Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Interleukin-17A Inhibitor Secukinumab Treatment in HIV-Positive Psoriasis Patient: A Case Report
Authors Gong J , Wu W , Qiu L, Wang X, Bao J, Wang J, Cheng L, Fu Z, Hu F
Received 10 November 2022
Accepted for publication 23 December 2022
Published 30 December 2022 Volume 2022:15 Pages 2949—2956
DOI https://doi.org/10.2147/CCID.S395348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Jian Gong,1,2,* Weiwei Wu,3,* Liguo Qiu,1,2 Xi Wang,1,2 Jianwei Bao,1,2 Jinjing Wang,4 Lifang Cheng,1,2 Zhiyuan Fu,1,5 Fengming Hu1,2
1Department of Integrated Traditional Chinese and Western Medicine of Dermatology, Dermatology Hospital of Jiangxi Province, Nanchang, Jiangxi, People’s Republic of China; 2Jiangxi Provincial Clinical Research Center for Skin Diseases, Nanchang, Jiangxi, People’s Republic of China; 3Department of Plastic and Dermatological Surgery, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 4Clinical School of Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi, People’s Republic of China; 5Department of Pharmacy, Dermatology Hospital of Jiangxi Province, Nanchang, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiyuan Fu, Department of Pharmacy, Dermatology Hospital of Jiangxi Province, Nanchang, Jiangxi, People’s Republic of China, Tel +86 13970827820, Fax +86-0791-85207512, Email [email protected] Fengming Hu, Department of Integrated Traditional Chinese and Western Medicine of Dermatology, Dermatology Hospital of Jiangxi Province, Nanchang, Jiangxi, People’s Republic of China, Tel +86 13970935828, Fax +86-0791-85207512, Email [email protected]
Abstract: Psoriasis is an immune-mediated chronic inflammatory dermatosis influenced by hereditary and environmental factors. Human immunodeficiency virus (HIV) infection affects the immune system and exacerbates psoriatic lesions. We report the case of a 33-year-old male patient diagnosed with psoriasis vulgaris, psoriatic arthritis and HIV infection. Acitretin capsules, etanercept and high-active antiretroviral therapy (HAART) were effective. Two months after etanercept was discontinued, his condition worsened. After switching to secukinumab combined with HAART, the symptoms of psoriatic arthritis resolved rapidly after four weeks, with a Disease Activity Index for Psoriatic Arthritis score of 0. The time to achieve psoriasis area and severity index 40, 75, 90, and 100 were 2, 4, 8, and 29 weeks. The treatment was maintained for 1 year with no adverse reactions. Regarding the stable CD4+ T lymphocyte count and the viral load, administering anti-IL-17 monoclonal antibodies is an effective treatment option for psoriasis patients.
Keywords: psoriasis, HIV, etanercept, secukinumab, biologics
Introduction
Psoriasis is an immune-mediated chronic inflammatory dermatosis influenced by the combined effects of hereditary and environmental factors, affecting 2–3% of the global population.1 Due to the high number of cases, patients with psoriasis often have other comorbidities, some of which greatly impact the disease condition, treatment and prognosis. Human immunodeficiency virus (HIV), a retrovirus, attacks and destroys the human immune system gradually, which causes the human immune system defect and leads to more severe or atypical psoriasis. The incidence of HIV-positive psoriasis has gradually increased. Statistically, by the end of 2017, there were 36.9 million HIV/AIDS patients worldwide.2 Studies have shown that the prevalence of psoriasis in people living with HIV (PLWH) ranges from 4% to 6%, is higher than the general population, which is 2–3%.3–5 In PLWH, psoriasis onset often companies with a poor prognosis. Without treatment, the average life expectancy of patients ranges from 4 to 24 months after diagnosis.6 Therefore, identifying safe and effective treatment options for patients with HIV-positive psoriasis is crucial.
Biologics have recently been widely used for treating moderate-to-severe plaque psoriasis and psoriatic arthritis. There are few case reports of biological therapy in HIV-positive psoriasis patients, at the same time, no data support from studies with larger sample sizes. Here, we report the management of an HIV-positive male psoriasis patient treated with the anti-TNF-α monoclonal antibody etanercept followed by the interleukin IL-17A monoclonal antibody secukinumab.
Case Presentation
The patient was a 31-year-old homosexual Chinese male with a 6-year history of multiple erythematous scaly plaques. Since June 2016, he has developed erythema, papules, plaques, and scales of various sizes throughout his body. Four months later, the rash increased rapidly, diffusing erythema all over the body, accompanied by a fever. He does not have a regular homosexual partners (regular homosexual partners), with some breaching the safe sex measures. The patient visited with the history of type 2 diabetes and penicillin allergy but no family history of psoriasis. A typical psoriatic rash covered over 90% of his body surface area, and he was admitted to the hospital with “erythrodermic psoriasis”. Erythrodermic psoriasis was diagnosed in the context of the pathological examination and clinical features during hospitalization. The pathological manifestations indicated hyperkeratosis, confluent parakeratosis, Munro microabscesses, acanthosis hypertrophy, regular elongation of the rete ridges, papillary dermal edema, mildly dilated blood vessels, scattered perivascular lymphocytes, and neutrophils infiltrated around the upper epidermis and superficial dermis (Figure 1), which are typical characteristics of erythrodermic psoriasis. Simultaneously, the patient tested positive for the human immunodeficiency virus (HIV) antibody. The CD4+ T lymphocyte count was 28 cells/μL, and the HIV viral load was 5.05 × 104 IU/mL. The diagnosis was “HIV infection”. The psoriasis was treated successively for 3 weeks with acitretin capsules, total glucosides of peony capsules, compound glycyrrhizin tablets, topical glucocorticoid cream, and moisturizers, and the condition was controlled. Oral tenofovir 200 mg once a day, 100 mg of oral lamivudine once a day, and 50 mg of oral lopinavir and ritonavir tablets twice a day were administered for continuous anti-HIV treatment, and the laboratory indicators were reviewed every six months. The re-examination results on December 11, 2019, showed that the CD4+ T cell count was greater than 200 cells/μL and the viral load was less than 100 IU/mL. During the period from November 2016 to March 2020, the patient took acitretin capsules orally for a long time for maintenance treatment. With the prolongation of the use time of acitretin A capsule, its efficacy gradually declined. The recurrence of psoriasis increased.
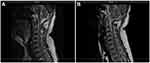
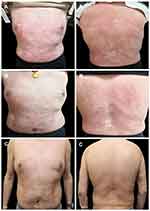
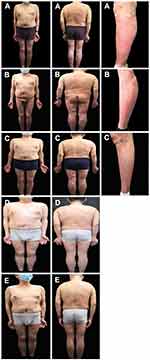
In March 2020, the psoriasis rash recurred and aggravated, accompanied by noticeable pain in the cervical spine and sacroiliac joints, with unfavorable flexion, extension and rotation. On April 8, 2020, the results of the spine and hip joint MRI performed in another hospital (Figure 2A) suggested abnormal changes in the bilateral sacroiliac and hip joints, slight swelling of the surrounding soft tissue, and right external obturator muscle swelling. Sacroiliitis was considered. On May 5, 2020, he was admitted to our hospital again and diagnosed with psoriatic arthritis (involvement of axial joints) and psoriasis vulgaris (Figure 2B). Laboratory data revealed an CD4+ T lymphocyte number of 278 cells/μL, HIV viral load was less than 100 IU/mL, the eosinophil number of 0×109/L↓, the red blood cell number of 2.66×1012/L↓, the level of hemoglobin was 101 g/l↓, the C-reactive protein level was 33.91 mg/l↑, the erythrocyte sedimentation rate was 138.00 mm/h↑, with negative serologic examination for hepatitis A virus, hepatitis B virus, hepatitis C virus, and syphilis. The remaining hematological, biochemical investigations and urine analysis were in the normal ranges. Pre-treatment screening was negative for tuberculosis, including chest X-rays, an interferon-gamma release assay, whereas the Echocardiography (ECG) showed sinus arrhythmia. The psoriasis area and severity index (PASI) score of the skin lesion was 14.4 (Figure 3A), and the Disease Activity Index for Psoriatic Arthritis (DAPSA) score was 52. On May 7, 2020, after the patient was fully informed and signed the informed consent form, subcutaneous injections of 25 mg of etanercept twice weekly combined with 20 mg of acitretin capsules per day were administered. After three weeks, erythema and scales were significantly ameliorated (Figure 3B). After four weeks, the joint symptoms had almost subsided, and the DAPSA score was 0. Acitretin capsules were discontinued, and subcutaneous injection of 25 mg etanercept twice weekly was continued. The rash largely resolved after 21 weeks (Figure 3C), and the PASI score was 1.4. Subsequently, the etanercept dose was modified to a subcutaneous injection of 25 mg once weekly for eight weeks and once every two weeks for another eight weeks. The patient spontaneously discontinued etanercept treatment in February 2021. After 2 months, the rash recurred and worsened (Figure 4A). The PASI score was 20.2, and mild pain was observed in the cervical spine and spinal joints. The DAPSA score was 34, CD4+ lymphocyte count was 353 cells/μL, and HIV viral load was less than 100 IU/mL. On May 31, 2021, after repeatedly excluding any abnormalities in the examinations for the administration of biologics, secukinumab 300 mg was prescribed for treatment in the 0th, 1st, 2nd, 3rd, and 4th weeks in the context of anti-HIV treatment. Subsequently, it was administered at a dose of 300 mg every four weeks. After four weeks, the patient’s joint symptoms completely disappeared, and the DAPSA score was 0. The times to achieve PASI 40, PASI 75, PASI 90, and PASI 100 were 2 weeks (Figure 4B), 4 weeks, 8 weeks (Figure 4C), and 29 weeks (Figure 4D), respectively. At present, secukinumab has been maintained for 1 year, and the clinical symptoms of psoriasis have stabilized (Figure 4E and the specific treatment timeline is shown in Figure 5). The CD4+ T lymphocyte count was 327 cells/μL, and the HIV viral load was less than 100 IU/mL. The results of the spine and hip joint MRI on April 25, 2022, indicated straightened physiological curvature of the cervical spine compared with the MRI results on April 8, 2020, before the use of biologics, with no abnormalities in height and signal of the vertebral body and less severe bilateral sacroiliac joint inflammation.
 |
Figure 5 Treatment timeline. |
The patient provided written informed permission to have any accompanying photos and case details published. The Hospital Ethics Committees of Dermatology Hospital of Jiangxi Province approved to publish the case details.
Discussion
In 1985, Johnson et al7 first reported HIV-infected patients with psoriasis and found that this infection may lead to the onset of psoriasis or aggravate the original disease condition. In our case, psoriasis might be the initial dermatological manifestation of HIV. Four months after the onset of rash, the disease exhibited rapid progression, with the development of psoriatic erythroderma, implying that HIV infection can further aggravate the symptoms of psoriasis. The pathogenesis of psoriasis is related to immunity and is accompanied by excessive activation of CD4+ T lymphocytes and upregulation of IL-17 and IL-23 secretion.6 A large decrease in CD4+ T lymphocytes and a large increase in the number of CD8+ T lymphocytes in PLWH patients often result in a decrease in the ratio of CD4+/CD8+ and an imbalance in the differentiation of T lymphocyte subsets, which leading to the (which lead to the) immunosuppression of the body.8 The immune status of the two diseases does not explain the phenomenon of HIV infection that causes the onset of psoriasis or exacerbates the original condition. However, the imbalance of immune cells and cytokines accompanying HIV infection, such as the imbalance of inflammatory factors related to the Th1/Th2 cell axis, may induce an outbreak of existing psoriatic skin lesions or the emergence of HIV-related psoriasis.9,10
In PLWH with moderate-to-severe psoriasis (BSA >10%), especially those with CD4+ T lymphocyte count of fewer than 350 cells/mm,3 topical medication combined with high-active antiretroviral therapy (HAART) is recommended.11 HAART can reduce HIV viral load. In contrast, psoriasis symptoms may be alleviated by inhibiting HIV stimulation of keratinocyte proliferation by interfering with HIV DNA synthesis.12 When HAART is ineffective, retinoids are used as second-line therapy, although theoretically, higher doses may be required to control the disease. Third-line treatment options may be adopted in PLWH with severe or difficult-to-control psoriasis, such as methotrexate, cyclosporine A, biological agents (TNF-α antagonists, IL-12/23, and IL-17 inhibitors), and other treatments. Currently, biologics are widely used in treating patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. Due to the inhibitory effect of biological agents on the body’s immunity, the risk of infection in the body is significantly increased, which is the main factor that limits the use of biological agents in HIV patients with psoriasis. According to the British Association of Dermatologists (BAD) guidelines, biologics can be used cautiously in HIV patients with moderate-to-severe psoriasis if HIV is adequately treated and monitored.13 In recent years, multiple case reports have demonstrated the safety of TNF-α antagonists in treating patients with HIV without increasing morbidity and mortality.14–16 TNF-α increases HIV expression via nuclear factor (NF)-κB. Therefore, when the TNF-α level is elevated, HIV viral load is increased, and the CD4+ T lymphocyte count is decreased, leading to HIV progression.6 Therefore, TNF-α antagonists, such as etanercept and infliximab, may help control HIV infection while stabilizing psoriatic skin lesions. However, some researchers suggest that TNF-α antagonists should only be administered to HIV-infected patients with psoriasis with a CD4+ T lymphocyte count of more than 200/μL to ensure patient safety.17 The American Academy of Dermatology and National Psoriasis Foundation (AAD-NPF) guidelines recommend that anti-IL-17 monoclonal antibodies can be used in HIV patients who have been receiving antiretroviral therapy and have a well-controlled viral load.18 There are also several case reports of successful treatment of HIV patients with psoriasis or psoriatic arthritis with secukinumab or ixekizumab. This suggests that anti-IL-17 monoclonal antibodies may serve as an effective treatment for patients with HIV psoriasis.19–22
In this case, we used acitretin capsules combined with HAART therapy in the early stage, effectively controlling the disease. The treatment regimen gradually failed to stabilize the rash over the time, and severe articular symptoms developed. Considering the limitation of applying immunosuppressive agents in HIV patients and the significant effectiveness of TNF-α antagonists in psoriatic arthropathy, we added etanercept to the HAART regimen. The joint pain symptoms almost subsided after approximately one month of medication, but the rash was not significantly ameliorated until after approximately five months. However, two months after discontinuation of etanercept, the rash recurred and was further aggravated, and mild pain occurred in some joints. This time, we used secukinumab combined with HAART regimen. His joint symptoms resolved rapidly after 4 weeks, and the rash diminished completely after 29 weeks. In this case, the patient was treated with etanercept and secukinumab for two years. During the treatment period, the patient’s CD4+ T lymphocyte count and viral load remained favorable, without biologics-related complications. However, previous studies have reported multiple infections with etanercept treatment and gastrointestinal Candida infections with secukinumab treatment,20,23 indicating that the use of biologics in HIV-associated psoriasis increases the risk of systemic bacterial or opportunistic fungal infections. Therefore, tuberculosis, hepatitis B and other opportunistic infections should be excluded before the drug administration.
Conclusion
Data on psoriasis complicated by HIV infection are mainly derived from case reports and small-scale, retrospective studies. The safety and efficacy of each treatment regimen remain to be confirmed. In moderate-to-severe refractory psoriasis with HIV infection and poor efficacy of HAART therapy and retinoic acid drugs, Secukinumab combined with HAART can be used as an appropriate treatment option when the HIV infection status of the patient is stable (CD4+ T lymphocyte count >200/mm3, stable viral load); however, strict monitoring of adverse reactions, CD4+ T lymphocyte count and viral load are required.
Ethics Statement
The publications of images were included with the patient’s consent.
Consent Statement
Informed consent was provided by the patient for publication of the case.
Acknowledgments
The authors would like to thank the patients recruited for participation in this study. Gong Jian and Wu Weiwei are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Construction Project of Hainan Province Clinical Medical Center, general projects of science and technology plan of Jiangxi Province Administration of Traditional Chinese Medicine (NO.2021B108) and Jiangxi Provincial Clinical Research Center for Skin Diseases (NO.20212BCG 74003).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Daniyal M, Akram M, Zainab R, et al. Progress and prospects in the management of psoriasis and developments in phyto-therapeutic modalities. Dermatol Ther. 2019;32:e12866. doi:10.1111/dth.12866
2. World Health Organization. WHO HIV update: global epidemic and progress in scale up and policy uptake [EB/OL]; [about 1 screen]. Geneva: World Health Organization; 2022. Available from: http://www.who.int/hiv/data/en/.
3. Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDs. 2000;14:239–246. doi:10.1089/108729100317696
4. Muñoz-Pérez MA, Rodriguez-Pichardo A, Camacho F. Dermatological findings correlated with CD4 lymphocyte counts in a prospective 3-year study of 1161 patients with human immunodeficiency virus disease predominantly acquired through intravenous drug abuse. Br J Dermatol. 1998;139:33–39. doi:10.1046/j.1365-2133.1998.02310.x
5. Yen YF, Jen IA, Chen M, et al. HIV infection increases the risk of incident psoriasis: a nationwide population-based cohort study in Taiwan. J Acquir Immune Defic Syndr. 2017;75:493–499. doi:10.1097/QAI.0000000000001431
6. Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis. 2010;10:470–478. doi:10.1016/S1473-3099(10)70101-8
7. Johnson TM, Duvic M, Rapini RP, Rios A. AIDS exacerbates psoriasis. N Engl J Med. 1985;313:1415.
8. Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV-associated psoriasis: epidemiology, pathogenesis, and management. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806
9. Graziosi C, Pantaleo G, Gantt KR, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi:10.1126/science.8023143
10. Clerici M, Shearer GM. The Th1–Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi:10.1016/0167-5699(94)90220-8
11. Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291–299. doi:10.1016/j.jaad.2009.03.047
12. Ochi SI, Hayashi M, Yamaoka T, et al. Occult HIV infection in Japanese rupioid psoriasis. J Dermatol. 2017;44:e172–e173. doi:10.1111/1346-8138.13850
13. Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association Of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628–636. doi:10.1111/bjd.15665
14. Fink D, Hedley L, Miller R. Systematic review of the efficacy and safety of biological therapy for inflammatory conditions in HIV-infected individuals. Int J STD AIDS. 2017;28:110. doi:10.1177/0956462416675109
15. Ting PT, Koo JY. Use of etanercept in human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) patients. Int J Dermatol. 2006;45:689–692. doi:10.1111/j.1365-4632.2005.02642.x
16. Myers B, Thibodeaux Q, Reddy V, et al. Biologic treatment of 4 HIV-positive patients: a case series and literature review. J Psoriasis Psoriatic Arthritis. 2020;6:19–26. doi:10.1177/2475530320954279
17. Cepeda EJ, Williams FM, Ishimori ML, Weisman MH, Reveille JD. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710–712. doi:10.1136/ard.2007.081513
18. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–1072. doi:10.1016/j.jaad.2018.11.057
19. Pangilinan MCG, Sermswan P, Asawanonda P. Use of anti-IL-17 monoclonal antibodies in HIV patients with erythrodermic psoriasis. Case Rep Dermatol. 2020;12:132–137. doi:10.1159/000508781
20. Vito DL, Casanova DM, Elisa G. Secukinumab in an HIV-positive patient with psoriasis. J Dtsch Dermatol Ges. 2019;17:646–668.
21. Bernardini N, Skroza N, Tolino E, et al. HIV positive patient treated with ixekizumab. Clin Ter. 2022;173:195–197. doi:10.7417/CT.2022.2416
22. Qin H, Lu J, Yi X, Ding Y, Shi Y. Secukinumab treatment for a psoriasis patient co-infected with HIV and latent tuberculosis: a case report. J Dermatol. 2022. doi:10.1111/1346-8138.16494
23. Nakamura M, Abrouk M, Farahnik B, Zhu TH, Bhutani T. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101(1):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.