Back to Journals » Psoriasis: Targets and Therapy » Volume 12
Efficacy and Safety of Anti-TNF Biosimilars for Psoriasis in Pediatric and Geriatric Populations: A 72-Week Real-Life Study
Authors Megna M, Fornaro L, Potestio L , Luciano MA, Nocerino M, Delfino M, Guarino M, Fabbrocini G, Camela E
Received 9 March 2022
Accepted for publication 19 May 2022
Published 9 July 2022 Volume 2022:12 Pages 199—204
DOI https://doi.org/10.2147/PTT.S365493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Matteo Megna, Luigi Fornaro, Luca Potestio, Maria Antonietta Luciano, Mariateresa Nocerino, Mario Delfino, Maria Guarino, Gabriella Fabbrocini, Elisa Camela
Section of Dermatology - Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, NA, Italy
Correspondence: Elisa Camela, Section of Dermatology - Department of Clinical Medicine and Surgery, University of Naples Federico II, Via Pansini 5, Naples, NA, 80131, Italy, Tel +39 - 081 – 7462457, Fax +39 - 081 – 7462442, Email [email protected]
Purpose: To determine the efficacy and safety of adalimumab (ADA) and etanercept (ETA) biosimilars in elderly and children with psoriasis.
Methods: A real-life retrospective observational study was conducted on pediatric (< 18 years) and geriatric (≥ 65 years) psoriasis patients treated with anti-TNF biosimilar agents referring to the Psoriasis Unit of the University of Naples Federico II, Italy, from January 2018 to January 2022. At baseline, demographic characteristics (age and sex), data on psoriasis duration and severity (measured by Psoriasis Area Severity Index [PASI] and body surface area [BSA]), presence of psoriatic arthritis if applicable, comorbidities, and previous psoriasis treatments were recorded. Patients were monitored by regular follow-ups (week 12, 24, 48 and 72) through clinical and haematological assessments and adverse events (AEs) were registered.
Results: A total of 11 children and 23 elderly psoriasis patients were enrolled. Concerning children, 6 (54.5%) were under ADA biosimilar and 5 (45.5%) under ETA biosimilar. ETA and ADA biosimilars were equally effective and safe for up to 72 weeks (mean PASI and BSA < 3). No significant AEs were reported, and none discontinued treatment. In the elderly, 15 (65.2%) were treated with ADA biosimilar and 8 (34.8%) with ETA biosimilar. ETA and ADA biosimilars were equally effective up to 72 weeks (mean PASI < 4 and mean BSA < 5%). AEs (mainly mild) were registered in 9 subjects (39.1%). Also, 4 (17.4%) patients discontinued biologicals for secondary lack of efficacy (3, 75%) or AEs (1, 25%).
Conclusion: Our study found that ADA and ETA biosimilars are effective and safe for the treatment of moderate-to-severe psoriasis in children and the elderly. No statistically significant efficacy and safety differences were found between ADA and ETA biosimilars in both children and the elderly. Geriatric patients displayed a higher discontinuation rate and side effects than the pediatric counterpart even if without approaching statistical significance.
Keywords: psoriasis, etanercept, adalimumab, biosimilar, treatment
Introduction
Biological agents represent an invaluable therapeutic opportunity for patients affected by moderate-to-severe psoriasis, especially if recalcitrant or if their comorbidities contraindicate conventional systemic treatments.1 Indeed, these drugs show an optimal safety and efficacy profile as well as a fast and durable action.1 Anyway, the costs of their production are huge, so that their prescription is subject to and limited by governmental health measures, leading to a non-equal access to care for patients.2 To date, 12 different drugs have been approved by the US FDA for the treatment of psoriasis and/or psoriatic arthritis and are categorized into four classes according to the target molecule: anti-Tumour Necrosis Factor (TNF), anti-Interleukin (IL)-12/23, anti-IL17 and anti-IL23. Anti-TNFs were the first to be developed and commercialized: in detail, adalimumab, etanercept and infliximab were approved more than 20 years ago (2002, 1998 and 1998, respectively), so that their biosimilars are currently available.3–5 This implies an important cost saving as well as improvement in patients’ access to treatment options worldwide.2,5 Several studies have already shown the efficacy and safety of biosimilars in treating psoriasis and even their comparability with originators.6–13 Anyway, since real-life data are scant, especially regarding special populations that are underrepresented in clinical trials, ie, children and elderly, we decide to perform a retrospective study to assess efficacy and safety of anti-TNF biosimilars in these categories of patients.
Materials and Methods
A real-life retrospective observational study was conducted on pediatric (<18 years) and geriatric (≥65 years) psoriasis patients treated with anti-TNF biosimilar agents referring to the Psoriasis Unit of the University of Naples Federico II, Italy, from January 2018 to January 2022. Data were gathered from the local computer database employed in daily clinical practice. Inclusion criteria were as follows: age <18 years or ≥65 years, a diagnosis of moderate-to-severe plaque psoriasis for at least 12 months and a minimum of 48 weeks of treatment with anti-TNF biosimilar agents (adalimumab or etanercept biosimilar). Infliximab biosimilar has not been considered in our study due to the very limited number of patients (this biosimilar is not approved for pediatric psoriasis and it is the only biologic administered intravenously, requiring hospital access which had been strictly limited due to COVID-19 pandemic). The study was drawn according to the ethical standards laid down in the World Medical Association Declaration of Helsinki and its later amendments and was approved by the local Ethical Committee, ie, Comitato Etico Università Federico II. Informed consent was obtained by participants and guardians prior to the study commencement. Adalimumab and etanercept biosimilars were administered subcutaneously at the label dosage: for adults 40 mg and 50 mg, and for children according to body weight, every 2 and 1 week, respectively. At baseline, demographic characteristics (age and sex), data on psoriasis duration and severity (measured by Psoriasis Area Severity Index [PASI] and body surface area [BSA]), presence of psoriatic arthritis if applicable, comorbidities, and previous psoriasis treatments were recorded. Patients were monitored by regular follow-ups (week 12, 24, 48 and 72) through clinical and haematological assessments (full blood count, glycemia, transaminases, gamma-glutamyl transpeptidase, erythrocyte sedimentation rate, C-reactive protein, urea, creatinine, cholesterol and triglycerides, protein electrophoresis) and any occurrence of treatment-emergent adverse events (AEs) was recorded. Effectiveness data were analysed using the last observation carried forward method, where, if a patient dropped out of the study, the last available value was “carried forward” until the end of the treatment.
Statistical Analysis
Continuous variables were displayed as mean ± standard deviation, and categorical variables or as the number and proportion of patients. Mann Whitney U-Test was used to calculate the significance of differences in mean values at the different time points of treatment. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
A total of 11 children and 23 elderly psoriasis patients were enrolled: mean age was 12.70 ± 3.8 (range 5–17) for the first and 73.5 ± 5.7 (range 65–88) for the second group. All data are illustrated in Table 1. Two young (18.1%) and seven old (30.4%) subjects had concomitant psoriatic arthritis. Concerning comorbidities, elderly was obviously more affected than young (21/23 [91.3%] vs 1/11 [9.1%], p < 0.001), and the most commonly reported were hypertension (86.9%), dyslipidaemia (65.2%) and diabetes (26.1%) in elderly compared to inflammatory bowel diseases (27.3%) in pediatric one. Previous treatments included topical therapies in all participants (100%); NB-UVB phototherapy was the most utilized treatment for children (5/11, 45.5%), while methotrexate for the elderly (12/23, 52.2%). Previous biologic failure was more common in elderly (5, 21.7%) compared to pediatric group (1, 9.1%) albeit this data did not reach significance (P = 0.56). At baseline, mean PASI was 16.1 ±4.7 for the first group and 17.6 ± 4.5 for the second, while mean BSA was 31.3 ±12.7 and 25.7 ± 3.6, respectively. Hence, both groups showed comparable disease severity at baseline.
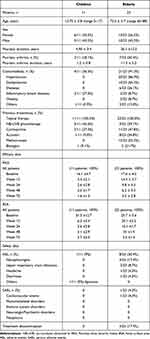 |
Table 1 Characteristics of the Study Population |
Children
Of the 11 enrolled patients, 6 (54.5%) were under adalimumab biosimilar and 5 (45.5%) under etanercept biosimilar. Concerning the first group, mean PASI decreased from 16.8 ± 5.5 at baseline to 2.1 ± 2.7 by week 72; similarly, BSA reduced from 26.4 ±12.1 to 2.9 ±2.7. As for the second group, mean PASI was 15.7 ±5.4 at baseline and reached 1.9 ±1.4 by the end of study (EoS). Likewise, BSA improved by around 90% from 33.3 ±9.9 to 3.0 ±2.5. The efficacy of adalimumab and etanercept was comparable at each follow-up visit. Safety monitoring was performed and throughout the study only one mild AE (epistaxis) was reported in one patient (9.1%) and no serious AEs were recorded. None had withdrawn treatment by EoS for secondary lack of efficacy or AEs.
Elderly
Of the 23 enrolled patients, 15 (65.2%) were under adalimumab biosimilar and 8 (34.8%) under etanercept biosimilar. Concerning the first group, mean PASI decreased from 16.1 ± 6.8 at baseline to 3.3 ± 1.1 by week 72; at the same time, BSA reduced from 26.3 ±8.1 to 4.1 ± 1.5. While for the etanercept biosimilar group, mean PASI was 15.6 ± 5.5 at baseline and reached 3.6 ± 1.1 by the EoS. Likewise, BSA improved by around 90% from 27.12 ±7.6 to 4.5 ± 2.1. Overall, no significant difference between adalimumab and etanercept was observed. As regards safety, 8 patients (34.8%) complained about mild AEs: 4 (17.4%) reported nasopharyngitis, 2 (8.7%) upper respiratory tract infections, 1 (4.3%) headache, 1 (4.3%) diarrhea. One (4.3%) experienced a serious AE (cardiovascular) for which treatment discontinuation was prescribed. Also, three other patients suspended treatment (n = 2 etanercept, n = 1 adalimumab) for secondary inefficacy. Hence, treatment discontinuation was observed in 4 (17.4%) subjects: 3 (75%) for secondary lack of efficacy and 1 (25%) for AE. AEs (9/23, 39.1% vs 1/11, 9.1%), as well as discontinuation rate (4/23, 17.4% vs 0/11, 0%), were higher in elderly with respect to children, however, without reaching statistical significance (P = 0.16 and P = 0.42, respectively).
Discussion
The availability of biosimilars for the treatment of psoriasis has boosted patients’ access to biotherapies all over the world, since the more sustainable costs for health care systems and private insurances.2,5 To date, physicians can rely on adalimumab, etanercept and infliximab biosimilars, for which consistent data concerning their comparability with the respective originators exist.6–13 Anyway, real-life experiences on the efficacy and safety of biosimilars in psoriasis patients are scant, especially regarding two categories of psoriasis population, ie, children and elderly, that are underrepresented in clinical trials. In this study, we report data on the efficacy and safety of adalimumab and etanercept biosimilars, in these fragile categories of subjects. Concerning adalimumab biosimilar, our study highlighted an excellent efficacy and safety profile in children as in the elderly. In detail, PASI and BSA decreased significantly from baseline to EoS in both groups (p < 0.001). Analogous findings were showed for etanercept biosimilar (p < 0.001). Hence, both biological agents are allowed to reach minimal disease activity with a mean PASI value <3 by the EoS in the analyzed cohorts. In detail, no statistically significant differences in terms of efficacy were observed between the two biosimilars in both children and the geriatric population. Concerning safety, the elderly population was more affected from AEs that children, especially regarding upper respiratory tract infections and pharyngitis, even if without reaching significance (9/23, 39.1% vs 1/11, 9.1%, P = 0.24). However, particular attention should be paid when administering anti-TNF agents to geriatric patients, considering individual predisposing factors for these events, especially in patients affected by multiple comorbidities and on polypharmacy. Treatment discontinuation was more common for the elderly with respect to pediatric group (4/23, 17.4% vs 0/11, 0%); however, these results did not approach statistical significance. In detail, 4 geriatric patients suspended treatment: three for secondary lack of efficacy and one for a serious AE. By contrast, all the included children did not undergo treatment withdrawal by week 72. This finding may be influenced by the lower number of pediatric subjects and the higher percentage of comorbidities as well as previous failure (including biologics) observed in the elderly. Overall, our findings are in line with those of clinical trials, as shown in the package insert of both drugs, where biosimilars are displayed to be superior to placebo in terms of short- and long-term efficacy regardless of patients’ age.14,15 Concerning safety, side effects were comparable between children and adults, provided the weight-dependent dosage, with both drugs, while in the comparison between elderly and adults, no difference was observed with etanercept, while an increased risk was highlighted with adalimumab. Anyway, no dosage adjustment was required in either of the two products.14,15 Concluding, several studies have proven the similarity in terms of efficacy, safety and immunogenicity between biosimilars and originators, allowing the switch from one to the other, without losing performance and increasing side effects.6–13 Hence, data on originators directly apply to the respective biosimilars.6–13 For example, Migliore et al, in a retrospective study to evaluate the efficacy of anti-TNF in the elderly with inflammatory diseases, of which psoriasis was included, reported that they are a safe option and increase quality of life, providing a thorough screening before prescription in order to reduce the well-known treatment-related side effects (infectious and cardiovascular).16 Also, the S2K guidelines on the treatment of psoriasis in children recommend the use of biosimilars within the approved indications and provide the required infectious screening.17
Limitations
Our study has some limitations: first, the design of the analysis, ie, retrospective, and second, the very small sample size of the population involved, especially the pediatric one.
Conclusion
Our analysis has shown an excellent efficacy and safety profile of adalimumab and etanercept biosimilars in children and elderly. No statistically significant efficacy and safety differences were found between ADA and ETA biosimilars in both children and the elderly. Geriatric patients displayed a higher discontinuation rate and side effects than the pediatric counterpart even if without approaching statistical significance. In any case, more real-life data are needed to support our findings.
Ethical Approval
The local ethical committee, Comitato Etico Università Federico II, approved the study.
Funding
AIFA fund 2012–2014 in the project: “farmaci biotecnologici: valutazione comparativa degli eventi avversi tra i farmaci bio-originators e i biosimilari”.
Disclosure
Doctor Matteo Megna acted as speaker or consultant for Novartis, Eli Lilly and AbbVie. Professor Gabriella Fabbrocini acted as speaker or consultant for Janssen, Leo Pharma, Novartis, Eli Lilly, AbbVie, and Almirall. The authors report no other potential conflicts of interest in relation to this work.
References
1. Dave R, Alkeswani A. An overview of biologics for psoriasis. J Drugs Dermatol. 2021;20:1246–1247. doi:10.36849/jdd.6040
2. Padda IS, Bhatt R, Rehman O, Parmar M. Biosimilars Use in Medicine for Inflammatory Diseases. Treasure Island (FL); 2022.
3. World Health Organization. Guidelines on Evaluation of Biosimilars. Geneva: WHO; 2022: 1–52.
4. Al-Janabi A, Yiu ZZN. Biologics in psoriasis: updated perspectives on long-term safety and risk management. Psoriasis. 2022;12:1–14. doi:10.2147/PTT.S328575
5. Singh SC, Bagnato KM. The economic implications of biosimilars. Am J Manag Care. 2015;21:s331–40.
6. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177:1562–1574. doi:10.1111/bjd.15857
7. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76:1093–1102. doi:10.1016/j.jaad.2016.12.014
8. Lund T, Sand C, Gniadecki R, Thomsen SF. Effectiveness and safety of switching to biosimilar infliximab and etanercept in patients with psoriasis. Dermatol Ther. 2019;32:e12846. doi:10.1111/dth.12846
9. Goll GL, Jørgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. 2019;285:653–669. doi:10.1111/joim.12880
10. Gerdes S, Thaçi D, Griffiths CEM, et al. Multiple switches between GP2015, an etanercept biosimilar, with originator product do not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis: 30-week results from the phase 3, confirmatory EGALITY study. J Eur Acad Dermatol Venereol. 2018;32:420–427. doi:10.1111/jdv.14605
11. Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–519. doi:10.1111/bjd.16102
12. Dapavo P, Vujic I, Fierro MT, Quaglino P, Sanlorenzo M. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:736–739. doi:10.1016/j.jaad.2016.04.068
13. Hercogová J, Papp KA, Chyrok V, Ullmann M, Vlachos P, Edwards CJ. AURIEL-PsO: a randomized, double-blind phase iii equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2020;182:316–326. doi:10.1111/bjd.18220
14. Adalimumab biosimilar (amgevita) - prescribing information; 2021. Available from: https://Www.Ema.Europa.Eu/En/Documents/Product-Information/Amgevita-Epar-Product-Information_en.Pdf.
15. Etanercept biosimilar (benepali) - prescribing information; 2019. Available from: https://Www.Ema.Europa.Eu/En/Documents/Product-Information/Benepali-Epar-Product-Information_en.Pdf.
16. Migliore A, Bizzi E, Laganà B, et al. The safety of anti-TNF agents in the elderly. Int J Immunopathol Pharmacol. 2009;22:415–426. doi:10.1177/039463200902200218
17. Lang BM, Balermpas P, Bauer A, et al. S2k guidelines for cutaneous basal cell carcinoma - part 2: treatment, prevention and follow-up. J Dtsch Dermatol Ges. 2019;17:214–230. doi:10.1111/ddg.13755
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
