Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Adalimumab, Ustekinumab, and Secukinumab in the Management of Hidradenitis Suppurativa: A Review of the Real-Life Experience
Authors Martora F , Megna M, Battista T, Potestio L , Annunziata MC, Marasca C, Villani A, Fabbrocini G
Received 17 November 2022
Accepted for publication 12 January 2023
Published 19 January 2023 Volume 2023:16 Pages 135—148
DOI https://doi.org/10.2147/CCID.S391356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Fabrizio Martora, Matteo Megna,* Teresa Battista,* Luca Potestio,* Maria Carmela Annunziata, Claudio Marasca, Alessia Villani, Gabriella Fabbrocini
Section of Dermatology - Department of Clinical Medicine and Surgery, University of Naples Federico II, Napoli, Italy
*These authors contributed equally to this work
Correspondence: Fabrizio Martora, Section of Dermatology - Department of Clinical Medicine and Surgery, University of Naples Federico II, Via Pansini 5, Napoli, 80131, Italy, Tel +39 - 081 – 7462457, Fax +39 - 081 – 7462442, Email [email protected]
Abstract: To date, adalimumab (ADA) is the only biotechnology drug approved for the management of hidradenitis suppurativa (HS), an inflammatory skin condition. However, it quickly became apparent that the efficacy of adalimumab in daily practice was highly variable. In our review, we highlighted the current evidence from literature on the use of biologics in HS in a real-life setting, particularly adalimumab, secukinumab and ustekinumab. Data on the effectiveness and safety of biologic drugs in HS management have been analyzed. Even if the results are promising, more studies are needed. In our opinion, the armamentarium of drugs for HS management is increasing, and treatment will be based on a tailored-tail approach, minimizing the risk of adverse events. In this context, we want to point out the reported effectiveness and safety data concerning adalimumab, ustekinumab and secukinumab as well as ixekizumab.
Keywords: hidradenitis suppurativa, adalimumab, ustekinumab, secukinumab, ixekizumab, guselkumab, real life evidence
Introduction
Hidradenitis Suppurativa (HS) is a chronic, relapsing inflammatory skin condition.1–3 It is characterized by the occurrence of nodules, often inflammatory, deep and painful, especially at skin regions with a high density of apocrine sweat glands such as the axillary, inguinal, and sub mammary regions.1
As regards HS pathogenesis, HS is a multifactorial disease in which genetic and environmental factors play a key role.4,5 The primary defect in HS pathophysiology involves follicular occlusion of the folliculopilosebaceous unit, followed by follicular rupture, and immune responses (perifollicular lympho-histiocytic inflammation), finally leading to the development of clinical HS lesions.4,5 Moreover, cutaneous microbiota seems to be involved in HS pathogenesis.6
HS is associated with several other pathological conditions. These include Obesity, Metabolic Syndrome, and autoimmune disorders such as Crohn’s Disease.7–10 Patients suffer as much physically as psychologically, with worsening quality of life and a decline in work productivity.7–10
To date, adalimumab (ADA) is the only biotechnology drug approved for the condition so registration in 2015 was a major step forward in the treatment of HS. However, it quickly became apparent that the efficacy of adalimumab in daily practice was highly variable.11–13
In recent years, studies have been directed toward new therapeutic targets for this condition; bimekizumab and secukinumab drugs are still being studied and would appear to show promising results.11,12
Several real-life studies on the use of ADAs in routine clinical practice have been published in the literature.14–16 Indeed, real-life data are mandatory to improve decision-making in the field of dermatology, as these studies also include patients typically excluded from clinical trials, such as subjects suffering from various comorbidities, subjects on polytherapies and multi-failure patients.17
In this review, we conducted a comprehensive literature review of real-life data on adalimumab, ustekinumab, and secukinumab; and a brief report of miscellaneous indicating small experiences with biologic drugs.
Methods
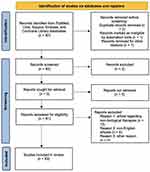
A comprehensive review of the English-language medical literature was performed using the PubMed, Ovid, Scopus, Embase, and Cochrane Library databases from their inception to October 1, 2022, using Medical Subject Headings (mesh) (if applicable) and medical terms for the concepts of adalimumab, ustekinumab, and secukinumab use in a real-life setting. Studies were identified, screened and extracted for relevant data following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1). The search strategy to identify articles was performed using the following search terms: “IL-17 inhibitors”, “adalimumab”, “secukinumab”, “ustekinumab”, “ixekizumab”, “guselkumab” AND “real life”, AND “real world evidence”, AND “hidradenitis suppurativa”, and combinations thereof. The search involved all fields, including title, abstract, keywords, and full text. Clinical and epidemiological studies, reviews, and systematic reviews on the use of adalimumab, secukinumab, ustekinumab, and other biologic drugs in a real-world setting were included. Articles published from the first papers published until October 2022 and from any source were considered. Non-English literature was excluded. The article is based on previously conducted studies.
Adalimumab
Adalimumab is an anti-TNF-α IgG1 monoclonal antibody, the only drug currently approved by the FDA in 2015 for hidradenitis suppurativa (HS), administration includes as initial dose 160 mg subcutaneously, followed by an 80 mg dose 2 weeks later and a maintenance dose of 40 mg weekly thereafter.18
A Phase II study by Kimball et al showed for the first time that a significantly greater proportion of patients receiving adalimumab weekly (17.6%) than placebo (3.9%) achieved the primary clinical endpoint of an HS-Physician’s Global Assessment (PGA) score with at least a two-grade improvement over baseline scores at week 16. This finding was not significantly confirmed in the group of patients who received adalimumab every other week (9.6%) (weekly vs placebo difference 13.7%, p = 0.025; every other week vs placebo difference 5.6%, p = 0.25).19
The subsequent Phase III PIONEER I and II studies involved a total of 633 patients with moderate to severe HS with inadequate response to oral antibiotics. A significantly higher percentage of patients administered adalimumab achieved a clinical response to suppurative hidradenitis (HiSCR), defined as a ≥50% reduction in abscess and inflammatory nodule counts, with no increase in abscess and draining fistula counts compared to baseline, compared to patients given placebo after 12 weeks of treatment (PIONEER I: 41.8% vs 26.0%, p = 2 weeks). Eight percent vs 26.0%, p = 0.003; PIONEER II: 58.9% vs 27.6%, p < 0.001).14 Secondary efficacy data also showed a higher percentage of subjects achieving ≥30% reduction in Patient’s Global Assessment of Skin Pain (PGA-SP) in both PIONEER I (adalimumab vs placebo [24.9%]; OR = 2.03, p = 0.004) and PIONEER II (adalimumab [61.2%] vs placebo [24.8%]; OR = 4.78, p < 0.001).14,20
An open-label extension study of the PIONEER I and II trials also confirmed previous data-in fact, patients who continued to receive adalimumab weekly had a HiSCR of 52.3% at week 168 and a decrease in Dermatology Life Quality Index (DLQI) of 5.1–6.8 points at week 72, with no new safety risks.21
Ryan et al analyzed safety data of adalimumab in HS, no new safety issues were identified with weekly dosing of adalimumab compared with dosing every other week.22
In 2021, Marzano et al conducted a multicenter real-life study to evaluate the impact of different clinical parameters on the clinical response to adalimumab in a large cohort of patients with moderate-severe HS at week 16 and week 52 after the initiation of adalimumab.23 In this multicenter retrospective real-life study, 21 Italian dermatology units were involved and provided demographic and clinical data of patients with moderate-severe HS being treated with adalimumab from January 2016 to December 2018. Data included gender, age at HS onset (≤30, >30–50, >50 years), age at diagnosis (≤29, >29 years), age at adalimumab initiation (≤30, >30–50, >50 years), body mass index (BMI; ≤25, >25–30, >30 kg m-2), smoking (never smoked, current smoker, former smoker), family history of HS, comorbidities, previous and concomitant treatments with adalimumab, and HS phenotypes according to van der Zee and Jemec classification.23
Adalimumab response rate was measured at week 16 and week 52 by HiSCR.23
Bettoli et al have previously stated that HS duration and diagnostic delay have a negative impact on disease severity.24 It has been suggested that early treatment with adalimumab positively affects the clinical response to the drug (window of opportunity).25
The group of 389 patients with moderate to severe HS treated with adalimumab analyzed by Marzano et al showed a significant inverse correlation between treatment delay and clinical response to the drug at week 16 of treatment 17. This finding confirms that early use of adalimumab in HS increases response to therapy and therefore provides evidence of a “window of opportunity” in this disease.24,25
Regarding ultrasound, it can be said that it is useful for diagnosis and treatment monitoring in patients with HS, in this regard Chiricozzi et al20 conducted a study to evaluate clinical response to adalimumab using ultrasound findings. The study included a total of 41 HS patients on treatment who were treated with adalimumab for a mean period of 50.8 ± 32.2 weeks; range 6–108 weeks).14 Clinical improvement was observed during adalimumab therapy, with progressively more patients achieving a HiSCR50 response (36.4% at week 52).26
HARMONY 21 is a multicenter, postmarketing observational study conducted in adult patients with moderate to severe HS. Disease severity and QoL parameters were assessed using validated measures at 12-week intervals for 52 weeks of treatment. The primary endpoint was the percentage of patients achieving HiSCR at 12 weeks.27 Secondary endpoints were HiSCR at 24, 36 and 52 weeks and changes in QoL parameters and work productivity ratings. Treatment of moderate to severe HS with adalimumab resulted in a reduction in disease severity and improvements in QoL and productivity.20 The response to adalimumab was rapid (within 12 weeks) and durable (52 weeks). No unexpected safety signals were reported.27
In the study conducted by Gulliver et al28 in 23 Canadian centers, 138 adults with moderate to severe HS who required modification of current therapy were treated with adalimumab for up to 52 weeks according to physician practice. Patient-reported outcome measures (PROMs) were obtained at baseline, weeks 24 and 52 to measure overall HRQoL, HS severity, anxiety and depression levels, HS impact and symptoms, work productivity, and activity impairment.28
From baseline to weeks 24 and 52, all overall PRO scores improved significantly (P ≤ 0.0023). The number of patients reporting “good disease control” and “complete disease control” increased from 9.7% to 66.4% over the 52 weeks.22 At week 24 and maintained at week 52 in a real-world setting, adalimumab significantly improved HRQoL, work productivity, and activity impairment in patients with moderate-to-severe HS.28
Muralidharan et al conducted a retrospective cross-sectional study to determine the effects of adalimumab on HS-PGA and DLQI scores in patients with HS who had been treated for at least 6 months.29 Approximately 77% (n = 78/101) of patients demonstrated improvements in HS-PGA scores. Significant improvements were also demonstrated in the DLQI scores of the patient cohort (P = 0.0001, 95% CI −12.8 to −5.9). A total of 31.7% (32/101) of patients experienced adverse effects (including fungal rash, worsening liver function tests, and depression) affecting multiple organ systems, with 27.7% (28/101) requiring discontinuation of treatment.29 Adalimumab was effective in reducing HS-PGA and DLQI scores.29
A retrospective observational study was conducted in 19 patients with clinically evident moderate to severe HS who were treated with adalimumab for at least 24 weeks.24 HS-PGA, modified Sartorius scale, and DLQI scores at baseline, week 4, week 12, and week 24 were retrieved from medical records. Both the modified Sartorius score and DLQI decreased significantly during the weeks of assessment (P < 0.001).30 The percentage of patients achieving a clinical response was 10.5% (n = 2) at week 4, 42.1% (n = 8) at week 12, and 63.2% (n = 12) at week 24. Treatment with adalimumab was associated with both clinical remission of HS and improvement in patients’ quality of life.30
Roccuzzo et al31 recently presented a retrospective real-world study designed to evaluate the safety and efficacy of switching from adalimumab originator to biosimilars in 37 patients, evaluated for 12 months in terms of IHS4 (International Hidradenitis Suppurativa Severity Score System) and HiSCR. Overall, no significant differences emerged between originator and biosimilar adalimumab in terms of clinical response following nonmedical switch.31
Another real-life study is this trial (ClinicalTrials.gov: NCT03894956) where the authors evaluated the safety and efficacy of adalimumab in routine clinical practice in Japan from March 2019 to May 2021.26 The primary endpoint was safety.32 Secondary endpoints assessed efficacy, reporting HiSCR and DLQI data. Eighty-three patients were enrolled. At week 12, 57.4% of patients achieved HiSCR and significant reductions from baseline in DLQI were observed (p < 0.0001).32
The treatment was well tolerated with no adverse events reported.32
Neves et al26 conducted a retrospective study in Lisbon to analyze HS patients treated with adalimumab between 2016 and 2019. Epidemiological, clinical, and therapeutic information was collected. HS activity and adalimumab response were monitored at baseline and at weeks 16 (W16), 24 (W24), and 52 (W52) 0.27 At W16, HISCR was achieved in 27 patients (75%). The iHS4 mean was reduced from 16.7 to 7.2 (p < 0.001). At W24, the mean iHS4 was 4.7 (p < 0.001) and HISCR was still achieved in 76.7% (n = 23/30) of patients. Between baseline and W52, DLQI and VAS pain increased from a mean value of 15.4 to 10.5 (p = 0.001) and 4.4 to 1.8 (p < 0.001), respectively. The results showed a greater response to adalimumab in patients with Hurley II compared to Hurley III. Also, in this study, the diagnostic delay was determined in fact these data confirm the hypothesis of the “window of opportunity”.25
This study showed superiority in terms of HISCR results at W12/16, W24 and W52 compared to the PIONEER I and II clinical studies14 and the multicenter study Marzano et al.23 However, the authors conclude that these improved results could be associated with the use of adjuvant intralesional, and systemic therapies taken by patients during treatment.33
Another hypothesis put forward was that the flare-up outbreak could be a possible predictor of response to adalimumab.34
Caposiena et al showed a positive correlation between relapse of exacerbation and non-response to adalimumab (P < 0.001).34 Among the identified causes associated with poor response to therapy was the onset of an exacerbation before week 12 which showed the highest risk of no response (P < 0.001).34
Nazzaro et al conducted a 12-week prospective study. There were 32 patients enrolled, two main parameters were evaluated at baseline and week 12: International Hidradenitis Suppurativa Severity Score System score (IHS4) and ultrasound (according to US HS-PGA)/Color Doppler. Regarding IHS4 the authors conclude by saying that it was 22.4 at baseline while at week 12 it had dropped to 14.7. Regarding IHS4 the authors conclude by saying that it was 22.4 at baseline while at week 12 it had dropped to 14.7.35 There were 78 (81.3%) intensely vascular lesions at baseline while at week 12 there were 25 (26.04%), which confirms the importance of ultrasound as a parameter for evaluating adalimumab therapy.35
In clinical cases, adalimumab has also been shown to be effective in treating HS.36–39 There are also cases of HS associated with ankylosing spondylitis, Crohn’s disease, pyoderma gangrenous, acne and psoriatic arthritis successfully treated with adalimumab.40–46 Main articles regarding the use of adalimumab for the management of hidradenitis suppurativa in real-life setting are reported in Table 1.
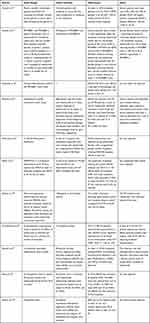 |
Table 1 The Use of Adalimumab for the Management of Hidradenitis Suppurativa in Real-Life Setting |
Ustekinumab
Ustekinumab is a human monoclonal antibody generally used to treat the following conditions: moderate to severe plaque psoriasis, psoriatic arthritis, moderate to severe Crohn’s disease or moderate to severe ulcerative colitis (inflammatory bowel disease).47 Ustekinumab is an interleukin-12 (IL12) and interleukin-23 (IL23) antagonist. FDA approval is indicated for use in moderate to severe plaque psoriasis (Ps) for patients 6 years of age and older.48,49 Main articles regarding the use of ustekinumab for the management of hidradenitis suppurativa in real-life setting are reported in Table 2.
 |
Table 2 The Use of Ustekinumab for the Management of Hidradenitis Suppurativa in Real-Life Setting |
To date ustekinumab has not received FDA approval for many other inflammatory-mediated diseases, however, there are studies and case reports in the literature where the drug is used off label for the treatment of hidradenitis suppurativa.50
Hollywood et al have conducted a retrospective review of patients treated with ustekinumab,51 sixteen patients received ustekinumab between January 2017 through September 2020, all the patients had failed the first lines treatments according HS guidelines, subjective clinical improvement was found in 9 patients (56%), the parameters that were used being reduction in the number of flares and improvement in quality of life. Four patients (25%) showed no improvement and the remaining 3 (19%) had good disease control.51
T Montero-Vichez et al have evaluated the therapeutic outcomes of 10 patients with HS treated with ustekinumab.52 All patients had moderate to severe HS (Hurley stage II–III) and had failed to respond to previous treatment. Disease severity was evaluated according to the physician’s global assessment (HS-PGA) and pain was assessed with the Numerical Pain Rating Scale (NPRS). The average duration of treatment was 48 (32–167) weeks.
A one-point reduction in HS-PGA was appreciated in seven (70%) patients and a ≥2-point reduction in NPRS in eight (80%) patients. In patients who did not respond to therapy, no worsening of HS severity or symptoms was observed.52
We reviewed all available literature on real-life studies with ustekinumab; in total, between case reports and case series there were 49 patients treated.53–62
Out of a total of 49 patients, the most encountered comorbidities were: 33 (67%) smokers or former smokers, 11 (22%) were obese, and 8 (16%) had additional components of the follicular occlusion tetrad: pilonidal sinus (n = 2) and acne conglobata (n = 6). Nine (18%) patients also had Crohn’s disease.53–62
Among the 49 patients, 32 (65%) had previously undergone biologic treatment; 27 (55%) had been treated with adalimumab and 14 (28%) with infliximab, while 17 (35%) had not received any biologic drug treatment prior to ustekinumab administration.53–62
The average duration of treatment was 40 (16–44) weeks. The instruments used to assess disease severity and treatment outcomes were different for the different cases.
Clinical improvement was measured using the International Hidradenitis Suppurativa Severity Score System (IHS4), the HS-PGA, the Hidradenitis Suppurativa Clinical Response (HiSCR) or the modified Sartorius Scale (mSS).63 Symptomatic improvement was assessed using the Visual Analog Scale (VAS) and the Dermatologic Quality of Life Index (DLQI). Of a total of 49 patients, a reduction in IHS4, HS-PGA, HiSCR or mSS was observed in 38 (78%). Of a total of 49 patients, a reduction in VAS or DLQI was observed in 42 (86%).
The real limitation of all reported real-life cases is the sampling number, which is very low to be able to establish the efficacy and safety of the drug.
Secukinumab
Secukinumab is a biological agent that acts selectively on interleukin 17 (IL-17).64 To date, several clinical studies confirm its efficacy in the management of plaque psoriasis and psoriatic arthritis and ankylosing spondylitis.64
FDA approved the drug for the following indications: moderate to severe psoriasis, hypertrophic palmoplantar psoriasis, generalized pustular psoriasis, psoriatic arthritis and ankylosing spondylitis.65 It has been used off-label for rheumatoid arthritis and hidradenitis suppurativa.66 Main articles regarding the use of secukinumab for the management of hidradenitis suppurativa in real-life setting are reported in Table 3.
 |
Table 3 The Use of Secukinumab for the Management of Hidradenitis Suppurativa in Real-Life Setting |
There is a lack of real-life data on HS or at least very few are available.
An open-label exploratory study evaluated the efficacy of secukinumab 300 mg per week for five weeks and then every four weeks thereafter in nine patients with moderate to severe HS (Hurley stage II or III).67 The authors conclude that six patients on nine (67%) achieved an improvement of at least 50% in total inflammatory nodule and abscess counts.
Ribero et al68 conducted a multi-center retrospective study with 31 patients treated with secukinumab, all patients had failed or had contraindications to at least one anti-TNF-alpha. In this study, the Hidradenitis Suppurativa Clinical Response Score (HiSCR) was achieved by 41% of patients at week 28. No adverse events were reported.68
Our research found no other monometric or multicenter real-life or open-label studies. We found several case reports and case series.
Marasca et al reported two cases;69 the first case involved a 63-year-old man with a history of Hurley’s stage III HS, initially treated with adalimumab who developed a bilateral psoriasiform rash on his lower limbs following treatment with adalimumab. The patient was treated with secukinumab 300 mg per week for the first four weeks and then 300 mg every four weeks, which resulted in complete resolution of the psoriasiform rash and partial resolution of the HS lesions.
The second reported case involved a 46-year-old man with a history of psoriasis for whom he had initiated secukinumab therapy and who subsequently paradoxically developed HS after therapy, this is one of the very few cases of secukinumab-induced HS.69
Thorlacius et al70 reported a case of a 47-year-old man with Hurley’s stage III HS treated with secukinumab with substantial disease improvement, particularly a decrease in the number of lesions from 23 to seven and on the analog pain scale visual score five to three after 12 weeks of therapy.70
Another case report of a 24-year-old man with recalcitrant HS reported by Schuch et al71 the authors indicate that the patient showed almost complete resolution of the inflammatory nodules, the authors however do not show scoring data but only clinical and photographic data.
Jørgensen et al72 reported the case of a 36-year-old woman with stage II Hurley HS treated with secukinumab 300 mg per week for the first four weeks and 300 mg every four weeks thereafter.
This patient was shown to have improved dermatological quality of life Index Score (DLQI) from 17 out of 30 to 5 out of 30, HSS from 76 to 19, Visual Analogue Score (VAS) from 10 out of 10 to 7 out of 10, International Hidradenitis Suppurativa Severity Score (IHS4) from 19 to 1, and the disappearance of most inflammatory lesions after six months of treatment. No adverse events were observed.72
Considering that serum levels of Il-17 are increased in patients with HS,69–73 the data emerging from these case reports would be confirmed by this hypothesis, however the sampling is still very scarce to demonstrate efficacy and safety, in large-scale studies are needed in the future.
Miscellaneous
We did not find real-life studies but only case reports or case series that involved the use of two other biologic drugs for HS: ixekizumab, guselkumab and brodalumab. Ixekizumab (an IL-17A antagonist) is a biologic therapeutic licensed for use in moderate-to-severe plaque psoriasis and psoriatic arthritis,73 represent a new generation of biologic therapy with rapid and high response rates, quickly becoming a crucial part of the psoriasis treatment armamentarium.74,75
Cotter et al76 reported two cases of Hurley’s stage III HS treated with ixekizumab 160 mg starting dose, 80 mg every 2 weeks for 3 months and 80 mg every four weeks thereafter.
The first case was a 42-year-old woman with Hurley’s stage III HS who achieved subjective improvement in the disease; she reported a reduction in swelling and suppuration of the lesions. Similar was the second case of a 34-year-old woman with Hurley’s stage III HS and Crohn’s disease who experienced a subjective improvement in disease severity and a reduction in groin swelling after treatment. We would like to point out, however, that the authors do not report accurate IHS4 or HSS score data.76
Esme et al reported on a case series of five patients with severe HS (Hurley stage III) who had been on adalimumab treatment for 3 months with poor results, these patients were switched to ixekizumab according to the psoriasis therapeutic schedule of administration.77 The primary endpoint was HiSCR after 12 weeks. The secondary endpoints were DLQI and VAS. Four out of 5 patients (80%) achieved HiSCR. While improvement was observed in VAS and DLQI scores of 4 patients. No adverse events to treatment were recorded.77
Megna et al69 reported the case of a 50-year-old man with HS and Psoriasis. Regarding HS lesions, the patient achieved HiSCR (Hidradenitis-Suppurativa-Clinical-Response) at week 10 of treatment and maintained it at the last follow-up visit (week 24). In addition, the pain analog-visual scale (VAS) score decreased from 4 to 1 and the pain/utility/handicap score decreased from 1 to 2.78
As regards anti-IL17, promising data have been reported also for brodalumab.79
Another interesting finding, we found in the literature was the article by Montero-Vichez et al, the authors reported a case series of 4 patients treated with Guselkumab, a fully human monoclonal antibody specifically targeting the p19 subunit of IL-23.80 The primary endpoint involved assessment of IHS4, DLQI and VAS after the 12 weeks of treatment. Two patients achieved 50% improvement while all had moderate improvement after treatment.80 No adverse events were recorded.80 The authors also conducted a systematic review of the literature where they reported another 16 case reports with discordant results-in fact, they conclude that further studies will be needed to establish and clarify the potential role of anti-IL-23 antibodies in this disease.80
Discussion
HS is a chronic, relapsing inflammatory skin condition that needs long-term management and frequent follow-ups.81 Unfortunately, current conventional available drugs (eg antibiotics), are ineffective for the severe forms of the disease as well as their use is contraindicated in several situations. In this scenario, the introduction of biologic drugs in HS treatment opened a new era in HS management. Indeed, these drugs have already been used in other dermatological conditions such as psoriasis and atopic dermatitis,82–86 reporting a high profile in terms of effectiveness and safety for these diseases,87–91 also during COVID-19 era.92–104 Since then, several clinical trials have been carried out to assess their use in HS management. Currently, adalimumab is the only drug approved for HS, but clinical trials and real-life experiences reported promising results also for other biologics.
In our review, we highlighted the current evidence from literature on the use of biologics in HS in a real-life setting, particularly adalimumab, secukinumab and ustekinumab. Data on the effectiveness and safety of biologic drugs in HS management have been analyzed.
In our opinion, the armamentarium of drugs for HS management is increasing, and treatment will be based on a personalized approach in order to choose the right treatment for the right patient at the right moment and to minimize the risk of adverse events. In this context, we want to point out the reported effectiveness and safety data concerning adalimumab, ustekinumab and secukinumab as well as ixekizumab. Of note, many data deriving from real-life, particularly for non-approved biologic drugs, are limited to few case reports and patients were usually affected by HS and other dermatological conditions (eg, psoriasis), requiring biologic treatment for the second one. Thus, the possible coincidence of HS resolution cannot be ruled out.
To sum up, the landscape of HS treatment is changing, also for the introduction of new personalized approaches (eg, telemedicine),105–107 and clinicians must be prepared for the revolution of HS treatment. Biological drugs seem to represent an extraordinary weapon among the armamentarium of HS treatment. Certainly, more studies, more clinical trials and more real-life experiences are required to confirm these promising data. Moreover, the introduction of new biologics for HS and the emerging data will lead to the need for new guidelines.
It is important to set new guidelines to well establish the “therapeutic window” for the biologic treatment because from all these data we can take out that previous is the treatment higher is the response. For this reason, ecografic panelist must set up new criteria that can be associated with the classification of severity of HS based on the real-life data.108–110
Strengths and Limitations
Main strengths of our review are the systematic method during the literature research and the elevated number of investigated articles. Main limitations should be reported. First, only adalimumab is currently approved for HS management. Thus, data from real-life for other biologics are limited. Moreover, several case reports reported the use of biologics for other diseases in patients also affected by HS. Therefore, the casual coincidence of HS improvement during treatment cannot be excluded.
Conclusion
New knowledge on HS pathogenesis is leading to the development of new selective and effective drugs, with a high profile in terms of safety. In this scenario, the introduction of biologic drugs is revolutionizing HS management. However, data based on real-life experiences are limited as only adalimumab is currently approved for HS. Fortunately, several studies on different biologic drugs are ongoing and the results are promising. Certainly, more data are needed.
Data Sharing Statement
Data are reported in the current study and are on request by corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chiricozzi A, Veraldi S, Fabbrocini G, et al. The Hidradenitis Suppurativa (HS) “multidisciplinary unit”: a rationale and practical proposal for an organised clinical approach. Eur J Dermatol. 2018;28(2):274–275. doi:10.1684/ejd.2018.3254
2. Martora F, Martora L, Fabbrocini G, Marasca C. A case of pemphigus vulgaris and hidradenitis suppurativa: may systemic steroids be considered in the standard management of hidradenitis suppurativa? Skin Appendage Disord. 2022;8(3):265–268. doi:10.1159/000521712
3. Jemec GB. Clinical practice Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–164. doi:10.1056/NEJMcp1014163
4. Zouboulis CC, Benhadou F, Byrd AS, et al. What causes hidradenitis suppurativa ?-15 years after. Exp Dermatol. 2020;29(12):1154–1170. doi:10.1111/exd.14214
5. de Oliveira ASLE, Bloise G, Moltrasio C, et al. Transcriptome meta-analysis confirms the hidradenitis suppurativa pathogenic triad: upregulated inflammation, altered epithelial organization, and dysregulated metabolic signaling. Biomolecules. 2022;12(10):1371. doi:10.3390/biom12101371
6. Benzecry V, Grancini A, Guanziroli E, et al. Hidradenitis suppurativa/acne inversa: a prospective bacteriological study and review of the literature. G Ital Dermatol Venereol. 2020;155(4):459–463. doi:10.23736/S0392-0488.18.05875-3
7. Kaleta KP, Nikolakis G, Hossini AM, et al. Metabolic disorders/obesity is a primary risk factor in hidradenitis suppurativa: an immunohistochemical real-world approach. Dermatology. 2022;238(2):251–259. doi:10.1159/000517017
8. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86(5):1092–1101. doi:10.1016/j.jaad.2021.01.059
9. Marasca C, Annunziata MC, Cacciapuoti S, et al. A dermatological questionnaire for general practitioners with a focus on hidradenitis suppurativa. Open Access Maced J Med Sci. 2018;6(10):1902–1905. doi:10.3889/oamjms.2018.358
10. Veraldi S, Guanziroli E, Benzecry V, Nazzaro G. Multidisciplinary approach for hidradenitis suppurativa patients. G Ital Dermatol Venereol. 2018;153(3 Suppl 2):18–19. doi:10.23736/S0392-0488.17.05779-0
11. Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30(Suppl 1):8–17. doi:10.1111/exd.14338
12. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82(5):1061–1082. doi:10.1016/j.jaad.2019.08.089
13. Martora F, Marasca C, Fabbrocini G, Ruggiero A. Strategies adopted in a southern Italian referral centre to reduce Adalimumab discontinuation: comment on ‘Can we increase the drug survival time of biologic therapies in hidradenitis suppurativa?’. Clin Exp Dermatol. 2022;47(10):1864–1865. doi:10.1111/ced.15291
14. Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422. doi:10.1056/NEJMoa1504370
15. Jemec GBE, Okun MM, Forman SB, et al. Adalimumab medium-term dosing strategy in moderate-to-severe hidradenitis suppurativa: integrated results from the phase III randomized placebo-controlled PIONEER trials. Br J Dermatol. 2019;181(5):967–975. doi:10.1111/bjd.17919
16. Martora F, Marasca C, Battista T, Fabbrocini G, Ruggiero A. Management of patients with hidradenitis suppurativa during COVID-19 vaccination: an experience from southern Italy. Comment on: ‘Evaluating the safety and efficacy of COVID-19 vaccination in patients with hidradenitis suppurativa’. Clin Exp Dermatol. 2022;47:2026–2028. doi:10.1111/ced.15306
17. Ruggiero A, Martora F, Picone V, Marano L, Fabbrocini G, Marasca C. Paradoxical hidradenitis suppurativa during biologic therapy, an emerging challenge: a systematic review. Biomedicines. 2022;10(2):455. doi:10.3390/biomedicines10020455
18. AbbVie. Humira; 2017. Available from: http://www.rxabbvie.com/pdf/humira.pdf.
19. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. doi:10.7326/0003-4819-157-12-201212180-00004
20. Kimball AB, Sundaram M, Shields AL, et al. Adalimumab alleviates skin pain in patients with moderate to severe hidradenitis suppurativa: secondary efficacy results from the PIONEER I and PIONEER II randomized controlled trials. J Am Acad Dermatol. 2018;79:1141–1143. doi:10.1016/j.jaad.2018.05.015
21. Zouboulis CC, Okun MM, Prens EP, et al. Long-term Adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80(1):60–69. e2. doi:10.1016/j.jaad.2018.05.040
22. Ryan C, Sobell JM, Leonardi CL, et al. Safety of adalimumab dosed every week and every other week: focus on patients with hidradenitis suppurativa or psoriasis. Am J Clin Dermatol. 2018;19(3):437–447. doi:10.1007/s40257-017-0341-6
23. Marzano AV, Genovese G, Casazza G, et al. Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with Adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol. 2021;184(1):133–140. doi:10.1111/bjd.18983
24. Bettoli V, Manfredini M, Calamo G, et al. Long-term Adalimumab treatment of hidradenitis suppurativa: results and practical insights from a real-life experience. Dermatol Ther. 2018;31(6):e12737. doi:10.1111/dth.12737
25. Martorell A, Caballero A, González Lama Y, et al. Management of patients with hidradenitis suppurativa. Manejo del paciente con hidradenitis supurativa. Actas Dermosifiliogr. 2016;107(Suppl 2):32–42. doi:10.1016/S0001-7310(17)30007-8
26. Chiricozzi A, Giovanardi G, Garcovich S, et al. Clinical and ultrasonographic profile of adalimumab-treated hidradenitis suppurativa patients: a real-life monocentric experience. Acta Derm Venereol. 2020;100(13):adv00172. doi:10.2340/00015555-3520
27. Hafner A, Ghislain PD, Kovács R, et al. Improvement in Hidradenitis Suppurativa and quality of life in patients treated with Adalimumab: real-world results from the HARMONY Study. J Eur Acad Dermatol Venereol. 2021;35(11):2277–2284. doi:10.1111/jdv.17551
28. Gulliver W, Alavi A, Wiseman MC, et al. Real-world moderate-to-severe hidradenitis suppurativa: decrease in disease burden with Adalimumab. J Cutan Med Surg. 2022;26(4):361–370. doi:10.1177/12034754221088584
29. Muralidharan V, Pathmarajah P, Peterknecht E, et al. Real-life data on the biopsychosocial effects of Adalimumab in the management of hidradenitis suppurativa: a multicenter cross-sectional analysis and consideration of a multisystem monitoring approach to follow up. Dermatol Ther. 2021;34(1):e14643. doi:10.1111/dth.14643
30. Kyriakou A, Trigoni A, Galanis N, Sotiriadis D, Patsatsi A. Efficacy of Adalimumab in moderate to severe hidradenitis suppurativa: real life data. Dermatol Reports. 2018;10(2):7859. doi:10.4081/dr.2018.7859
31. Roccuzzo G, Rozzo G, Burzi L, et al. Switching from Adalimumab originator to biosimilars in hidradenitis suppurativa: what’s beyond cost-effectiveness? Dermatol Ther. 2022;35:e15803. doi:10.1111/dth.15803
32. Hayashi N, Hayama K, Takahashi K, et al. Real-world safety and effectiveness of Adalimumab in patients with hidradenitis suppurativa: 12-week interim analysis of post-marketing surveillance in Japan. J Dermatol. 2022;49(4):411–421. doi:10.1111/1346-8138.16297
33. Neves JM, Cunha N, Lencastre A, Cabete J. Treating hidradenitis suppurativa patients with Adalimumab: a real-life experience of a tertiary care center in Lisboa, Portugal. An Bras Dermatol. 2022;97(6):816–819. doi:10.1016/j.abd.2021.12.004
34. Caposiena Caro RD, Chiricozzi A, Sechi A, et al. Flares as dynamic predictive factor of response to Adalimumab in hidradenitis suppurativa: real-life data. Ital J Dermatol Venerol. 2022;157(3):240–246. doi:10.23736/S2784-8671.21.07049-3
35. Nazzaro G, Calzari P, Passoni E, et al. Vascularization and fibrosis are important ultrasonographic tools for assessing response to Adalimumab in hidradenitis suppurativa: prospective study of 32 patients. Dermatol Ther. 2021;34(1):e14706. doi:10.1111/dth.14706
36. Bahillo Monné C, Honorato Guerra S, Schoendorff Ortega C, Gargallo Quintero AB. Management of hidradenitis suppurativa with biological therapy: report of four cases and review of the literature. Dermatology. 2014;229(4):279–287. doi:10.1159/000365076
37. Gorovoy I, Berghoff A, Ferris L. Successful treatment of recalcitrant hidradenitis suppurativa with Adalimumab. Case Rep Dermatol. 2009;1(1):71–77. doi:10.1159/000251217
38. Harde V, Mrowietz U. Treatment of severe recalcitrant hidradenitis suppurativa with Adalimumab. J Dtsch Dermatol Ges. 2009;7(2):139–141. doi:10.1111/j.1610-0387.2008.06918.x
39. Yamauchi PS, Mau N. Hidradenitis suppurativa managed with Adalimumab. J Drugs Dermatol. 2009;8(2):181–183.
40. Bosnić D, Žarković B, Barešić M, Zarkovic M, Anic B. Improvement of overlapping hidradenitis suppurativa and ankylosing spondylitis after the introduction of Adalimumab. Reumatologia. 2016;54(6):321–325. doi:10.5114/reum.2016.64910
41. Diamantova D, Lomickova I, Cetkovska P. Adalimumab treatment for hidradenitis suppurativa associated with Crohn’s disease. Acta Dermatovenerol Croat. 2014;22(4):291–293.
42. Murphy B, Morrison G, Podmore P. Successful use of Adalimumab to treat pyoderma gangrenosum, acne and suppurative hidradenitis (PASH syndrome) following colectomy in ulcerative colitis. Int J Colorectal Dis. 2015;30(8):1139–1140. doi:10.1007/s00384-014-2110-9
43. Reddick CL, Singh MN, Chalmers RJ. Successful treatment of superficial pyoderma gangrenosum associated with hidradenitis suppurativa with Adalimumab. Dermatol Online J. 2010;16(8):15. doi:10.5070/D34PH5R1M2
44. Scheinfeld N. Treatment of coincident seronegative arthritis and hidradentis supprativa with Adalimumab. J Am Acad Dermatol. 2006;55(1):163–164. doi:10.1016/j.jaad.2006.01.024
45. De Wet J, Jordaan HF, Kannenberg SM, Tod B, Glanzmann B, Visser WI. Pyoderma gangrenosum, acne, and suppurative hidradenitis syndrome in end-stage renal disease successfully treated with Adalimumab. Dermatol Online J. 2017;23:12. doi:10.5070/D32312037669
46. Saraceno R, Babino G, Chiricozzi A, Zangrilli A, Chimenti S. PsAPASH: a new syndrome associated with hidradenitis suppurativa with response to tumor necrosis factor inhibition. J Am Acad Dermatol. 2015;72(1):e42–44. doi:10.1016/j.jaad.2014.10.002
47. Yiu ZZN, Becher G, Kirby B, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158(10):1131–1141. doi:10.1001/jamadermatol.2022.2909
48. Yu Q, Ge X, Jing M, et al. A systematic review with meta-analysis of comparative efficacy and safety of risankizumab and ustekinumab for psoriasis treatment. J Immunol Res. 2022;2022:2802892. doi:10.1155/2022/2802892
49. Ye L, Wu Z, Li C, Zhao X, Wan M, Wang L. Off-label uses of ustekinumab. Dermatol Ther. 2022;35:e15910. doi:10.1111/dth.15910
50. Hollywood A, Murray G, Fleming S, Kirby B, Hughes R. Ustekinumab in the management of hidradenitis suppurativa: a retrospective study. J Drugs Dermatol. 2022;21(3):319–320. doi:10.36849/JDD.6298
51. Montero-Vilchez T, Pozo-Román T, Sánchez-Velicia L, Vega-Gutiérrez J, Arias-Santiago S, Molina-Leyva A. Ustekinumab in the treatment of patients with hidradenitis suppurativa: multicenter case series and systematic review. J Dermatolog Treat. 2022;33(1):348–353. doi:10.1080/09546634.2020.1755008
52. Baerveldt EM, Kappen JH, Thio HB, van Laar JA, van Hagen PM, Prens EP. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72(4):626–627. doi:10.1136/annrheumdis-2012-202392
53. Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174(4):839–846. doi:10.1111/bjd.14338
54. Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26(7):911–914. doi:10.1111/j.1468-3083.2011.04123.x
55. Santos-Pérez MI, García-Rodicio S, Del Olmo-Revuelto MA, Pozo-Román T. Ustekinumab for hidradenitis suppurativa: a case report. Actas Dermosifiliogr. 2014;105(7):720–722. doi:10.1016/j.ad.2013.09.011
56. Sharon VR, Garcia MS, Bagheri S, et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92(3):320–321. doi:10.2340/00015555-1229
57. Romaní J, Vilarrasa E, Martorell A, Fuertes I, Ciudad C, Molina-Leyva A. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236(1):21–24. doi:10.1159/000501075
58. Scholl L, Hessam S, Garcovich S, Bechara FG. High-dosage ustekinumab for the treatment of severe hidradenitis suppurativa. Eur J Dermatol. 2019;29(6):659–661. doi:10.1684/ejd.2019.3663
59. Jiang SW, Kwock JT, Liu B, et al. High-dose, high-frequency ustekinumab therapy for patients with severe hidradenitis suppurativa. Br J Dermatol. 2022;187(3):417–419. doi:10.1111/bjd.21066
60. Takeda K, Kikuchi K, Kanazawa Y, Yamasaki K, Aiba S. Ustekinumab treatment for hidradenitis suppurativa. J Dermatol. 2019;46(12):1215–1218. doi:10.1111/1346-8138.15122
61. Martora F, Picone V, Fabbrocini G, Marasca C. Hidradenitis suppurativa flares following COVID-19 vaccination: a case series. JAAD Case Rep. 2022;23:42–45. doi:10.1016/j.jdcr.2022.03.008
62. Ruggiero A, Marasca C, Villani A, Fabbrocini G, Martora F. Tacrolimus ointment may improve the effectiveness of Adalimumab in patients with hidradenitis suppurativa: a novel promising treatment. Clin Exp Dermatol. 2022;47(10):1871–1872. doi:10.1111/ced.15299
63. Napolitano M, Fabbrocini G, Martora F, Picone V, Morelli P, Patruno C. Role of aryl hydrocarbon receptor activation in inflammatory chronic skin diseases. Cells. 2021;10(12):3559. doi:10.3390/cells10123559
64. Secukinumab. Drugs and Lactation Database (Lactmed). Bethesda (MD): National Library of Medicine (US); 2022.
65. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi:10.1056/NEJMoa1314258
66. Wu KK, Dao H. Off-label dermatologic uses of IL-17 inhibitors. J Dermatolog Treat. 2022;33(1):41–47. doi:10.1080/09546634.2020.1737638
67. Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609–611. doi:10.1111/bjd.17822
68. Ribero S, Ramondetta A, Fabbrocini G, et al. Effectiveness of Secukinumab in the treatment of moderate-severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol. 2021;35. doi:10.1111/jdv.17178
69. Marasca C, Megna M, Balato A, Balato N, Napolitano M, Fabbrocini G. Secukinumab and hidradenitis suppurativa: friends or foes? JAAD Case Rep. 2019;5(2):184–187. doi:10.1016/j.jdcr.2018.12.002
70. Thorlacius L, Theut Riis P, Jemec GBE. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2018;179(1):182–185. doi:10.1111/bjd.15769
71. Schuch A, Fischer T, Boehner A, Biedermann T, Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. 2018;98(1):151–152. doi:10.2340/00015555-2794
72. Jørgensen AR, Yao Y, Thomsen SF. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018;2018:8685136. doi:10.1155/2018/8685136
73. Ixekizumab. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2017.
74. Marasca C, Fornaro L, Martora F, Picone V, Fabbrocini G, Megna M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol Ther. 2021;34(5):e15102. doi:10.1111/dth.15102
75. Martora F, Battista T, Fornaro L, et al. Generalized versus localized vitiligo after ixekizumab: may previous treatment affect the clinical presentation? Dermatol Ther. 2022;35:e15874. doi:10.1111/dth.15874
76. Cotter C, Tobin AM, O’Connor R, et al. Severe refractory hidradenitis suppurativa: treatment with ixekizumab, two case reports. Bristol Cup. 2018;179:24–73.
77. Esme P, Botsali A, Akoglu G, Caliskan E. An anti-interleukin-17A monoclonal antibody, ixekizumab, in the treatment of resistant hidradenitis suppurativa: a case series. Skin Appendage Disord. 2022;8(4):342–345. doi:10.1159/000521860
78. Megna M, Ruggiero A, Di Guida A, Patrì A, Fabbrocini G, Marasca C. Ixekizumab: an efficacious treatment for both psoriasis and hidradenitis suppurativa. Dermatol Ther. 2020;33(4):e13756. doi:10.1111/dth.13756
79. Navrazhina K, Frew JW, Grand D, et al. Interleukin-17RA blockade by brodalumab decreases inflammatory pathways in hidradenitis suppurativa skin and serum. Br J Dermatol. 2022;187(2):223–233. doi:10.1111/bjd.21060
80. Montero-Vilchez T, Martinez-Lopez A, Salvador-Rodriguez L, Arias-Santiago S, Molina-Leyva A. The use of guselkumab 100 mg every 4 weeks on patients with hidradenitis suppurativa and a literature review. Dermatol Ther. 2020;33(3):e13456. doi:10.1111/dth.13456
81. Megna M, Potestio L, Fabbrocini G, et al. Tildrakizumab: a new therapeutic option for erythrodermic psoriasis? Dermatol Ther. 2021;34(5):e15030. doi:10.1111/dth.15030
82. Megna M, Potestio L, Ruggiero A, et al. Risankizumab treatment in psoriasis patients who failed anti-IL17: a 52-week real-life study. Dermatol Ther. 2022;35(7):e15524. doi:10.1111/dth.15524
83. Tsai YC, Tsai TF. Switching biologics in psoriasis - practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13(5):493–503. doi:10.1080/17512433.2020.1767590
84. Megna M, Potestio L, Fabbrocini G, et al. Treating psoriasis in the elderly: biologics and small molecules. Expert Opin Biol Ther. 2022;22:1–18.
85. Napolitano M, Maffei M, Patruno C, et al. Dupilumab effectiveness for the treatment of patients with concomitant atopic dermatitis and chronic rhinosinusitis with nasal polyposis. Dermatol Ther. 2021;34(6):e15120. doi:10.1111/dth.15120
86. Megna M, Potestio L, Battista T, et al. Immune response to COVID-19 mRNA vaccination in patients with psoriasis undergoing treatment with biologics. Clin Exp Dermatol. 2022;47:2310–2312. doi:10.1111/ced.15395
87. Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis: a retrospective cohort study. J Am Acad Dermatol. 2022;S0190–9622(22):2417
88. He M, Ferris LK, Gabriel N, et al. COVID-19 and adherence to biologic therapies for psoriasis: an analysis of nationwide pharmacy claims data. J Manag Care Spec Pharm. 2022;28(11):1213–1218. doi:10.18553/jmcp.2022.28.11.1213
89. Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi:10.2147/CCID.S364640
90. Megna M, Tommasino N, Potestio L, et al. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an Italian 28-week retrospective study. J Dermatolog Treat. 2022;33(6):2813–2820. doi:10.1080/09546634.2022.2081655
91. Potestio L, Villani A, Fabbrocini G, et al. Cutaneous reactions following booster dose of COVID-19 mRNA vaccination: what we should know? J Cosmet Dermatol. 2022;21:5339–5340. doi:10.1111/jocd.15331
92. Martora F, Villani A, Battista T, et al. COVID-19 vaccination and inflammatory skin diseases. J Cosmet Dermatol. 2022. doi:10.1111/jocd.15414
93. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11(6):1511. doi:10.3390/jcm11061511
94. Megna M, Camela E, Villani A, et al. Teledermatology: a useful tool also after COVID-19 era? J Cosmet Dermatol. 2022;21(6):2309–2310. doi:10.1111/jocd.14938
95. Martora F, Fabbrocini G, Marasca C. Pityriasis rosea after Moderna mRNA-1273 vaccine: a case series. Dermatol Ther. 2022;35(2):e15225. doi:10.1111/dth.15225
96. Martora F, Fabbrocini G, Nappa P, Megna M. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani et al. J Eur Acad Dermatol Venereol. 2022;36(10):e750–e751. doi:10.1111/jdv.18302
97. Picone V, Martora F, Fabbrocini G, Marano L. ”Covid arm”: abnormal side effect after Moderna COVID-19 vaccine. Dermatol Ther. 2022;35(1):e15197. doi:10.1111/dth.15197
98. Ruggiero A, Megna M, Fabbrocini G. Video, and telephone teledermatology consultations during COVID-19 in comparison: patient satisfaction, doubts and concerns. Clin Exp Dermatol. 2022;47(10):1863–1864. doi:10.1111/ced.15286
99. Camela E, Potestio L, Fabbrocini G, et al. New frontiers in personalized medicine in psoriasis. Expert Opin Biol Ther. 2022;22:1–3.
100. Mintoff D, Benhadou F. Guselkumab does not appear to influence the IgG antibody response to SARS-CoV-2. Dermatol Ther. 2022;35(2):e15246. doi:10.1111/dth.15246
101. Martora F, Battista T, Marasca C, Genco L, Fabbrocini G, Potestio L. Cutaneous reactions following COVID-19 vaccination: a review of the current literature. Clin Cosmet Investig Dermatol. 2022;15:2369–2382. doi:10.2147/CCID.S388245
102. De Lucia M, Potestio L, Costanzo L, et al. Scabies outbreak during COVID-19: an Italian experience. Int J Dermatol. 2021;60(10):1307–1308. doi:10.1111/ijd.15809
103. Picone V, Fabbrocini G, Martora L, Martora F. A case of new-onset lichen planus after COVID-19 vaccination. Dermatol Ther. 2022;12(3):801–805. doi:10.1007/s13555-022-00689-y
104. Martora F, Picone V, Fornaro L, Fabbrocini G, Marasca C. Can COVID-19 cause atypical forms of pityriasis rosea refractory to conventional therapies? J Med Virol. 2022;94(4):1292–1293. doi:10.1002/jmv.27535
105. Camela E, Potestio L, Ruggiero A, et al. Towards Personalized Medicine in Psoriasis: current Progress. Psoriasi. 2022;12:231–250. doi:10.2147/PTT.S328460
106. Ruggiero A, Martora F, Picone V, et al. The impact of COVID-19 infection on patients with psoriasis treated with biologics: an Italian experience. Clin Exp Dermatol. 2022;47:2280–2282. doi:10.1111/ced.15336
107. Marasca C, Marasca D, Megna M, Annunziata MC, Fabbrocini G. Ultrasound: an indispensable tool to evaluate the outcome of surgical approaches in patients affected by hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(8):e413–e414. doi:10.1111/jdv.16361
108. Krajewski PK, Jfri A, Ochando-Ibernón G, Martorell A. Ultrasonographic railway sign in tunnels as a new independent risk factor of Adalimumab failure in hidradenitis suppurativa. J Am Acad Dermatol. 2022. doi:10.1016/j.jaad.2022.08.064
109. Nazzaro G, Passoni E, Calzari P, Marzano AV. Ultrasonographic assessment of fibrosis in hidradenitis suppurativa fistulae helps in addressing treatment. Skin Res Technol. 2020;26(3):445–446. doi:10.1111/srt.12805
110. Nazzaro G, Passoni E, Calzari P, et al. Color Doppler as a tool for correlating vascularization and pain in hidradenitis suppurativa lesions. Skin Res Technol. 2019;25(6):830–834. doi:10.1111/srt.12729
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.