JR, Sullivan JT, Chen L, Dias C. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult subjects. Clin Pharmacol Ther. 2019;106(2):441–449. doi:10.1002/cpt.1401
15. Corren J, Garcia Gil E, Griffiths JM, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021;126(2):187–193. doi:10.1016/j.anai.2020.10.008
16. Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi:10.1016/S2213-2600(21)00226-5
17. Emson C, Corren J, Sałapa K, Hellqvist Å, Parnes JR, Colice G. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without nasal polyposis: a post hoc analysis of the phase 2b PATHWAY study. J Asthma Allergy. 2021;14:91–99. doi:10.2147/JAA.S288260
18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
19. Rohatgi A. WebplotDigitizer (Version 4.6) {Computer software}; 2021. Available from: http://arohatgi.info/WebPlotDigitizer.2022.
20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
21. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi:10.1002/14651858.ED000142
22. Study to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma (SOURCE). Available from: https://clinicaltrials.gov/ct2/show/NCT03406078.
23. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi:10.1056/NEJMoa2034975
24. Chapman KR, Albers FC, Chipps B, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy. 2019;74(9):1716–1726. doi:10.1111/all.13850
25. Rogliani P, Calzetta L, Matera MG, et al. Severe Asthma and Biological Therapy: when, Which, and for Whom. Pulm Ther. 2020;6(1):47–66. doi:10.1007/s41030-019-00109-1
26. Edris A, De Feyter S, Maes T, Joos G, Lahousse L. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respir Res. 2019;20(1):179. doi:10.1186/s12931-019-1138-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


