Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Successful Treatment of Refractory Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis (SAPHO) Syndrome and Paradoxical Psoriasis with Secukinumab: A Case Report
Authors Fan D, Li F , Liu Z, Tang Z, Lv S
Received 8 December 2023
Accepted for publication 21 February 2024
Published 5 March 2024 Volume 2024:17 Pages 547—552
DOI https://doi.org/10.2147/CCID.S454057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Danyang Fan, Fuqiu Li, Zhe Liu, Zhanhan Tang, Sha Lv
Department of Dermatology, The Second Hospital of Jilin University, Changchun, Jilin, 130000, People’s Republic of China
Correspondence: Sha Lv, Department of Dermatology, The second hospital of Jilin University, Changchun, Jilin, 130000, People’s Republic of China, Email [email protected]
Abstract: Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is a rare chronic inflammatory disease mainly manifested as skin and osteoarticular lesions. Herein, we describe a female patient with SAPHO syndrome exhibited paradoxical psoriasis and primary palmoplantar pustulosis (PPP) worsened during treatment with adalimumab. We then switched to secukinumab and obtained significant improvement in both skin lesions and osteoarticular pain. These findings suggest that secukinumab might be an appropriate option for patients with SAPHO syndrome who present with TNF-α-inhibitor-induced paradoxical psoriasis.
Keywords: SAPHO syndrome, psoriasis, adalimumab, secukinumab
Introduction
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is a rare autoinflammatory disease characterized by dermatological and musculoskeletal manifestations.1 While the pathogenesis of SAPHO syndrome has not been fully elucidated, individual genetic differences, inflammatory factors, and microorganisms might play roles in its mechanism.2 Notably, Brandao et al identified three enriched exclusive pathways (eEP) associated with extracellular matrix organization in patients with SAPHO syndrome (R-HSA-8941237, R-HSA-2243919, and R-HSA-1474290), suggesting these pathways may be related to the pathogenesis of SAPHO syndrome.3 Additionally, multiple inflammatory factors, such as TNF-α, IL-1β, IL-8, IL-17, IL-18, are related to SAPHO syndrome, contributing to the complexity of treatment.4
At present, adalimumab has been used for SAPHO syndrome and showed efficacy in both osteoarticular pain and cutaneous lesions. However, patients might develop psoriasiform rash during treatment with TNF-α inhibitors. These adverse effects lead physicians to select appropriate therapeutic options to treat SAPHO syndrome while alleviating paradoxical cutaneous lesions caused by TNF-α inhibitors.
Secukinumab is a fully human IgG1/κ monoclonal antibody which specifically binds to IL-17A and inhibits its interaction with the IL-17 receptor, thereby playing a role in treating IL-17A-mediated inflammatory diseases. Herein, we describe a patient with refractory SAPHO syndrome and TNF-α-inhibitor-induced paradoxical skin lesions, whose cutaneous and osteoarticular symptoms improved without side effects after treatment with secukinumab.
Case Report
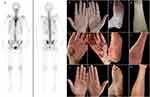
A 31-year-old female patient presented with erythema and pustules on the palmoplantar area without obvious causes for 2 months, along with pain in the spine, sternoclavicular joints, and sacroiliac joints for the past 1 month. Medical history revealed that she was diagnosed with palmoplantar pustulosis (PPP) at a local clinic 2 months ago and was given oral glucocorticoids (precise dosage unclear), and her lesions were not in remission. The patient was previously healthy and did not have a history of psoriasis. A dermatological examination revealed that the palms and soles had erythematous patches with well-defined boundaries. Pustules on the surface were partially fused, and obsolete pustules had dried up, with mild desquamation. The palmoplantar psoriasis area and severity index (PPPASI) was 6.6 points and the overall pain intensity score was 6 points according to visual analog scale (VAS). Laboratory tests revealed the following: erythrocyte sedimentation rate, 50 mm/h [normal range <20 mm/h]; C-reactive protein, 19.6 mg/L [normal range <6 mg/L]; human leukocyte antigen-B27, positive; rheumatoid factor, negative; and anti-nuclear antibodies, negative. 99mTc-methylene diphosphonate whole-body bone scintigraphy revealed inflammatory changes in the osternal angle, bilateral sternoclavicular joints, upper edge of the third lumbar vertebrae, and bilateral sacroiliac joints. The pathological uptake of the anterior chest wall presented the “bull’s head” sign. Based on the comprehensive clinical assessment, we diagnosed the patient with SAPHO syndrome (Figure 1a–e).
Following a definitive diagnosis, we administered adalimumab (80 mg for the first week, followed by 40 mg after a week and 40 mg every 2 weeks thereafter). Initially, the PPP and joint pain improved after three injections. However, cutaneous lesions worsened and developed psoriasiform rash after 26 days (Figure 1f–i). Therefore, we added adalimumab (80 mg every 2 weeks) and minocycline (100 mg, twice daily). However, lesions remained unmanaged after 28 days. As a result, methylprednisolone pills (16 mg, daily) were administered; however, pustules still gradually increased, and the maximal PPPASI was 21 points. After 42 days, adalimumab, minocycline, and methylprednisolone were discontinued, and the patient was administered secukinumab (started with 300 mg weekly over 5 weeks, followed by 300 mg every 4 weeks). After treatment with secukinumab, significant improvement in PPP and paradoxical psoriasis were observed, and the patient did not exhibit recurrence of cutaneous manifestations or osteoarticular pain (Figure 1j–m). The changes in disease evolution and treatment options of the patient are shown in Figure 2.
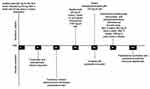 |
Figure 2 Disease evolution and treatment options of the patient. |
Discussion
Here, we have presented a patient with refractory SAPHO syndrome who developed paradoxical skin lesions, which manifested as new-onset psoriasiform rash and exacerbations of primary lesions after treated with adalimumab for 3 successive injections. Adalimumab was then discontinued, and secukinumab was administered; new-onset paradoxical psoriasis was controlled, and the SAPHO syndrome improved.
SAPHO syndrome is a rare spectrum of inflammatory diseases characterized by osteoarticular and dermatologic manifestations.5 The most widely used diagnostic criteria proposed by Kahn et al concentrates on the skin and osteoarticular lesions.6 Osteitis and hyperostosis involving the anterior chest wall and axial skeleton (including the spine and sacroiliac joints) are the core clinical manifestations of SAPHO syndrome, and the skin involvement is mostly palmoplantar pustulosis and severe acne.4 In the present report, the patient had typical clinical features: PPP; pain in the spine, sternoclavicular joints, and sacroiliac joints; and inflammatory changes showed on the 99mTc-methylene diphosphonate whole-body bone scintigraphy. There was no evidence of infectious disease or bone tumor as well as other spondyloarthropathy, thus confirming the diagnosis of SAPHO syndrome.
At present, there is no standard treatment method for SAPHO syndrome based on limited experience, and the main purpose of treatment is to relieve clinical symptoms and to delay disease progression. The commonly used treatment methods conclude non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), corticosteroids, and antibiotics. Recently, biologics have been used in cases refractory to conventional treatment with noteworthy results. Moreover, the combination of biological agents and conventional systemic treatment is a safe and effective therapeutic option for patients with SAPHO syndrome.7 TNF-α inhibitors are the most widely used biologics in the treatment of SAPHO syndrome and show improvement in both skin and osteoarticular manifestations.8 Currently used TNF-α inhibitors include infliximab, adalimumab, certolizumab pegol, golimumab, and etanercept, and these agents are proved to be well tolerated.9 However, Li et al reported dermatologic complications during treatment with TNF-α inhibitors, with psoriasiform lesions as the most common skin-related side effect, mainly have been associated with infliximab or etanercept.10 In this report, during the treatment with adalimumab, the patient developed paradoxical psoriasis and pre-existing PPP worsened.
At present, the following hypotheses have been proposed for the causes of paradoxical psoriasis. First, TNF-α inhibitors increase the ability of plasmacytoid dendritic cells (pDC) to secrete interferon (IFN) by inhibiting their maturation.11 The excessive level of IFN-α activates myeloid dendritic cells and thereby stimulating pathogenic T cells. This leads to increased release of inflammatory cytokines (including TNF-α, IL-23, and IL-12) and dysregulation of keratinocytes.12 Secondly, the levels of IFN-γ-secreting Th1 cells and IL-17/IL-22-secreting Th17 cells increase in patients who developed TNF-α-inhibitor-induced psoriasis.13 Th17 cytokines, such as IL-17A, IL-17F, and IL-22, induce hyperproliferation of epidermis, attract neutrophils to the skin, and activate the production of chemokines and antimicrobial peptides by keratinocytes, thereby directly driving the development of the psoriatic phenotype.14 Some authors associate PPP a disease in the spectrum of psoriasis, and recently the IL-23/IL-17 inflammatory pathway has been suggested to play an important role in PPP.15 Therefore, we assumed that the factors that lead to the appearance of paradoxical psoriasis are also involved in the mechanism of the exacerbation of PPP.
Secukinumab is a fully human monoclonal IL-17A-targeted antibody, which is FDA approved for the treatment of plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis (AS) in adults. In recent reports, the efficacy of secukinumab in SAPHO syndrome was indicated. Ji et al reported a female patient with SAPHO syndrome with PPP who was treated with secukinumab when conventional treatment regimens were ineffective. During the 7-month follow-up, her symptoms had resolved completely without complications or adverse reactions.16 Sun et al described a male patient with SAPHO syndrome who presented with significant left jaw pain and mouth opening limitation. Despite undergoing successive treatments with pamidronate, tofacitinib, and adalimumab without achieving long-term remission, significant improvement in symptoms and remarkable remission on MRI were observed after treatment with secukinumab.17 Tu et al reported a male patient with SAPHO syndrome/AS overlapping. Despite a limited response to traditional treatment regimens and adalimumab, both skin and osteoarticular symptoms improved after switching to secukinumab.18 Cheng et al found that in patients with SAPHO syndrome, secukinumab demonstrated a higher improvement rate in cutaneous symptoms compared to osteoarticular symptoms.19 This difference may be attributed to the involvement of distinct inflammatory pathways in cutaneous and osteoarthritis symptoms among patients with SAPHO syndrome. In this report, in view of the role of IL-17A plays in both SAPHO syndrome and paradoxical psoriasis, we chose to use secukinumab. The psoriasiform rash, PPP, and osteoarthritis pain of the patient improved after intensive injections, which suggested that secukinumab could be an optional treatment method for paradoxical psoriasis induced by TNF-α inhibitors for patients with SAPHO syndrome. Given the increasing evidence supporting the involvement of IL-17 in SAPHO syndrome, secukinumab emerges as a potential treatment option. However, further prospective studies are required to validate our findings.
Conclusion
There are few reports of patients with SAPHO syndrome who developed paradoxical psoriasis during treatment with adalimumab and were successfully treated by switching to secukinumab. We described in detail the successful treatment of primary lesions and paradoxical psoriasis with secukinumab in a patient with refractory SAPHO syndrome. Therefore, secukinumab might be an effective method for patients with SAPHO syndrome combined with TNF-α-inhibitor-induced paradoxical psoriasis. We hope this report provides valuable information on the pathogenesis and treatment option of paradoxical psoriasis caused by TNF-α inhibitor in patients with SAPHO syndrome. However, as a follow-up of 5 months is short to draw definitive conclusions, further reports and long-term follow-up are needed to clarify the efficacy of secukinumab in SAPHO syndrome.
Consent
Written informed consent for publication of the case details and any accompanying images was obtained from the patient. This study was performed according to the convention of the Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of the Second Hospital of Jilin University [Approval No.: 2024-026]. Institutional approval was not required to publish the case details.
Acknowledgments
The authors thank the patient for participation in this study and providing written consent for publication.
Funding
This work was supported by Jilin Science and Technology Development Program Funded Projects [grant numbers YDZJ202102CXJD074].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marzano AV, Genovese G, Moltrasio C, et al. Whole-Exome sequencing in 10 unrelated patients with syndromic hidradenitis suppurativa: a preliminary step for a genotype-phenotype correlation. Dermatology. 2022;238(5):860–869. doi:10.1159/000521263
2. Guo C, Tian X, Han F, Liu L, Gao J, Ma X. Copy number variation of multiple genes in SAPHO syndrome. J Rheumatol. 2020;47(9):1323–1329. doi:10.3899/jrheum.181393
3. Brandao LAC, de Moura RR, Marzano AV, Moltrasio C, Tricarico PM, Crovella S. Variant enrichment analysis to explore pathways functionality in complex autoinflammatory skin disorders through whole exome sequencing analysis. Int J Mol Sci. 2022;23:4.
4. Liu S, Tang M, Cao Y, Li C. Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: review and update. Ther Adv Musculoskelet Dis. 2020;12:1759720x20912865. doi:10.1177/1759720x20912865
5. Demirci Yildirim T, Sari I. SAPHO syndrome: current clinical, diagnostic and treatment approaches. Rheumatol Int. 2023. doi:10.1007/s00296-023-05491-3
6. Kahn M Proposed classification criteria of SAPHO syndrome; 2003.
7. Genovese G, Caorsi R, Moltrasio C, Marzano AV. Successful treatment of co-existent SAPHO syndrome and hidradenitis suppurativa with Adalimumab and methotrexate. J Eur Acad Dermatol Venereol. 2019;33:40–41. doi:10.1111/jdv.15849
8. Daoussis D, Konstantopoulou G, Kraniotis P, Sakkas L, Liossis SN. Biologics in SAPHO syndrome: a systematic review. Semin Arthritis Rheum. 2019;48(4):618–625. doi:10.1016/j.semarthrit.2018.04.003
9. Chokshi A, Beckler MD, Laloo A, Kesselman MM. Paradoxical Tumor Necrosis Factor-Alpha (TNF- a) inhibitor-induced psoriasis: a systematic review of pathogenesis, clinical presentation, and treatment. Cureus J Med Sci. 2023;15(8).
10. Li C, Wu X, Cao Y, et al. Paradoxical skin lesions induced by anti-TNF-α agents in SAPHO syndrome. Clin Rheumatol. 2019;38(1):53–61. doi:10.1007/s10067-018-4083-5
11. Hu JZ, Billings SD, Yan D, Fernandez AP. Histologic comparison of tumor necrosis factor-α inhibitor–induced psoriasis and psoriasis vulgaris. J Am Acad Dermatol. 2020;83(1):71–77. doi:10.1016/j.jaad.2020.01.006
12. Chokshi A, Demory Beckler M, Laloo A, Kesselman MM. Paradoxical Tumor Necrosis Factor-Alpha (TNF-α) inhibitor-induced psoriasis: a systematic review of pathogenesis, clinical presentation, and treatment. Cureus. 2023;15(8):e42791. doi:10.7759/cureus.42791
13. Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63(4):567–577. doi:10.1136/gutjnl-2012-302853
14. Mylonas A, Conrad C. Psoriasis: classical vs. Paradoxical. The Yin-Yang of TNF and Type I Interferon. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02746
15. Yamamoto T. Similarity and difference between palmoplantar pustulosis and pustular psoriasis. J Dermatol. 2021;48(6):750–760. doi:10.1111/1346-8138.15826
16. Ji Q, Wang Q, Pan WP, et al. Exceptional response of skin symptoms to secukinumab treatment in a patient with SAPHO syndrome: case report and literature review. Medicine. 2022;101:33.
17. Sun B, Cao Y, Wang L, Wang M, Li C. Successful treatment of refractory mandibular lesions in SAPHO syndrome with secukinumab. Rheumatology. 2021;60(1):473–474. doi:10.1093/rheumatology/keaa352
18. Tu W, Nie D, Chen YX, Wen C, Zeng ZP. Successful treatment of SAPHO syndrome complicated with ankylosing spondylitis by secukinumab: a case report. J Person Med. 2023;13(3).
19. Cheng W, Li F, Tian J, et al. New insights in the treatment of SAPHO syndrome and medication recommendations. J Inflamm Res. 2022;15:2365–2380. doi:10.2147/jir.S353539
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Efficacy and Safety of Anti-TNF Biosimilars for Psoriasis in Pediatric and Geriatric Populations: A 72-Week Real-Life Study
Megna M, Fornaro L, Potestio L, Luciano MA, Nocerino M, Delfino M, Guarino M, Fabbrocini G, Camela E
Psoriasis: Targets and Therapy 2022, 12:199-204
Published Date: 9 July 2022
Drug Survival Outcomes Associated with the Real-World Use of Ixekizumab, Secukinumab, Guselkumab, and Adalimumab for the Treatment of Plaque Psoriasis in China: A 52-Week Single-Center Retrospective Study
Li Y, Lu JJ, Zhong XY, Yu YY, Yu N, Wang Y, Yi XM, Ding YF, Shi YL
Clinical, Cosmetic and Investigational Dermatology 2022, 15:2245-2252
Published Date: 20 October 2022
Interleukin-17A Inhibitor Secukinumab Treatment in HIV-Positive Psoriasis Patient: A Case Report
Gong J, Wu W, Qiu L, Wang X, Bao J, Wang J, Cheng L, Fu Z, Hu F
Clinical, Cosmetic and Investigational Dermatology 2022, 15:2949-2956
Published Date: 30 December 2022
Adalimumab, Ustekinumab, and Secukinumab in the Management of Hidradenitis Suppurativa: A Review of the Real-Life Experience
Martora F, Megna M, Battista T, Potestio L, Annunziata MC, Marasca C, Villani A, Fabbrocini G
Clinical, Cosmetic and Investigational Dermatology 2023, 16:135-148
Published Date: 19 January 2023
Efficacy and Safety of Secukinumab in Elderly Patients with Moderate to Severe Plaque-Type Psoriasis: Post-Hoc Analysis of the SUPREME Study
Talamonti M, Russo F, Malara G, Hansel K, Papini M, Cattaneo A, Parodi A, Chiricozzi A, Malagoli P, Bardazzi F, Brazzelli V, Dapavo P, Gisondi P, Zane C, Potenza C, Cantoresi F, Fargnoli MC, Trevisini S, Brianti P, Pescitelli L, Gigante G, Bartezaghi M, Caputo L, Aloisi E, Costanzo A
Clinical, Cosmetic and Investigational Dermatology 2023, 16:847-852
Published Date: 1 April 2023